Working Memory in Children with Learning Disorders: An EEG Power Spectrum Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Working Memory Task
2.3. EEG Recording and Data Analysis
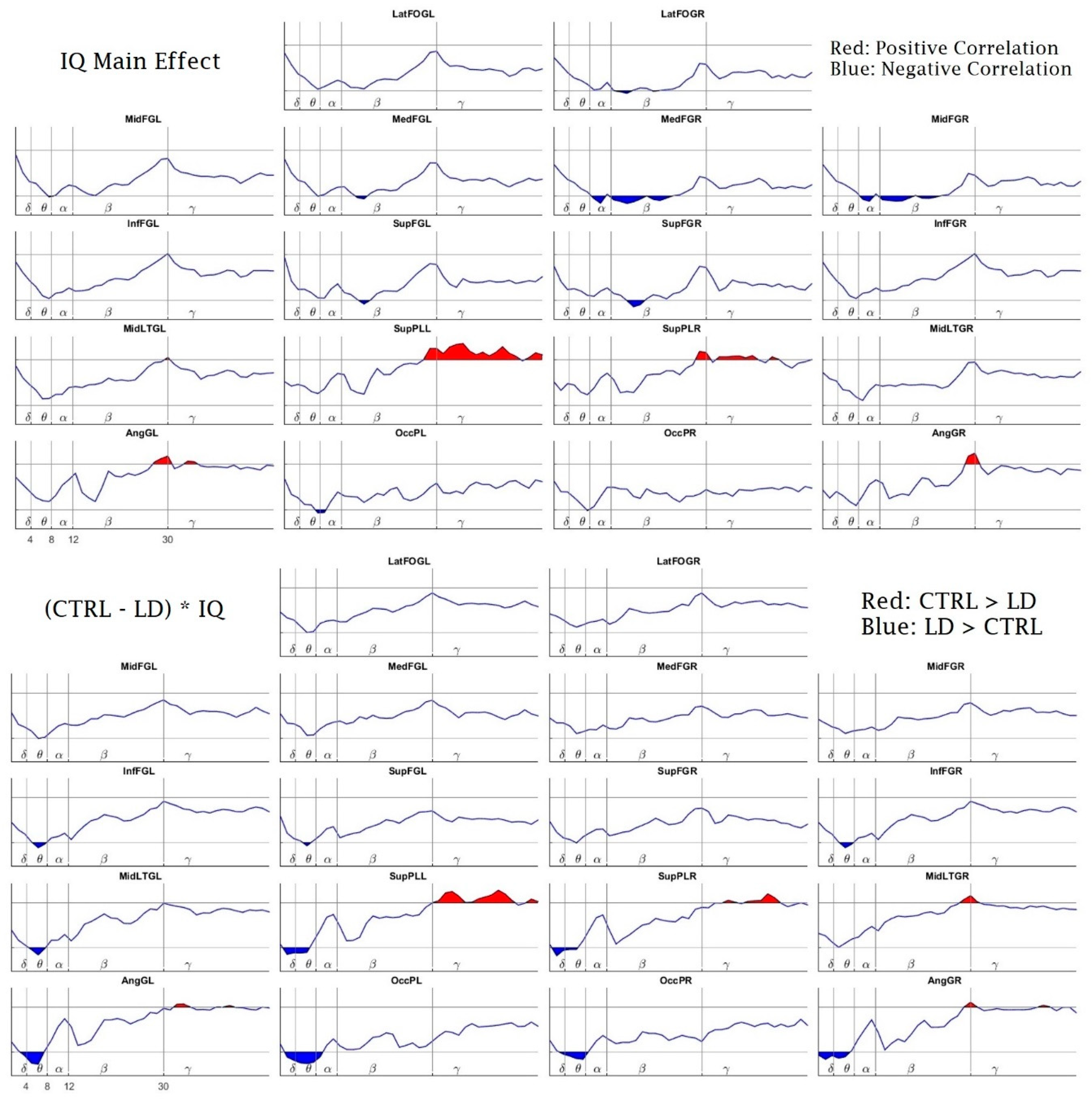
3. Results
3.1. Behavioral Results
3.2. Power Spectral Density Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| LD (# of Selected Epochs) | CTRL (# of Selected Epochs) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 19 | 20 | 21 | 22 | 23 | 24 | 19 | 20 | 21 | 22 | 23 | 24 | |
| Low Load | 1 | 0 | 0 | 0 | 1 | 17 | 1 | 2 | 0 | 1 | 1 | 16 |
| High Load | 0 | 0 | 1 | 0 | 1 | 17 | 0 | 0 | 0 | 1 | 0 | 20 |
| Contrast | p of PCR | p of RT |
|---|---|---|
| (HL-LL) * CTR | 0.050717 | 0.033811 |
| (HL-LL) * LD | 0.000001 | 0.000653 |
| (CTR-LD) * HL | 0.047681 | 0.454841 |
| (CTR-LD) * LL | 0.143291 | 0.383543 |
| (HL-LL) * CTR–(HL-LL) * LD | 0.021140 | 0.212304 |
| IQ | 0.361491 | 0.336430 |
| CTR * IQ | 0.129421 | 0.482411 |
| LD * IQ | 0.167774 | 0.397886 |
| (CTR-LD) * IQ | 0.143015 | 0.429394 |
References
- Altarac, M.; Saroha, E. Lifetime Prevalence of Learning Disability Among US Children. Pediatrics 2007, 119 (Suppl. 1), S77–S83. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders; American Psychiatric Association: Arlington, VA, USA, 2013. [Google Scholar] [CrossRef]
- Lagae, L. Learning Disabilities: Definitions, Epidemiology, Diagnosis, and Intervention Strategies. Pediatr. Clin. N. Am. 2008, 55, 1259–1268. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed.; American Psychiatric Association: Arlington, VA, USA, 2000; Volume 1, p. 943. [Google Scholar] [CrossRef]
- Willcutt, E.G.; Petrill, S.A.; Wu, S.; Boada, R.; DeFries, J.C.; Olson, R.K.; Pennington, B.F. Comorbidity Between Reading Disability and Math Disability. J. Learn. Disabil. 2013, 46, 500–516. [Google Scholar] [CrossRef] [PubMed]
- Schuchardt, K.; Maehler, C.; Hasselhorn, M. Working Memory Deficits in Children with Specific Learning Disorders. J. Learn. Disabil. 2008, 41, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Baddeley, A.D.; Hitch, G. Working Memory. Psychol. Learn. Motiv. 1974, 8, 47–89. [Google Scholar] [CrossRef]
- De Weerdt, F.; Desoete, A.; Eroeyers, H. Working Memory in Children with Reading Disabilities and/or Mathematical Disabilities. J. Learn. Disabil. 2012, 46, 461–472. [Google Scholar] [CrossRef]
- Siegel, L.S.; Ryan, E.B. The Development of Working Memory in Normally Achieving and Subtypes of Learning Disabled Children. Child Dev. 1989, 60, 973. [Google Scholar] [CrossRef]
- Swanson, H.L. Intelligence, Working Memory, and Learning Disabilities; Elsevier Inc.: Amsterdam, The Netherlands, 2015. [Google Scholar] [CrossRef]
- Schuchardt, K.; Bockmann, A.-K.; Bornemann, G.; Mähler, C. Working Memory Functioning in Children with Learning Disorders and Specific Language Impairment. Top. Lang. Disord. 2013, 33, 298–312. [Google Scholar] [CrossRef]
- Alloway, T.P. Working Memory, but Not IQ, Predicts Subsequent Learning in Children with Learning Difficulties. Eur. J. Psychol. Assess. 2009, 25, 92–98. [Google Scholar] [CrossRef]
- Roca-Stappung, M.; Fernández, T.; Bosch-Bayard, J.; Harmony, T.; Ricardo-Garcell, J. Electroencephalographic characterization of subgroups of children with learning disorders. PLoS ONE 2017, 12, e0179556. [Google Scholar] [CrossRef]
- Fernández, T.; Harmony, T.; Fernández-Bouzas, A.; Silva, J.; Herrera, W.; Santiago-Rodríguez, E.; Sánchez, L. Sources of EEG activity in learning disabled children. Clin. Electroencephalogr. 2002, 33, 160–164. [Google Scholar] [CrossRef]
- Fonseca, L.C.; Tedrus, G.M.; Chiodi, M.G.; Cerqueira, J.N.; Tonelotto, J.M. Quantitative EEG in children with learning disabilities: Analysis of band power. Arq. Neuro-Psiquiatr. 2006, 64, 376–381. [Google Scholar] [CrossRef]
- Chabot, R.J.; Di Michele, F.; Prichep, L.; John, E.R. The Clinical Role of Computerized EEG in the Evaluation and Treatment of Learning and Attention Disorders in Children and Adolescents. J. Neuropsychiatry Clin. Neurosci. 2001, 13, 171–186. [Google Scholar] [CrossRef]
- Schapkin, S.A.; Raggatz, J.; Hillmert, M.; Böckelmann, I. EEG correlates of cognitive load in a multiple choice reaction task. Acta Neurobiol. Exp. 2020, 80, 76–89. [Google Scholar] [CrossRef]
- Fernandez, T.; Harmony, T.; Gersenowies, J.; Silva-Pereyra, J.; Fernández-Bouzas, A.; Galán, L.; Díaz-Comas, L. Sources of EEG activity during a verbal working memory task in adults and children. Suppl. Clin. Neurophysiol. 2002, 54, 269–283. [Google Scholar] [CrossRef]
- Dimitriadis, S.I.; Laskaris, N.A.; Tsirka, V.; Vourkas, M.; Micheloyannis, S. What does delta band tell us about cognitive processes: A mental calculation study. Neurosci. Lett. 2010, 483, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Harmony, T. The functional significance of delta oscillations in cognitive processing. Front. Integr. Neurosci. 2013, 7, 83. [Google Scholar] [CrossRef]
- Klimesch, W. EEG alpha and theta oscillations reflect cognitive and memory performance: A review and analysis. Brain Res. Rev. 1999, 29, 169–195. [Google Scholar] [CrossRef]
- Gevins, A.; Smith, M.E.; McEvoy, L.; Yu, D. High-resolution EEG mapping of cortical activation related to working memory: Effects of task difficulty, type of processing, and practice. Cereb. Cortex 1997, 7, 374–385. [Google Scholar] [CrossRef] [PubMed]
- Brzezicka, A.; Kamiński, J.; Reed, C.M.; Chung, J.M.; Mamelak, A.N.; Rutishauser, U. Working Memory Load-related Theta Power Decreases in Dorsolateral Prefrontal Cortex Predict Individual Differences in Performance. J. Cogn. Neurosci. 2019, 31, 1290–1307. [Google Scholar] [CrossRef]
- Maurer, U.; Brem, S.; Liechti, M.; Maurizio, S.; Michels, L.; Brandeis, D. Frontal Midline Theta Reflects Individual Task Performance in a Working Memory Task. Brain Topogr. 2015, 28, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Jensen, O.; Tesche, C.D. Frontal theta activity in humans increases with memory load in a working memory task. Eur. J. Neurosci. 2002, 15, 1395–1399. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, D.J.; McNaughton, N.; Flanagan, D.; Kirk, I.J. Frontal-midline theta from the perspective of hippocampal “theta”. Prog. Neurobiol. 2008, 86, 156–185. [Google Scholar] [CrossRef]
- Eschmann, K.C.; Bader, R.; Mecklinger, A. Topographical differences of frontal-midline theta activity reflect functional differences in cognitive control abilities. Brain Cogn. 2018, 123, 57–64. [Google Scholar] [CrossRef]
- Cavanagh, J.F.; Frank, M.J. Frontal theta as a mechanism for cognitive control. Trends Cogn. Sci. 2014, 18, 414–421. [Google Scholar] [CrossRef]
- Collado-Mateo, D.; Villafaina, S.; Collado-Mateo, D.; Cano-Plasencia, R.; Gusi, N. Chess Players Increase the Theta Power Spectrum When the Difficulty of the Opponent Increases: An EEG Study. Int. J. Environ. Res. Public Health 2019, 17, 46. [Google Scholar] [CrossRef]
- Pfurtscheller, G.; Stancák, A.; Neuper, C. Event-related synchronization (ERS) in the alpha band—An electrophysiological correlate of cortical idling: A review. Int. J. Psychophysiol. 1996, 24, 39–46. [Google Scholar] [CrossRef]
- Singh, Y.; Singh, J.; Sharma, R.; Talwar, A. FFT transformed quantitative EEG analysis of short term memory load. Ann. Neurosci. 2015, 22, 176–179. [Google Scholar] [CrossRef] [PubMed]
- Jensen, O.; Gelfand, J.; Kounios, J.; Lisman, J.E. Oscillations in the Alpha Band (9–12 Hz) Increase with Memory Load during Retention in a Short-Term Memory Task. Cereb. Cortex 2002, 12, 877–882. [Google Scholar] [CrossRef] [PubMed]
- Klimesch, W.; Sauseng, P.; Hanslmayr, S. EEG alpha oscillations: The inhibition–timing hypothesis. Brain Res. Rev. 2007, 53, 63–88. [Google Scholar] [CrossRef]
- Michels, L.; Moazami-Goudarzi, M.; Jeanmonod, D.; Sarnthein, J. EEG alpha distinguishes between cuneal and precuneal activation in working memory. NeuroImage 2008, 40, 1296–1310. [Google Scholar] [CrossRef]
- Wang, R.; Kamezawa, R.; Watanabe, A.; Iramina, K. EEG alpha power change during working memory encoding in adults with different memory performance levels. In Proceedings of the 2017 39th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Seogwipo, Korea, 11–15 July 2017. [Google Scholar] [CrossRef]
- Klimesch, W. The frequency architecture of brain and brain body oscillations: An analysis. Eur. J. Neurosci. 2018, 48, 2431–2453. [Google Scholar] [CrossRef]
- Stipacek, A.; Grabner, R.; Neuper, C.; Fink, A.; Neubauer, A. Sensitivity of human EEG alpha band desynchronization to different working memory components and increasing levels of memory load. Neurosci. Lett. 2003, 353, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Rypma, B.; Berger, J.S.; D’Esposito, M. The Influence of Working-Memory Demand and Subject Performance on Prefrontal Cortical Activity. J. Cogn. Neurosci. 2002, 14, 721–731. [Google Scholar] [CrossRef]
- Neubauer, A.C.; Fink, A. Intelligence and neural efficiency. Neurosci. Biobehav. Rev. 2009, 33, 1004–1023. [Google Scholar] [CrossRef]
- Grabner, R.H.; Fink, A.; Stipacek, A.; Neuper, C.; Neubauer, A.C. Intelligence and working memory systems: Evidence of neural efficiency in alpha band ERD. Cogn. Brain Res. 2004, 20, 212–225. [Google Scholar] [CrossRef] [PubMed]
- Nussbaumer, D.; Grabner, R.H.; Stern, E. Neural efficiency in working memory tasks: The impact of task demand. Intelligence 2015, 50, 196–208. [Google Scholar] [CrossRef]
- Doppelmayr, M.; Klimesch, W.; Sauseng, P.; Hödlmoser, K.; Stadler, W.; Hanslmayr, S. Intelligence related differences in EEG-bandpower. Neurosci. Lett. 2005, 381, 309–313. [Google Scholar] [CrossRef]
- Capotosto, P.; Perrucci, M.G.; Brunetti, M.; Del Gratta, C.; Doppelmayr, M.; Grabner, R.H.; Klimesch, W.; Neubauer, A.; Neuper, C.; Pfurtscheller, G.; et al. Is there “neural efficiency” during the processing of visuo-spatial information in male humans? An EEG study. Behav. Brain Res. 2009, 205, 468–474. [Google Scholar] [CrossRef]
- Pavlov, Y.G.; Kotchoubey, B. EEG correlates of working memory performance in females. BMC Neurosci. 2017, 18, 1–14. [Google Scholar] [CrossRef]
- Hwang, G.M.; Jacobs, J.; Geller, A.S.; Danker, J.; Sekuler, R.; Kahana, M.J. EEG correlates of verbal and nonverbal working memory. Behav. Brain Funct. 2005, 1, 20. [Google Scholar] [CrossRef]
- Jokisch, D.; Jensen, O. Modulation of Gamma and Alpha Activity during a Working Memory Task Engaging the Dorsal or Ventral Stream. J. Neurosci. 2007, 27, 3244–3251. [Google Scholar] [CrossRef] [PubMed]
- Jensen, O.; Kaiser, J.; Lachaux, J.-P. Human gamma-frequency oscillations associated with attention and memory. Trends Neurosci. 2007, 30, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Popov, T.; Popova, P.; Harkotte, M.; Awiszus, B.; Rockstroh, B.; Miller, G.A. Cross-frequency interactions between frontal theta and posterior alpha control mechanisms foster working memory. NeuroImage 2018, 181, 728–733. [Google Scholar] [CrossRef]
- Honkanen, R.; Rouhinen, S.; Wang, S.H.; Palva, S.; Palva, S. Gamma Oscillations Underlie the Maintenance of Feature-Specific Information and the Contents of Visual Working Memory. Cereb. Cortex 2014, 25, 3788–3801. [Google Scholar] [CrossRef]
- Tallon-Baudry, C.; Bertrand, O.; Peronnet, F.; Pernier, J. Induced γ-Band Activity during the Delay of a Visual Short-Term Memory Task in Humans. J. Neurosci. 1998, 18, 4244–4254. [Google Scholar] [CrossRef] [PubMed]
- Rippon, G. Trait and state EEG indices of information processing in developmental dyslexia. Int. J. Psychophysiol. 2000, 36, 251–265. [Google Scholar] [CrossRef]
- Spironelli, C.; Penolazzi, B.; Vio, C.; Angrilli, A. Inverted EEG theta lateralization in dyslexic children during phonological processing. Neuropsychologia 2006, 44, 2814–2821. [Google Scholar] [CrossRef]
- Klimesch, W.; Doppelmayr, M.; Wimmer, H.; Gruber, W.; Röhm, D.; Schwaiger, J.; Hutzler, F. Alpha and beta band power changes in normal and dyslexic children. Clin. Neurophysiol. 2001, 112, 1186–1195. [Google Scholar] [CrossRef]
- James, W. Memory. In Talks to Teachers on Psychology—And to Students on Some of Life’s Ideals; Metropolitan Books/Henry Holt and Company: New York, NY, USA, 1899; pp. 116–143. [Google Scholar] [CrossRef]
- Silva-Pereyra, J.; Fernández, T.; Harmony, T.; Bernal, J.; Galán, L.; Díaz-Comas, L.; Fernández-Bouzas, A.; Yáñez, G.; Rivera-Gaxiola, M.; Rodríguez, M.; et al. Delayed P300 during Sternberg and color discrimination tasks in poor readers. Int. J. Psychophysiol. 2001, 40, 17–32. [Google Scholar] [CrossRef]
- Luck, S.J.; Kappenman, E.S.; Cacioppo, J.T.; Tassinary, L.G.; Berntson, G.G. Electroencephalography and Event-Related Brain Potentials. In Handbook of Psychophysiology; Cambridge University Press: Cambridge, UK, 2016; pp. 74–100. [Google Scholar]
- Koerner, T.K.; Zhang, Y. Application of Linear Mixed-Effects Models in Human Neuroscience Research: A Comparison with Pearson Correlation in Two Auditory Electrophysiology Studies. Brain Sci. 2017, 7, 26. [Google Scholar] [CrossRef]
- Nunez, P.L.; Srinivasan, R. Electric Fields of the Brain: The Neurophysics of EEG, 2nd ed.; Oxford University Press: New York, NY, USA, 2006; ISBN 0-19-505038. [Google Scholar]
- He, B.; Lian, J.; Li, G. High-resolution EEG: A new realistic geometry spline Laplacian estimation technique. Clin. Neurophysiol. 2001, 112, 845–852. [Google Scholar] [CrossRef]
- Pascual-Marqui, R.D.; Faber, P.L.; Kinoshita, T.; Kochi, K.; Milz, P.; Nishida, K.; Yoshimura, M. Comparing EEG/MEG neuroimaging methods based on localization error, false positive activity, and false positive connectivity. bioRxiv 2018, 269753. [Google Scholar] [CrossRef]
- World Medical Association World Medical Association Declaration of Helsinki. JAMA 2013, 310, 2191–2194. [CrossRef] [PubMed]
- Wechsler, D.; Flanagan, D.P. WISC-IV: Escala de Inteligencia de Wechsler Para Niños-IV.; Departamento I+D de TEA Ediciones: Madrid, Spain, 2007. [Google Scholar]
- Harter, L.P.; Gross, B.H.; Callen, P.W.; Barth, R.A. Ultrasonic evaluation of abdominal aortic thrombus. J. Ultrasound Med. 1982, 1, 315–318. [Google Scholar] [CrossRef]
- Silva-Pereyra, J.; Rivera-Gaxiola, M.; Fernández, T.; Díaz-Comas, L.; Harmony, T.; Fernández-Bouzas, A.; Rodríguez, M.; Bernal, J.; Marosi, E. Are poor readers semantically challenged? An event-related brain potential assessment. Int. J. Psychophysiol. 2003, 49, 187–199. [Google Scholar] [CrossRef]
- Holcomb, P.J.; Ackerman, P.T.; Dykman, R.A. Auditory event-related potentials in attention and reading disabled boys. Int. J. Psychophysiol. 1986, 3, 263–273. [Google Scholar] [CrossRef]
- Hernández-Barros, D.; Savio, G.; Pérez, M. Evaluación de La Percepción Auditiva Con El Sistema Medicid 3E. Rev. CENIC Cienc. Biol. 2002, 33, 93–99. [Google Scholar]
- Bosch-Bayard, J.; Valdés-Sosa, P.; Virues-Alba, T.; Aubert-Vázquez, E.; John, E.R.; Harmony, T.; Riera-Díaz, J.; Trujillo-Barreto, N.J. 3D statistical parametric mapping of EEG source spectra by means of variable resolution electromagnetic tomography (VARETA). Clin. Electroencephalogr. 2001, 32, 47–61. [Google Scholar] [CrossRef]
- Biscay, R.J.; Bosch-Bayard, J.F.; Pascual-Marqui, R.D. Unmixing EEG Inverse Solutions Based on Brain Segmentation. Front. Neurosci. 2018, 12, 325. [Google Scholar] [CrossRef]
- Pascual-Marqui, R.D. Discrete, 3D Distributed, Linear Imaging Methods of Electric Neuronal Activity. Part 1: Exact, Zero Error Localization. arXiv 2007, arXiv:0710.3341. [Google Scholar]
- Lohmann, G.; Margulies, D.S.; Horstmann, A.; Pleger, B.; Lepsien, J.; Goldhahn, D.; Schloegl, H.; Stumvoll, M.; Villringer, A.; Turner, R. Eigenvector Centrality Mapping for Analyzing Connectivity Patterns in fMRI Data of the Human Brain. PLoS ONE 2010, 5, e10232. [Google Scholar] [CrossRef] [PubMed]
- Worsley, K.; Taylor, J.; Carbonell, F.; Chung, M.; Duerden, E.; Bernhardt, B.; Lyttelton, O.; Boucher, M.; Evans, A. SurfStat: A Matlab toolbox for the statistical analysis of univariate and multivariate surface and volumetric data using linear mixed effects models and random field theory. NeuroImage 2009, 47, S102. [Google Scholar] [CrossRef]
- Suckling, J.; Bullmore, E.; Bullmore, E.T. Permutation tests for factorially designed neuroimaging experiments. Hum. Brain Mapp. 2004, 22, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Sarnthein, J.; Petsche, H.; Rappelsberger, P.; Shaw, G.L.; Von Stein, A. Synchronization between prefrontal and posterior association cortex during human working memory. Proc. Natl. Acad. Sci. USA 1998, 95, 7092–7096. [Google Scholar] [CrossRef]
- Owen, A.M. The role of the lateral frontal cortex in mnemonic processing: The contribution of functional neuroimaging. Exp. Brain Res. 2000, 133, 33–43. [Google Scholar] [CrossRef]
- Bell, M.A.; Wolfe, C.D. Changes in Brain Functioning From Infancy to Early Childhood: Evidence from EEG Power and Coherence Working Memory Tasks. Dev. Neuropsychol. 2007, 31, 21–38. [Google Scholar] [CrossRef]
- Chai, W.J.; Hamid, A.I.A.; Abdullah, J.M. Working Memory from the Psychological and Neurosciences Perspectives: A Review. Front. Psychol. 2018, 9, 401. [Google Scholar] [CrossRef]
- Gulbinaite, R.; Van Rijn, H.; Cohen, M.X. Fronto-parietal network oscillations reveal relationship between working memory capacity and cognitive control. Front. Hum. Neurosci. 2014, 8, 761. [Google Scholar] [CrossRef]
- Schack, B.; Vath, N.; Petsche, H.; Geissler, H.-G.; Moller, E. Phase-Coupling of Theta-Gamma EEG Rhythms during Short-Term Memory Processing. Int. J. Psychophysiol. 2002, 44, 143–163. [Google Scholar] [CrossRef]








| Ctrl Group n = 22 | LD Group n = 23 | Statistical Differences between Groups | |||
|---|---|---|---|---|---|
| Mean | Sd | Mean | Sd | ||
| Age | 9.5 | 0.9 | 9.4 | 1.10 | t = 0.31 (NS) |
| WISC test: | |||||
| Full Scale IQ | 109.3 | 16.4 | 88.5 | 7.9 | t = 5.46, p < 0.001 |
| Working Memory Index | 105.7 | 16.5 | 89 | 8.8 | t = 4.25, p < 0.001 |
| Female/Male ratio | 14/8 | 12/11 | OR = 0.62; CI: (0.18, 2.05); (NS) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Briones, B.J.; Fernández-Harmony, T.; Garófalo Gómez, N.; Biscay-Lirio, R.J.; Bosch-Bayard, J. Working Memory in Children with Learning Disorders: An EEG Power Spectrum Analysis. Brain Sci. 2020, 10, 817. https://doi.org/10.3390/brainsci10110817
Martínez-Briones BJ, Fernández-Harmony T, Garófalo Gómez N, Biscay-Lirio RJ, Bosch-Bayard J. Working Memory in Children with Learning Disorders: An EEG Power Spectrum Analysis. Brain Sciences. 2020; 10(11):817. https://doi.org/10.3390/brainsci10110817
Chicago/Turabian StyleMartínez-Briones, Benito J., Thalía Fernández-Harmony, Nicolás Garófalo Gómez, Rolando J. Biscay-Lirio, and Jorge Bosch-Bayard. 2020. "Working Memory in Children with Learning Disorders: An EEG Power Spectrum Analysis" Brain Sciences 10, no. 11: 817. https://doi.org/10.3390/brainsci10110817
APA StyleMartínez-Briones, B. J., Fernández-Harmony, T., Garófalo Gómez, N., Biscay-Lirio, R. J., & Bosch-Bayard, J. (2020). Working Memory in Children with Learning Disorders: An EEG Power Spectrum Analysis. Brain Sciences, 10(11), 817. https://doi.org/10.3390/brainsci10110817





