Brain Oscillatory Activity during Tactile Stimulation Correlates with Cortical Thickness of Intact Areas and Predicts Outcome in Post-Traumatic Comatose Patients
Abstract
:1. Introduction
2. Methods
2.1. Participants
2.2. Procedure
2.3. Stimuli
- (1)
- Slow stroking (2–3 cm/s) with a soft brush (60 mm, squirrel hair);
- (2)
- Fast stroking (12–15 cm/s) with a soft brush (60 mm, squirrel hair);
- (3)
- Slow stroking (2–3 cm/s) with a hard brush (60 mm, natural bristle);
- (4)
- Fast stroking (12–15 cm/s) with a hard brush (60 mm, natural bristle).
2.4. Subjective Assessment of Stimuli
2.5. EEG Registration
2.6. EEG Data Analysis
2.7. MRI Acquisition
2.8. Structural Morphometry
2.9. Statistical Analysis
2.9.1. Between and Within-Group Comparison
2.9.2. Within-Group Correlations
2.9.3. Comparison of PSD in Rest and Stimulation at the Individual Level
3. Results
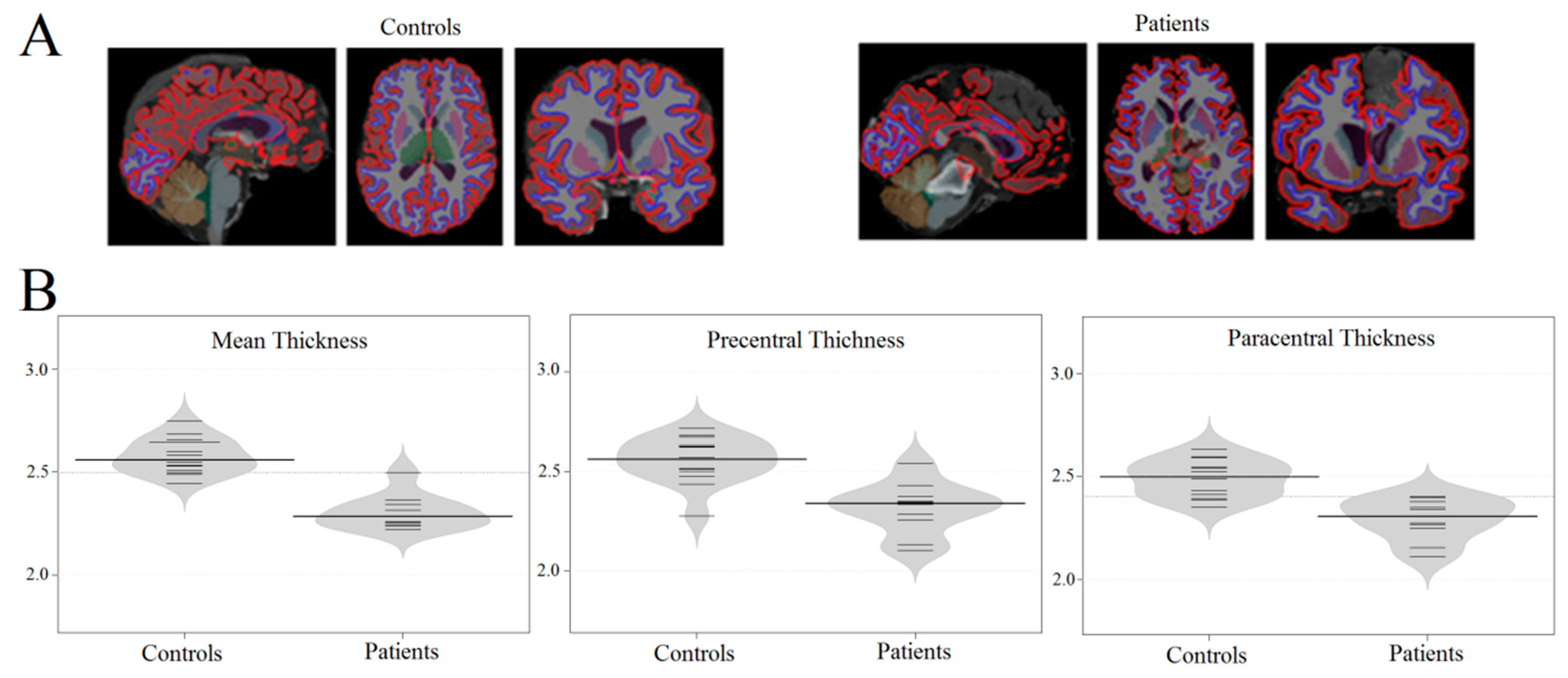
3.1. Brain Atrophy in Coma Patients
3.2. Altered Resting-State EEG in Comatose Patients
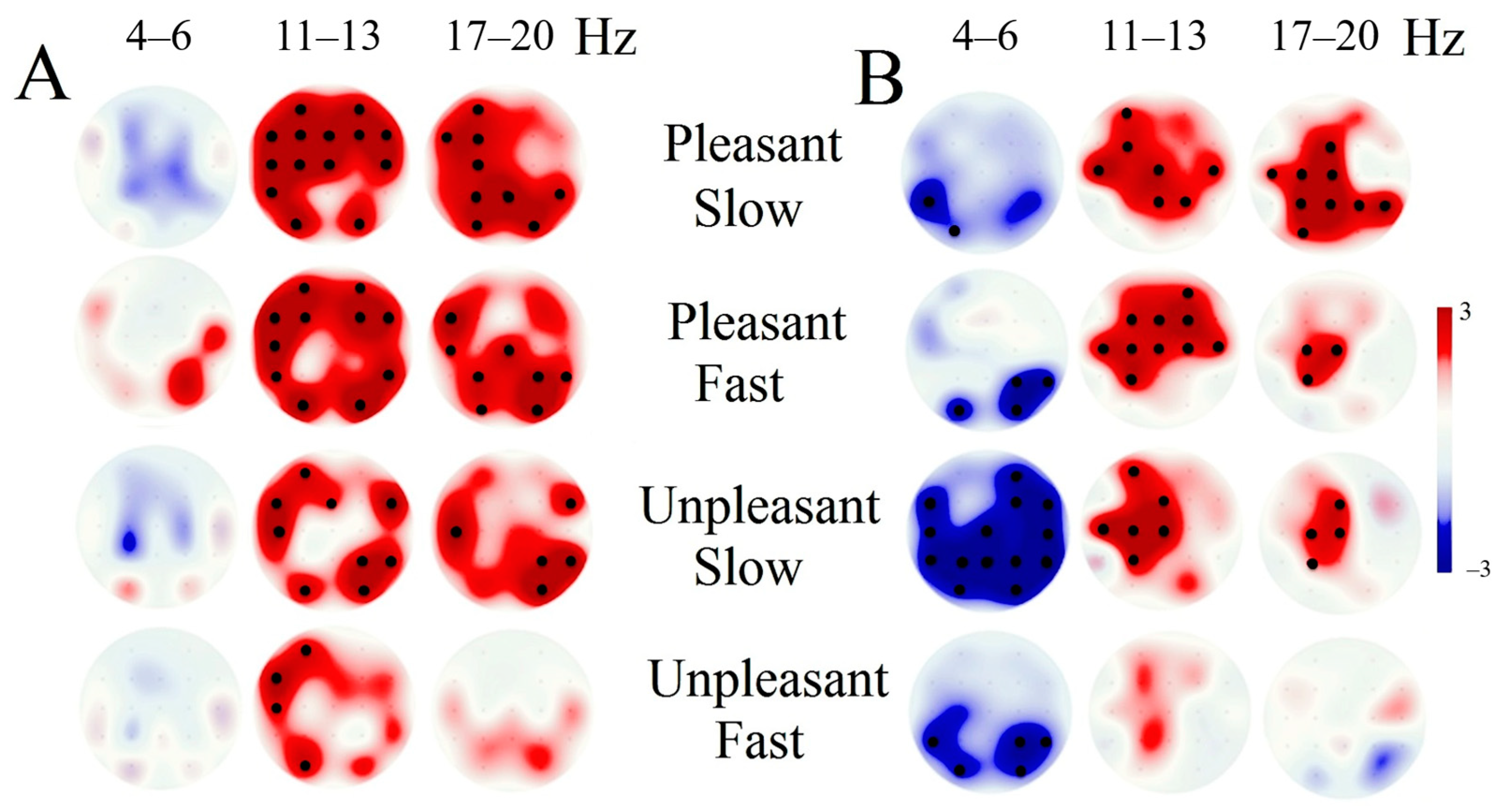
3.3. EEG Response Tactile Stimulation
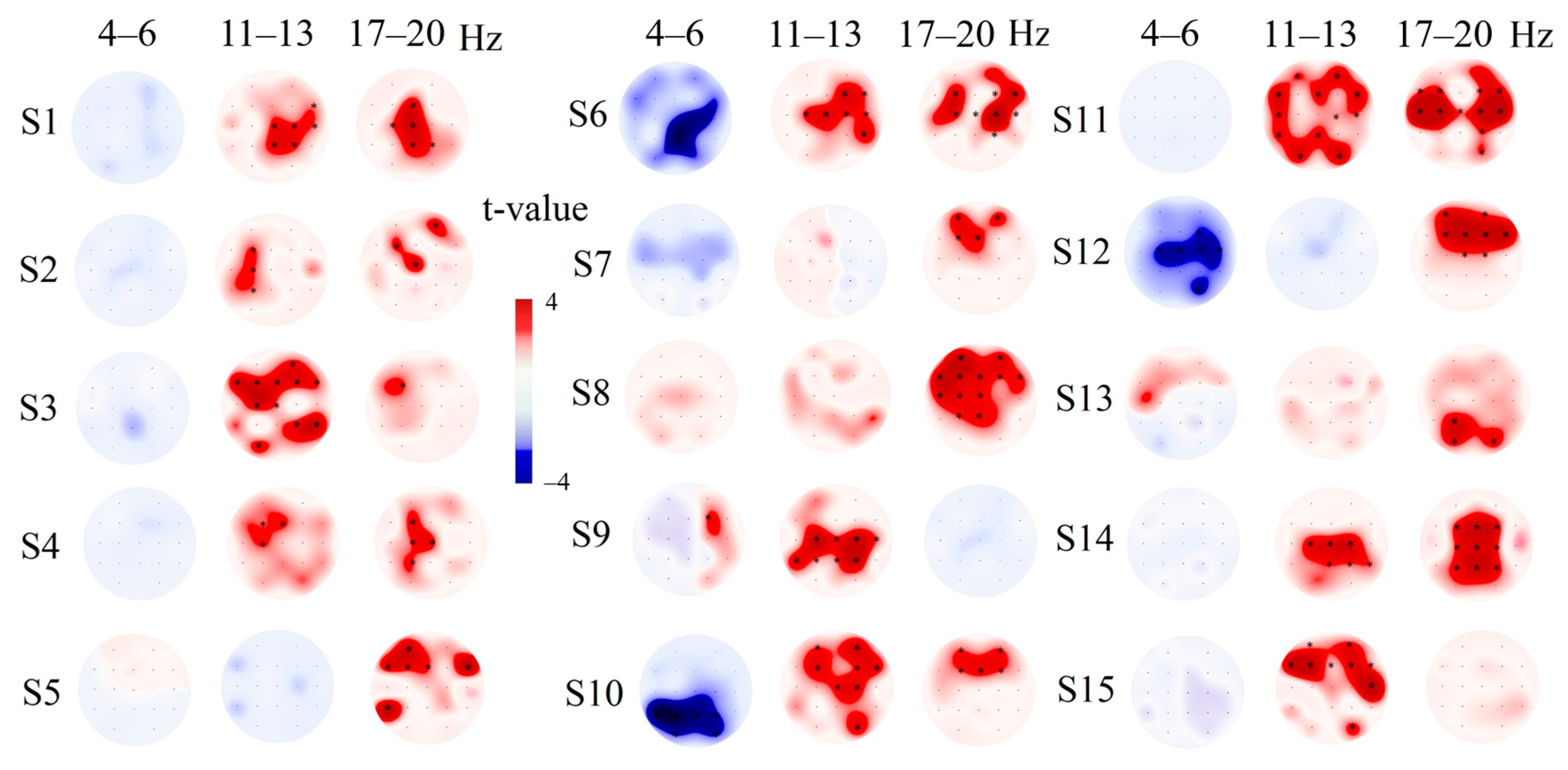
3.4. Individual EEG Response Tactile Stimulation
3.5. Correlation of Tactile EEG Response with Morphometric Data
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lutkenhoff, E.S.; Ma, J.C.; Tshibanda, L.; Kamau, E.; Kirsch, M.; Pickard, J.D.; Laureys, S.; Owen, A.M.; Monti, M.M. Thalamic and extrathalamic mechanisms of consciousness after severe brain injury. Ann. Neurol. 2015, 78, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Lazarus, M.; Chen, J.-F.; Urade, Y.; Huang, Z.-L. Role of the basal ganglia in the control of sleep and wakefulness. Curr. Opin. Neurobiol. 2013, 23, 780–785. [Google Scholar] [CrossRef] [Green Version]
- Lazarus, M.; Huang, Z.-L.; Lu, J.; Urade, Y.; Chen, J.-F. How do the basal ganglia regulate sleep–wake behavior? Trends Neurosci. 2012, 35, 723–732. [Google Scholar] [CrossRef]
- Schiff, N.D. Central thalamic contributions to arousal regulation and neurological disorders of consciousness. Ann. N. Y. Acad. Sci. 2008, 1129, 105–118. [Google Scholar] [CrossRef] [Green Version]
- Tomaiuolo, F.; Carlesimo, G.A.; Di Paola, M.; Petrides, M.; Fera, F.; Bonanni, R.; Formisano, R.; Pasqualetti, P.; Caltagirone, C. Gross morphology and morphometric sequelae in the hippocampus, fornix, and corpus callosum of patients with severe non-missile traumatic brain injury without macroscopically detectable lesions: A T1 weighted MRI study. J. Neurol. Neurosurg. Psychiatry 2004, 75, 1314–1322. [Google Scholar] [CrossRef] [Green Version]
- Crick, F.; Koch, C. A framework for consciousness. Nat. Neurosci. 2003, 6, 119–126. [Google Scholar] [CrossRef]
- Dehaene, S.; Changeux, J.-P. Experimental and Theoretical Approaches to Conscious Processing. Neuron 2011, 70, 200–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, J.-H.; Yang, Y.; Zhang, Y.; Qiu, S.-Y.; Zhou, Z.-Y.; Dang, Y.-Y.; Dai, Y.-W.; Liu, Y.-J.; Xu, R.-X. Hyperactive external awareness against hypoactive internal awareness in disorders of consciousness using resting-state functional MRI: Highlighting the involvement of visuo-motor modulation. NMR Biomed. 2014, 27, 880–886. [Google Scholar] [CrossRef] [PubMed]
- Hannawi, Y.; Lindquist, M.A.; Caffo, B.S.; Sair, H.I.; Stevens, R.D. Resting brain activity in disorders of consciousness: A systematic review and meta-analysis. Neurol. 2015, 84, 1272–1280. [Google Scholar] [CrossRef] [Green Version]
- Laureysab, S.S.; Goldman, S.; Phillips, C.; Van Bogaert, P.; Aerts, J.; Luxen, A.; G.Franckab, G.; P.Maquetab, P. Impaired Effective Cortical Connectivity in Vegetative State: Preliminary Investigation Using PET. NeuroImage 1999, 9, 377–382. [Google Scholar] [CrossRef] [Green Version]
- Jang, S.; Kwak, S. The Upper Ascending Reticular Activating System between Intralaminar Thalamic Nuclei and Cerebral Cortex in the Human Brain. J. Korean Phys. Ther. 2017, 29, 109–114. [Google Scholar] [CrossRef]
- Laureys, S. The neural correlate of (un)awareness: Lessons from the vegetative state. Trends Cogn. Sci. 2005, 9, 556–559. [Google Scholar] [CrossRef] [PubMed]
- Laureys, S.; Faymonville, M.E.; Luxen, A.; Lamy, M.; Franck, G.; Maquet, P. Restoration of thalamocortical connectivity after recovery from persistent vegetative state. Lancet 2000, 355, 1790–1791. [Google Scholar] [CrossRef] [Green Version]
- Tshibanda, L.; Vanhaudenhuyse, A.; Galanaud, D.; Boly, M.; Laureys, S.; Puybasset, L. Magnetic resonance spectroscopy and diffusion tensor imaging in coma survivors: Promises and pitfalls. Sleep Deprivation Cogn. 2009, 177, 215–229. [Google Scholar] [CrossRef] [Green Version]
- Potapov, A.A.; Zakharova, N.E.; Kornienko, V.N.; Pronin, I.N.; Alexandrova, E.V.; Zaitsev, O.S.; Likhterman, L.B.; Gavrilov, A.G.; Danilov, G.V.; Oshorov, A.V.; et al. [Neuroanatomical basis for traumatic coma: Clinical and magnetic resonance correlates]. Vopr. neirokhirurgii Im. N.N. Burdenko 2014, 78. [Google Scholar]
- Woischneck, D.; Klein, S.; Peters, B.; Firsching, R.; Döhring, W.; Reißberg, S. Classification of Severe Head Injury Based on Magnetic Resonance Imaging. Acta Neurochir. 2001, 143, 263–271. [Google Scholar] [CrossRef]
- Brenner, R.P. The Interpretation of the EEG in Stupor and Coma. Neurol. 2005, 11, 271–284. [Google Scholar] [CrossRef]
- Xie, Q.; Ni, X.; Yu, R.; Li, Y.; Huang, R. Chronic disorders of consciousness. Exp. Ther. Med. 2017, 14, 1277–1283. [Google Scholar] [CrossRef] [Green Version]
- Emidio, A.C.; Faria, R.; Patricio, P.; Canas, N.; Messias, A.; Meneses-Oliveira, C. Spindle Coma in the Intensive Care Unit: Different Aetiologies—Different Outcomes. Eur. J. Case Rep. Intern. Med. 2019, 6, 001316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ardeshna, N.I. EEG and Coma. Neurodiagnostic J. 2016, 56, 1–16. [Google Scholar] [CrossRef]
- Husain, A.M. Electroencephalographic Assessment of Coma. J. Clin. Neurophysiol. 2006, 23, 208–220. [Google Scholar] [CrossRef] [PubMed]
- Weiss, N.; Galanaud, D.; Carpentier, A.; De Montcel, S.T.; Naccache, L.; Coriat, P.; Puybasset, L. A combined clinical and MRI approach for outcome assessment of traumatic head injured comatose patients. J. Neurol. 2008, 255, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Bekinschtein, T.; Gleichgerrcht, E.; Manes, F.; Tristán, B. Acute loss of consciousness. In Handbook of Clinical Neurology; Elsevier BV: Amsterdam, The Netherlands, 2015; Volume 127, pp. 195–204. [Google Scholar]
- Abraira, V.E.; Ginty, D.D. The Sensory Neurons of Touch. Neuron 2013, 79, 618–639. [Google Scholar] [CrossRef] [Green Version]
- Vallbo, A.; Olausson, H.; Wessberg, J.; Norrsell, U. A system of unmyelinated afferents for innocuous mechanoreception in the human skin. Brain Res. 1993, 628, 301–304. [Google Scholar] [CrossRef]
- Olausson, H.; Lamarre, Y.; Backlund, H.; Morin, C.; Wallin, B.; Starck, G.; Ekholm, S.; Strigo, I.; Worsley, K.; Vallbo, Å.B.; et al. Unmyelinated tactile afferents signal touch and project to insular cortex. Nat. Neurosci. 2002, 5, 900–904. [Google Scholar] [CrossRef]
- Björnsdotter, M.; Löken, L.; Olausson, H.; Vallbo, Å.; Wessberg, J. Somatotopic Organization of Gentle Touch Processing in the Posterior Insular Cortex. J. Neurosci. 2009, 29, 9314–9320. [Google Scholar] [CrossRef] [Green Version]
- Goodwin, A.; Wheat, H. Physiological Responses of Sensory Afferents in Glabrous and Hairy Skin of Humans and Monkeys. The Senses: A Comprehensive Reference 2008, 6, 39–54. [Google Scholar] [CrossRef]
- Morrison, I. CT afferents. Curr. Biol. 2010, CB 22, R77–R78. [Google Scholar] [CrossRef] [Green Version]
- Fischl, B.; Salat, D.H.; Busa, E.; Albert, M.; Dieterich, M.; Haselgrove, C.; Van Der Kouwe, A.; Killiany, R.; Kennedy, D.; Klaveness, S.; et al. Whole Brain Segmentation. Neuron 2002, 33, 341–355. [Google Scholar] [CrossRef] [Green Version]
- Gordon, I.; Voos, A.C.; Bennett, R.H.; Bolling, D.Z.; Pelphrey, K.A.; Kaiser, M.D. Brain mechanisms for processing affective touch. Hum. Brain Mapp. 2011, 34, 914–922. [Google Scholar] [CrossRef]
- Obadia, N.A.; Zyss, J.; Gavaret, M.; Lefaucheur, J.-P.; Azabou, E.; Boulogne, S.; Guérit, J.-M.; McGonigal, A.; Merle, P.; Mutschler, V.; et al. Recommendations for the use of electroencephalography and evoked potentials in comatose patients. Neurophysiol. Clin. Neurophysiol. 2018, 48, 143–169. [Google Scholar] [CrossRef] [PubMed]
- Eackerley, R.; Eriksson, E.; Wessberg, J. Ultra-late EEG potential evoked by preferential activation of unmyelinated tactile afferents in human hairy skin. Neurosci. Lett. 2013, 535, 62–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hawryluk, G.W.J.; Ullman, J.S.; Totten, A.M.; Ghajar, J. In Reply: Guidelines for the Management of Severe Traumatic Brain Injury, Fourth Edition. Neurosurg. 2017, 81, E3–E4. [Google Scholar] [CrossRef] [PubMed]
- Schaer, M.; Cuadra, M.B.; Schmansky, N.; Fischl, B.; Thiran, J.-P.; Eliez, S. How to Measure Cortical Folding from MR Images: A Step-by-Step Tutorial to Compute Local Gyrification Index. J. Vis. Exp. 2012, 59, e3417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fischl, B.; Salat, D.H.; Van Der Kouwe, A.J.; Makris, N.; Ségonne, F.; Quinn, B.T.; Dale, A.M. Sequence-independent segmentation of magnetic resonance images. NeuroImage 2004, 23, S69–S84. [Google Scholar] [CrossRef] [Green Version]
- Sled, J.G.; Zijdenbos, A.; Evans, A. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans. Med Imaging 1998, 17, 87–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fischl, B.; Liu, A.; Dale, A. Automated manifold surgery: Constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Trans. Med Imaging 2001, 20, 70–80. [Google Scholar] [CrossRef] [Green Version]
- Segonne, F.; Pacheco, J.; Fischl, B. Geometrically Accurate Topology-Correction of Cortical Surfaces Using Nonseparating Loops. IEEE Trans. Med Imaging 2007, 26, 518–529. [Google Scholar] [CrossRef]
- Dale, A.M.; Sereno, M.I. Improved Localizadon of Cortical Activity by Combining EEG and MEG with MRI Cortical Surface Reconstruction: A Linear Approach. J. Cogn. Neurosci. 1993, 5, 162–176. [Google Scholar] [CrossRef]
- Dale, A.M.; Fischla, B.; Sereno, M.I. Cortical Surface-Based Analysis. NeuroImage 1999, 9, 179–194. [Google Scholar] [CrossRef]
- Fischl, B.; Dale, A.M. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc. Natl. Acad. Sci. USA 2000, 97, 11050–11055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fischl, B.; Sereno, M.I.; Dale, A.M. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage 1999, 9, 195–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fischl, B.; Sereno, M.I.; Tootell, R.B.; Dale, A.M. High-resolution intersubject averaging and a coordinate system for the cortical surface. Hum. Brain Mapp. 1999, 8, 272–284. [Google Scholar] [CrossRef]
- Fischl, B.; Van Der Kouwe, A.; Destrieux, C.; Halgren, E.; Ségonne, F.; Salat, D.H.; Busa, E.; Seidman, L.J.; Goldstein, J.; Kennedy, D.; et al. Automatically Parcellating the Human Cerebral Cortex. Cereb. Cortex 2004, 14, 11–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desikan, R.S.; Ségonne, F.; Fischl, B.; Quinn, B.T.; Dickerson, B.C.; Blacker, D.; Buckner, R.L.; Dale, A.M.; Maguire, R.P.; Hyman, B.T.; et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage 2006, 31, 968–980. [Google Scholar] [CrossRef] [PubMed]
- Kitada, R.; Doizaki, R.; Kwon, J.; Tanigawa, T.; Nakagawa, E.; Kochiyama, T.; Kajimoto, H.; Sakamoto, M.; Sadato, N. Brain networks underlying tactile softness perception: A functional magnetic resonance imaging study. NeuroImage 2019, 197, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.-X.; Preuss, T.; Kaas, J. Somatosensory Areas of the Cerebral Cortex: Architectonic Characteristics and Modular Organization; Elsevier BV: Amsterdam, The Netherlands, 2008; pp. 143–169. [Google Scholar]
- Pfurtscheller, G.; Da Silva, F.L. Event-related EEG/MEG synchronization and desynchronization: Basic principles. Clin. Neurophysiol. 1999, 110, 1842–1857. [Google Scholar] [CrossRef]
- Jeffry, J.; Kim, S.; Chen, Z.-F. Itch Signaling in the Nervous System. Physiol. 2011, 26, 286–292. [Google Scholar] [CrossRef]
- Trinka, E.; Leitinger, M. Which EEG patterns in coma are nonconvulsive status epilepticus? Epilepsy Behav. 2015, 49, 203–222. [Google Scholar] [CrossRef] [Green Version]
- Young, G.; Blume, W.; Campbell, V.; DeMelo, J.; Leung, L.; McKeown, M.J.; McLachlan, R.; Ramsay, D.; Schieven, J. Alpha, theta and alpha-theta coma: A clinical outcome study utilizing serial recordings. Electroencephalogr. Clin. Neurophysiol. 1994, 91, 93–99. [Google Scholar] [CrossRef]
- Fernández-Torre, J.L.; López-Delgado, A.; Hernández-Hernández, M.A.; Paramio-Paz, A.; Pía-Martínez, C.; Orizaola, P.; Martín-García, M. Postanoxic alpha, theta or alpha-theta coma: Clinical setting and neurological outcome. Resusc. 2018, 124, 118–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitchell, D.J.; McNaughton, N.; Flanagan, D.; Kirk, I.J. Frontal-midline theta from the perspective of hippocampal “theta. ” Prog. Neurobiol. 2008, 86, 156–185. [Google Scholar] [CrossRef] [PubMed]
- Di Perri, C.; Stender, J.; Laureys, S.; Gosseries, O. Functional neuroanatomy of disorders of consciousness. Epilepsy Behav. 2014, 30, 28–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanhaudenhuyse, A.; Demertzi, A.; Schabus, M.; Noirhomme, Q.; Bredart, S.; Boly, M.; Phillips, C.; Soddu, A.; Luxen, A.; Moonen, G.; et al. Two Distinct Neuronal Networks Mediate the Awareness of Environment and of Self. J. Cogn. Neurosci. 2011, 23, 570–578. [Google Scholar] [CrossRef] [Green Version]
- Vanhaudenhuyse, A.; Noirhomme, Q.; Tshibanda, L.J.-F.; Bruno, M.-A.; Boveroux, P.; Schnakers, C.; Soddu, A.; Perlbarg, V.; LeDoux, D.; Brichant, J.-F.; et al. Default network connectivity reflects the level of consciousness in non-communicative brain-damaged patients. Brain 2009, 133, 161–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Patient. | Cause of Trauma | Age | Time from Accident to EEG Recording | Coma Glasgow Scale | MRI Results (Regions of Brain Contusions) | Outcome (GOSe Rate) |
|---|---|---|---|---|---|---|
| 1 | car accident | 18 | 7 days | 5 | right frontal and the corpus callosum | 6 |
| 2 | car accident | 41 | 28 days | 7 | right temporal–frontal region left temporal–frontal region, right hemisphere of the cerebellum | 3 |
| 3 | car accident | 43 | 12 days | 7 | front temporal lobes of the right hemisphere left temporal and occipital lobes | 5 |
| 4 | car accident | 26 | 2 months 2 days | 6 | left frontal and right occipital lobe | 3 |
| 5 | Beating | 32 | 1 month 18 days | 5 | brain stem left basal temporal region | 3 |
| 6 | fall from a height | 51 | 7 days | 6 | left frontal and temporal regions, diffuse axonal damage | 5 |
| 7 | car accident | 34 | 2 months 25 days | 7 | diffuse axonal damage | 6 |
| 8 | car accident | 34 | 4 days | 5 | the corpus callosum, diffuse axonal damage | 3 |
| 9 | car accident | 38 | 8 days | 6 | brain stem | 5 |
| 10 | car accident | 26 | 4 days | 5 | the corpus callosum, diffuse axonal damage | 7 |
| Area | Thickness (mm) in Control Group (mean ± std) | Thickness (mm) in Comatose Patients (mean ± std) | Z (Mann–Whitney U Test) | p-Value | |
|---|---|---|---|---|---|
| Right | Anterior cingulate | 2.7 ± 0.3 | 2.5 ± 0.2 | 1.36 | 0.17 |
| Posterior cingulate | 2.5 ± 0.2 | 2.5 ± 0.2 | 1.16 | 0.24 | |
| Middle-frontal | 2.6 ± 0.1 | 2.6 ± 0.2 | 0.78 | 0.44 | |
| Parahippocampal | 2.8 ± 0.3 | 2.8 ± 0.3 | −1.14 | 0.26 | |
| Inferior-parietal | 2.5 ± 0.1 | 2.4 ± 0.1 | 1.83 | 0.07 | |
| Superior parietal | 2.2 ± 0.1 | 2.2 ± 0.1 | −0.08 | 0.93 | |
| Paracentral | 2.5 ± 0.1 | 2.3 ± 0.1 | 2.22 | 0.03 | |
| Postcentral | 2.0 ± 0.1 | 2.0 ± 0.1 | 1.00 | 0.32 | |
| Precentral | 2.6 ± 0.1 | 2.3 ± 0.2 | 3.00 | 0.00 | |
| Precuneus | 2.4 ± 0.1 | 2.4 ± 0.1 | −1.55 | 0.12 | |
| Supramarginal | 2.6 ± 0.1 | 2.4 ± 0.2 | 2.77 | 0.01 | |
| Insula | 3.1 ± 0.1 | 2.9 ± 0.2 | 3.16 | 0.00 | |
| Mean Thickness | 2.6 ± 0.1 | 2.1 ± 0.1 | 3.54 | 0.00 | |
| Left | Anterior cingulate | 2.8 ± 0.2 | 2.7 ± 0.4 | 1.11 | 0.27 |
| Posterior cingulate | 2.6 ± 0.1 | 2.4 ± 0.2 | 1.94 | 0.05 | |
| Middle-frontal | 2.5 ± 0.1 | 2.4 ± 0.2 | 0.89 | 0.37 | |
| Parahippocampal | 2.8 ± 0.3 | 2.9 ± 0.2 | −0.83 | 0.41 | |
| Inferior–parietal | 2.5 ± 0.1 | 2.4 ± 0.1 | 1.22 | 0.22 | |
| Superior–parietal | 2.2 ± 0.1 | 2.1 ± 0.1 | 1.58 | 0.11 | |
| Paracentral | 2.4 ± 0.2 | 2.3 ± 0.1 | 1.55 | 0.12 | |
| Postcentral | 2.0 ± 0.1 | 2.0 ± 0.1 | 1.05 | 0.29 | |
| Precentral | 2.6 ± 0.1 | 2.3 ± 0.2 | 3.36 | 0.00 | |
| Precuneus | 2.4 ± 0.1 | 2.4 ± 0.1 | 0.64 | 0.52 | |
| Supramarginal | 2.6 ± 0.1 | 2.4 ± 0.2 | 2.58 | 0.01 | |
| Insula | 3.1 ± 0.1 | 2.8 ± 0.2 | 2.55 | 0.01 | |
| Mean Thickness | 2.6 ± 0.1 | 2.4 ± 0.1 | 3.05 | 0.00 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Portnova, G.; Girzhova, I.; Filatova, D.; Podlepich, V.; Tetereva, A.; Martynova, O. Brain Oscillatory Activity during Tactile Stimulation Correlates with Cortical Thickness of Intact Areas and Predicts Outcome in Post-Traumatic Comatose Patients. Brain Sci. 2020, 10, 720. https://doi.org/10.3390/brainsci10100720
Portnova G, Girzhova I, Filatova D, Podlepich V, Tetereva A, Martynova O. Brain Oscillatory Activity during Tactile Stimulation Correlates with Cortical Thickness of Intact Areas and Predicts Outcome in Post-Traumatic Comatose Patients. Brain Sciences. 2020; 10(10):720. https://doi.org/10.3390/brainsci10100720
Chicago/Turabian StylePortnova, Galina, Irina Girzhova, Daria Filatova, Vitaliy Podlepich, Alina Tetereva, and Olga Martynova. 2020. "Brain Oscillatory Activity during Tactile Stimulation Correlates with Cortical Thickness of Intact Areas and Predicts Outcome in Post-Traumatic Comatose Patients" Brain Sciences 10, no. 10: 720. https://doi.org/10.3390/brainsci10100720
APA StylePortnova, G., Girzhova, I., Filatova, D., Podlepich, V., Tetereva, A., & Martynova, O. (2020). Brain Oscillatory Activity during Tactile Stimulation Correlates with Cortical Thickness of Intact Areas and Predicts Outcome in Post-Traumatic Comatose Patients. Brain Sciences, 10(10), 720. https://doi.org/10.3390/brainsci10100720




