Constitutive Genetic Deletion of Hcn1 Increases Alcohol Preference during Adolescence
Abstract
1. Introduction
2. Materials and Methods
3. Results
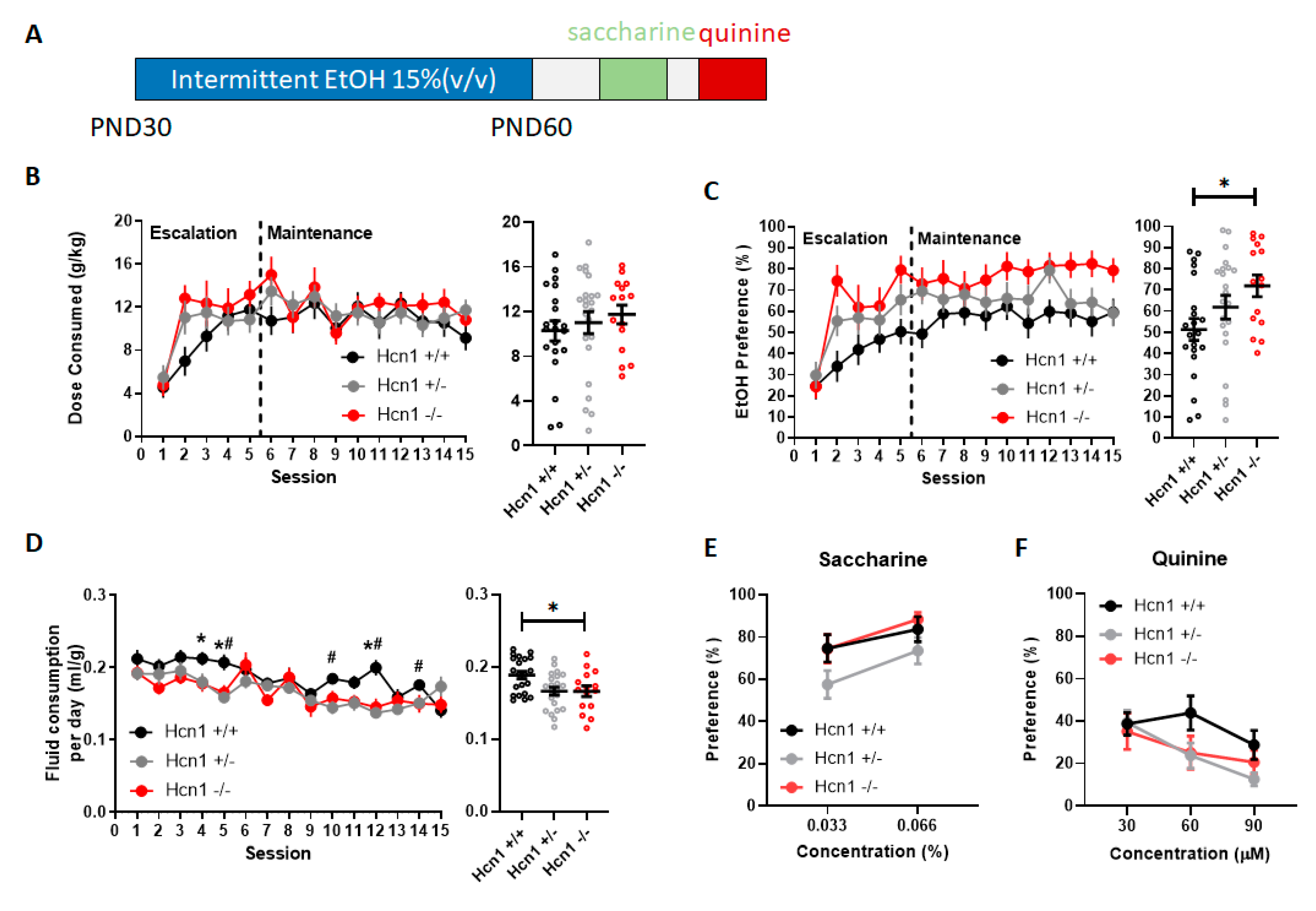
3.1. Hcn1 Gene Deletion Caused an Overall Increase in Alcohol Preference in Adolescence
3.2. Hcn1 Gene Deletion Does Not Affect Sweet or Bitter Taste Sensitivity
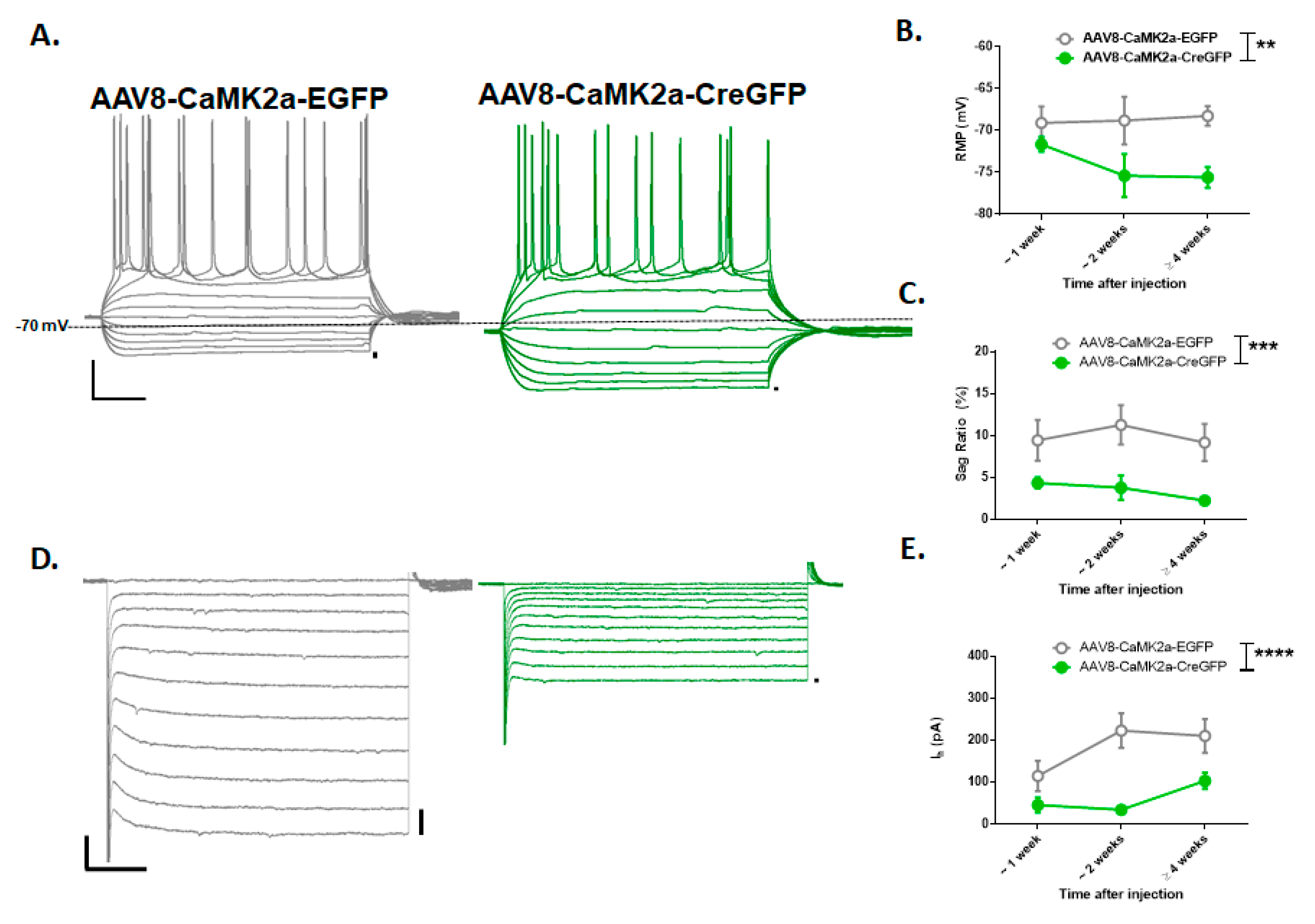
3.3. Viral Deletion of HCN1 Was Effective in the Prefrontal Cortex
3.4. PFC Viral KO of HCN1 Does Not Regulate Intermittent Alcohol Consumption
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Grant, B.F.; Dawson, D.A. Age at onset of alcohol use and its association with DSM-IV alcohol abuse and dependence: Results from the national longitudinal alcohol epidemiologic survey. J. Subst. Abus. 1997, 9, 103–110. [Google Scholar] [CrossRef]
- Squeglia, L.M.; Jacobus, J.; Tapert, S.F. The effect of alcohol use on human adolescent brain structures and systems. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2014; Volume 125, pp. 501–510. [Google Scholar]
- Casey, B.J.; Jones, R.M. Neurobiology of the Adolescent Brain and Behavior: Implications for Substance Use Disorders. J. Am. Acad. Child Adolesc. Psychiatry 2010, 49, 1189–1201. [Google Scholar] [CrossRef] [PubMed]
- Pfefferbaum, A.; Kwon, D.; Brumback, T.; Thompson, W.K.; Cummins, K.; Tapert, S.F.; Brown, S.A.; Colrain, I.M.; Baker, F.C.; Prouty, D.; et al. Altered Brain Developmental Trajectories in Adolescents After Initiating Drinking. Am. J. Psychiatry 2018, 175, 370–380. [Google Scholar] [CrossRef] [PubMed]
- Meda, S.A.; Dager, A.D.; Hawkins, K.A.; Tennen, H.; Raskin, S.; Wood, R.M.; Austad, C.S.; Fallahi, C.R.; Pearlson, G.D. Heavy Drinking in College Students Is Associated with Accelerated Gray Matter Volumetric Decline over a 2 Year Period. Front. Behav. Neurosci. 2017, 11, 176. [Google Scholar] [CrossRef]
- Bellis, M.D.; Narasimhan, A.; Thatcher, D.L.; Keshavan, M.S.; Soloff, P.; Clark, D.B. Prefrontal Cortex, Thalamus, and Cerebellar Volumes in Adolescents and Young Adults with Adolescent-Onset Alcohol Use Disorders and Comorbid Mental Disorders. Alcohol. Clin. Exp. Res. 2005, 29, 1590–1600. [Google Scholar] [CrossRef]
- Sullivan, E.V.; Brumback, T.; Tapert, S.F.; Brown, S.A.; Baker, F.C.; Colrain, I.M.; Prouty, D.; De Bellis, M.D.; Clark, D.B.; Nagel, B.J.; et al. Disturbed Cerebellar Growth Trajectories in Adolescents Who Initiate Alcohol Drinking. Biol. Psychiatry 2020, 87, 632–644. [Google Scholar] [CrossRef]
- Medina, K.L.; McQueeny, T.; Nagel, B.J.; Hanson, K.L.; Schweinsburg, A.D.; Tapert, S.F. Prefrontal cortex volumes in adolescents with alcohol use disorders: Unique gender effects. Alcohol. Clin. Exp. Res. 2008, 32, 386–394. [Google Scholar] [CrossRef]
- Courtney, K.E.; Infante, M.A.; Bordyug, M.; Simmons, A.N.; Tapert, S.F. Prospective Associations between BOLD Markers of Response Inhibition and the Transition to Frequent Binge Drinking. Alcohol. Clin. Exp. Res. 2020, 44, 463–469. [Google Scholar] [CrossRef]
- Crego, A.; Holguín, S.R.; Parada, M.; Mota, N.; Corral, M.; Cadaveira, F. Binge Drinking Affects Attentional and Visual Working Memory Processing in Young University Students. Alcohol. Clin. Exp. Res. 2009, 33, 1870–1879. [Google Scholar] [CrossRef]
- Squeglia, L.M.; Schweinsburg, A.D.; Pulido, C.; Tapert, S.F. Adolescent Binge Drinking Linked to Abnormal Spatial Working Memory Brain Activation: Differential Gender Effects. Alcohol. Clin. Exp. Res. 2011, 35, 1831–1841. [Google Scholar] [CrossRef]
- Tapert, S.F.; Cheung, E.H.; Brown, G.G.; Frank, L.R.; Paulus, M.P.; Schweinsburg, A.D.; Meloy, M.J.; Brown, S.A. Neural Response to Alcohol Stimuli in Adolescents With Alcohol Use Disorder. Arch. Gen. Psychiatry 2003, 60, 727–735. [Google Scholar] [CrossRef]
- Abrahao, K.P.; Salinas, A.G.; Lovinger, D.M. Alcohol and the Brain: Neuronal Molecular Targets, Synapses, and Circuits. Neuron 2017, 96, 1223–1238. [Google Scholar] [CrossRef] [PubMed]
- Cannady, R.; Rinker, J.A.; Nimitvilai, S.; Woodward, J.J.; Mulholland, P.J. Chronic Alcohol, Intrinsic Excitability, and Potassium Channels: Neuroadaptations and Drinking Behavior. Bile Acids Their Recept. 2018, 248, 311–343. [Google Scholar] [CrossRef]
- Cannady, R.; Nimitvilai-Roberts, S.; Jennings, S.D.; Woodward, J.J.; Mulholland, P.J. Distinct Region- and Time-Dependent Functional Cortical Adaptations in C57BL/6J Mice after Short and Prolonged Alcohol Drinking. eNeuro 2020, 7. [Google Scholar] [CrossRef] [PubMed]
- Nimitvilai, S.; Lopez, M.F.; Mulholland, P.J.; Woodward, J.J. Chronic Intermittent Ethanol Exposure Enhances the Excitability and Synaptic Plasticity of Lateral Orbitofrontal Cortex Neurons and Induces a Tolerance to the Acute Inhibitory Actions of Ethanol. Neuropsychopharmacology 2015, 41, 1112–1127. [Google Scholar] [CrossRef]
- Varodayan, F.P.; Sidhu, H.; Kreifeldt, M.; Roberto, M.; Contet, C. Morphological and functional evidence of increased excitatory signaling in the prelimbic cortex during ethanol withdrawal. Neuropharmacology 2018, 133, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Pleil, K.E.; Lowery-Gionta, E.G.; Crowley, N.A.; Li, C.; Marcinkiewcz, C.A.; Rose, J.H.; McCall, N.M.; Maldonado-Devincci, A.M.; Morrow, A.L.; Jones, S.R.; et al. Effects of chronic ethanol exposure on neuronal function in the prefrontal cortex and extended amygdala. Neuropharmacology 2015, 99, 735–749. [Google Scholar] [CrossRef]
- Kroener, S.; Mulholland, P.J.; New, N.N.; Gass, J.T.; Becker, H.C.; Chandler, L.J. Chronic Alcohol Exposure Alters Behavioral and Synaptic Plasticity of the Rodent Prefrontal Cortex. PLoS ONE 2012, 7, e37541. [Google Scholar] [CrossRef]
- Barker, J.M.; Bryant, K.G.; Osborne, J.I.; Chandler, L.J. Age and Sex Interact to Mediate the Effects of Intermittent, High-Dose Ethanol Exposure on Behavioral Flexibility. Front. Pharmacol. 2017, 8, 450. [Google Scholar] [CrossRef]
- Salling, M.C.; Skelly, M.J.; Avegno, E.; Regan, S.; Zeric, T.; Nichols, E.; Harrison, N.L. Alcohol Consumption during Adolescence in a Mouse Model of Binge Drinking Alters the Intrinsic Excitability and Function of the Prefrontal Cortex through a Reduction in the Hyperpolarization-Activated Cation Current. J. Neurosci. 2018, 38, 6207–6222. [Google Scholar] [CrossRef]
- Lee, C.-H.; MacKinnon, R. Structures of the Human HCN1 Hyperpolarization-Activated Channel. Cell 2017, 168, 111–120. [Google Scholar] [CrossRef]
- Biel, M.; Wahl-Schott, C.; Michalakis, S.; Zong, X. Hyperpolarization-Activated Cation Channels: From Genes to Function. Physiol. Rev. 2009, 89, 847–885. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.M. Cortical HCN channels: Function, trafficking and plasticity. J. Physiol. 2014, 592, 2711–2719. [Google Scholar] [CrossRef]
- Yang, S.-S.; Li, Y.-C.; Coley, A.A.; Chamberlin, L.A.; Yu, P.; Gao, W.-J. Cell-Type Specific Development of the Hyperpolarization-Activated Current, Ih, in Prefrontal Cortical Neurons. Front. Synaptic Neurosci. 2018, 10, 7. [Google Scholar] [CrossRef]
- Lee, C.-H.; Park, J.H.; Won, M.-H. Protein expression changes of HCN1 and HCN2 in hippocampal subregions of gerbils during the normal aging process. Iran J. Basic Med. Sci. 2019, 22, 1308–1313. [Google Scholar] [PubMed]
- Nolan, M.; Malleret, G.; Dudman, J.; Buhl, D.; Santoro, B.; Gibbs, E.; Vronskaya, S.; Buzsaki, G.; Siegelbaum, S.; Kandel, E. A Behavioral Role for Dendritic IntegrationHCN1 Channels Constrain Spatial Memory and Plasticity at Inputs to Distal Dendrites of CA1 Pyramidal Neurons. Cell 2004, 119, 719–732. [Google Scholar] [CrossRef][Green Version]
- Thuault, S.J.; Malleret, G.; Constantinople, C.M.; Nicholls, R.; Chen, I.; Zhu, J.; Panteleyev, A.; Vronskaya, S.; Nolan, M.F.; Bruno, R.; et al. Prefrontal Cortex HCN1 Channels Enable Intrinsic Persistent Neural Firing and Executive Memory Function. J. Neurosci. 2013, 33, 13583–13599. [Google Scholar] [CrossRef]
- Nolan, M.F.; Malleret, G.; Lee, K.H.; Gibbs, E.; Dudman, J.T.; Santoro, B.; Yin, D.; Thompson, R.F.; Siegelbaum, S.A.; Kandel, E.R.; et al. The hyperpolarization-activated HCN1 channel is important for motor learning and neuronal integration by cerebellar Purkinje cells. Cell 2003, 115, 551–564. [Google Scholar] [CrossRef]
- Melendez, R.I. Intermittent (Every-Other-Day) Drinking Induces Rapid Escalation of Ethanol Intake and Preference in Adolescent and Adult C57BL/6J Mice. Alcohol. Clin. Exp. Res. 2011, 35, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.P.; Zou, M.E.; Janak, P.H.; Messing, R.O. Responses to ethanol in C57BL/6 versus C57BL/6 × 129 hybrid mice. Brain Behav. 2012, 2, 22–31. [Google Scholar] [CrossRef]
- Hwa, L.S.; Chu, A.; Levinson, S.A.; Kayyali, T.M.; DeBold, J.F.; Miczek, K.A. Persistent Escalation of Alcohol Drinking in C57BL/6J Mice With Intermittent Access to 20% Ethanol. Alcohol. Clin. Exp. Res. 2011, 35, 1938–1947. [Google Scholar] [CrossRef] [PubMed]
- Stevens, D.R.; Seifert, R.; Bufe, B.; Müller, F.; Kremmer, E.; Gauss, R.; Meyerhof, W.; Kaupp, U.B.; Lindemann, B. Hyperpolarization-activated channels HCN1 and HCN4 mediate responses to sour stimuli. Nat. Cell Biol. 2001, 413, 631–635. [Google Scholar] [CrossRef] [PubMed]
- Linsenbardt, D.N.; Timme, N.M.; Lapish, C.C. Encoding of the Intent to Drink Alcohol by the Prefrontal Cortex Is Blunted in Rats with a Family History of Excessive Drinking. eNeuro 2019, 6. [Google Scholar] [CrossRef] [PubMed]
- Siciliano, C.A.; Noamany, H.; Chang, C.-J.; Brown, A.R.; Chen, X.; Leible, D.; Lee, J.J.; Wang, J.; Vernon, A.N.; Weele, C.M.V.; et al. A cortical-brainstem circuit predicts and governs compulsive alcohol drinking. Science 2019, 366, 1008–1012. [Google Scholar] [CrossRef] [PubMed]
- Hartog, C.D.; Zamudio-Bulcock, P.; Nimitvilai, S.; Gilstrap, M.; Eaton, B.; Fedarovich, H.; Motts, A.; Woodward, J.J. Inactivation of the lateral orbitofrontal cortex increases drinking in ethanol-dependent but not non-dependent mice. Neuropharmacology 2016, 107, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Dembrow, N.C.; Chitwood, R.A.; Johnston, D. Projection-Specific Neuromodulation of Medial Prefrontal Cortex Neurons. J. Neurosci. 2010, 30, 16922–16937. [Google Scholar] [CrossRef]
- Hughes, B.A.; Crofton, E.J.; O’Buckley, T.K.; Herman, M.A.; Morrow, A.L. Chronic ethanol exposure alters prelimbic prefrontal cortical Fast-Spiking and Martinotti interneuron function with differential sex specificity in rat brain. Neuropharmacology 2019, 162, 107805. [Google Scholar] [CrossRef]
- Capuzzo, G.; Floresco, S.B. Prelimbic and Infralimbic Prefrontal Regulation of Active and Inhibitory Avoidance and Reward-Seeking. J. Neurosci. 2020, 40, 4773–4787. [Google Scholar] [CrossRef]
- Fenno, L.E.; Ramakrishnan, C.; Kim, Y.S.; Evans, K.E.; Lo, M.; Vesuna, S.; Inoue, M.; Cheung, K.Y.; Yuen, E.; Pichamoorthy, N.; et al. Comprehensive Dual- and Triple-Feature Intersectional Single-Vector Delivery of Diverse Functional Payloads to Cells of Behaving Mammals. Neuron 2020, 107, 836–853.e11. [Google Scholar] [CrossRef]
- Nakayama, H.; Ibañez-Tallon, I.; Heintz, N. Cell-Type-Specific Contributions of Medial Prefrontal Neurons to Flexible Behaviors. J. Neurosci. 2018, 38, 4490–4504. [Google Scholar] [CrossRef]
- Moaddab, M.; Mangone, E.; Ray, M.H.; McDannald, M.A. Adolescent Alcohol Drinking Renders Adult Drinking BLA-Dependent: BLA Hyper-Activity as Contributor to Comorbid Alcohol Use Disorder and Anxiety Disorders. Brain Sci. 2017, 7, 151. [Google Scholar] [CrossRef] [PubMed]
- Joffe, M.E.; Winder, D.G.; Conn, P.J. Contrasting sex-dependent adaptations to synaptic physiology and membrane properties of prefrontal cortex interneuron subtypes in a mouse model of binge drinking. Neuropharmacology 2020, 178, 108126. [Google Scholar] [CrossRef]
- Knoll, A.T.; Halladay, L.R.; Holmes, A.J.; Levitt, P. Quantitative Trait Loci and a Novel Genetic Candidate for Fear Learning. J. Neurosci. 2016, 36, 6258–6268. [Google Scholar] [CrossRef]
- Greenwood, T.A.; Lazzeroni, L.C.; Maihofer, A.X.; Swerdlow, N.R.; Calkins, M.E.; Freedman, R.; Green, M.F.; Light, G.A.; Nievergelt, C.M.; Nuechterlein, K.H.; et al. Genome-wide Association of Endophenotypes for Schizophrenia From the Consortium on the Genetics of Schizophrenia (COGS) Study. JAMA Psychiatry 2019, 76, 1274. [Google Scholar] [CrossRef]
- Santoro, B.; Shah, M.M. Hyperpolarization-Activated Cyclic Nucleotide-Gated Channels as Drug Targets for Neurological Disorders. Annu. Rev. Pharmacol. Toxicol. 2020, 60, 109–131. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salling, M.C.; Harrison, N.L. Constitutive Genetic Deletion of Hcn1 Increases Alcohol Preference during Adolescence. Brain Sci. 2020, 10, 763. https://doi.org/10.3390/brainsci10110763
Salling MC, Harrison NL. Constitutive Genetic Deletion of Hcn1 Increases Alcohol Preference during Adolescence. Brain Sciences. 2020; 10(11):763. https://doi.org/10.3390/brainsci10110763
Chicago/Turabian StyleSalling, Michael C., and Neil L. Harrison. 2020. "Constitutive Genetic Deletion of Hcn1 Increases Alcohol Preference during Adolescence" Brain Sciences 10, no. 11: 763. https://doi.org/10.3390/brainsci10110763
APA StyleSalling, M. C., & Harrison, N. L. (2020). Constitutive Genetic Deletion of Hcn1 Increases Alcohol Preference during Adolescence. Brain Sciences, 10(11), 763. https://doi.org/10.3390/brainsci10110763



