Changes in Balance, Gait and Electroencephalography Oscillations after Robot-Assisted Gait Training: An Exploratory Study in People with Chronic Stroke
Abstract
:1. Introduction
2. Materials and Methods
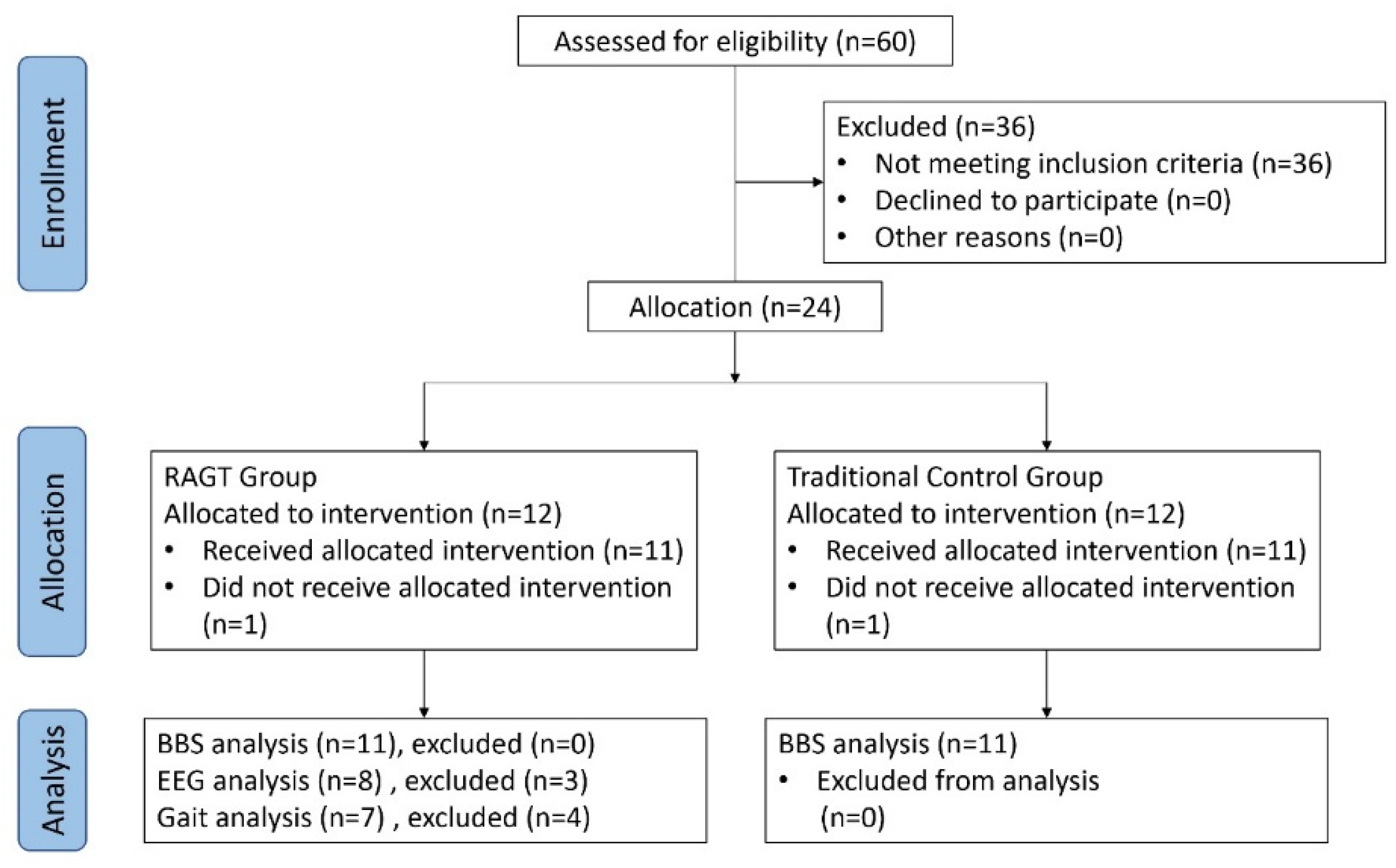
2.1. Participants
2.1.1. Protocol
2.1.2. Primary Clinical Scores
2.2. Secondary Parameter Measurements
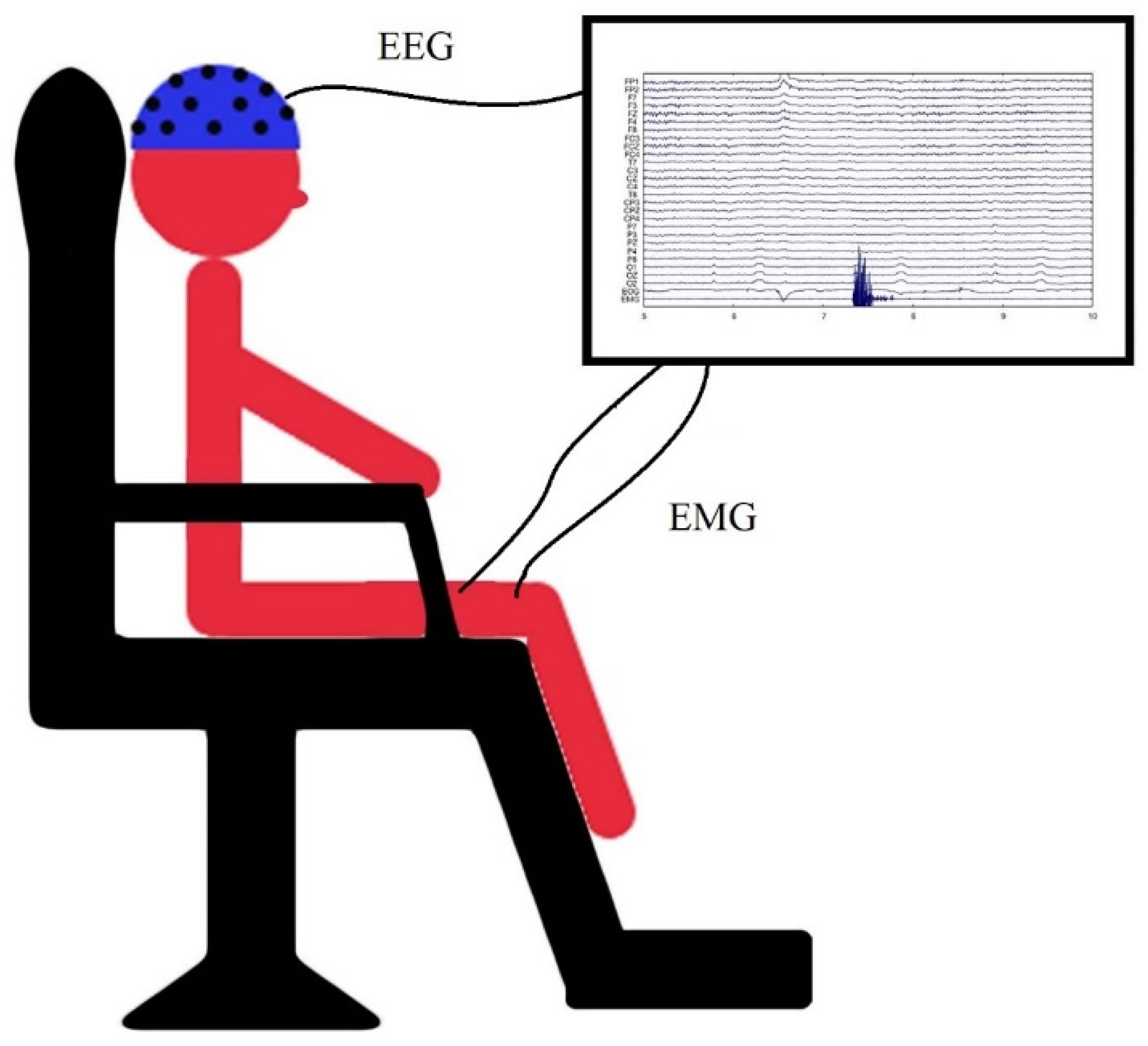
2.2.1. EEG and EMG Recordings
2.2.2. ERD/ERS Analyses
2.2.3. Gait Analysis
2.3. Statistics
3. Results
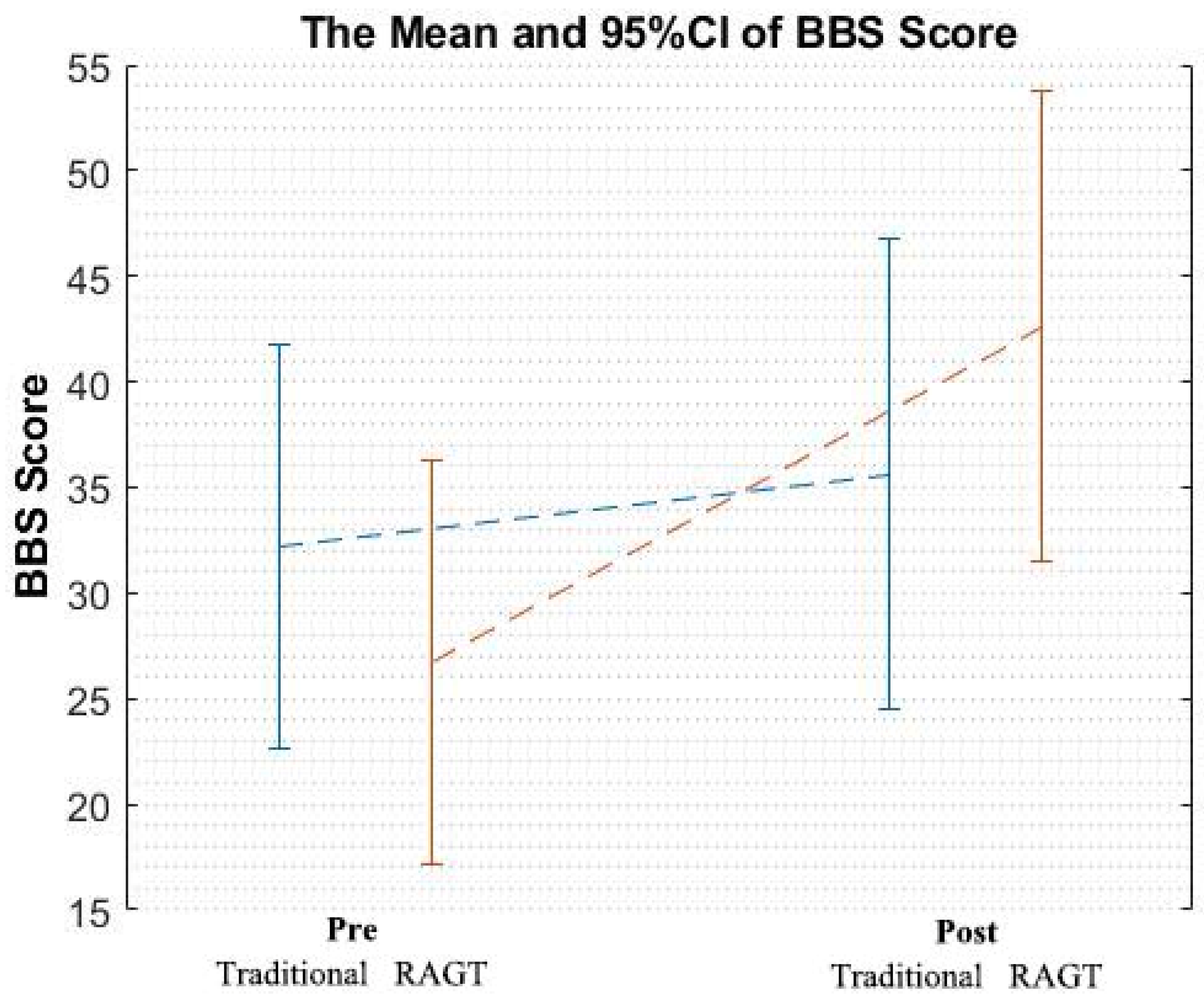
3.1. Primary Clinical Scores
3.2. Secondary Parameters
3.2.1. Gait Analysis
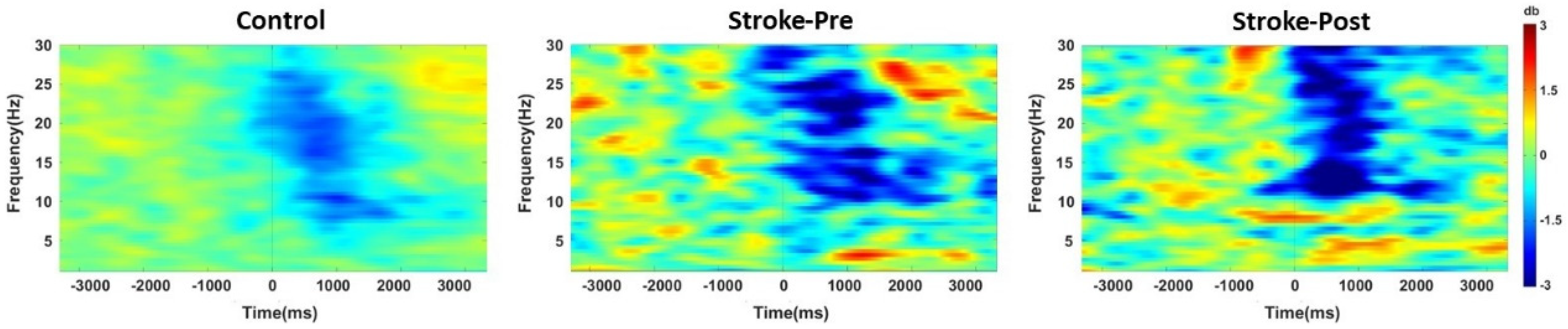
3.2.2. Changes in ERD and ERS
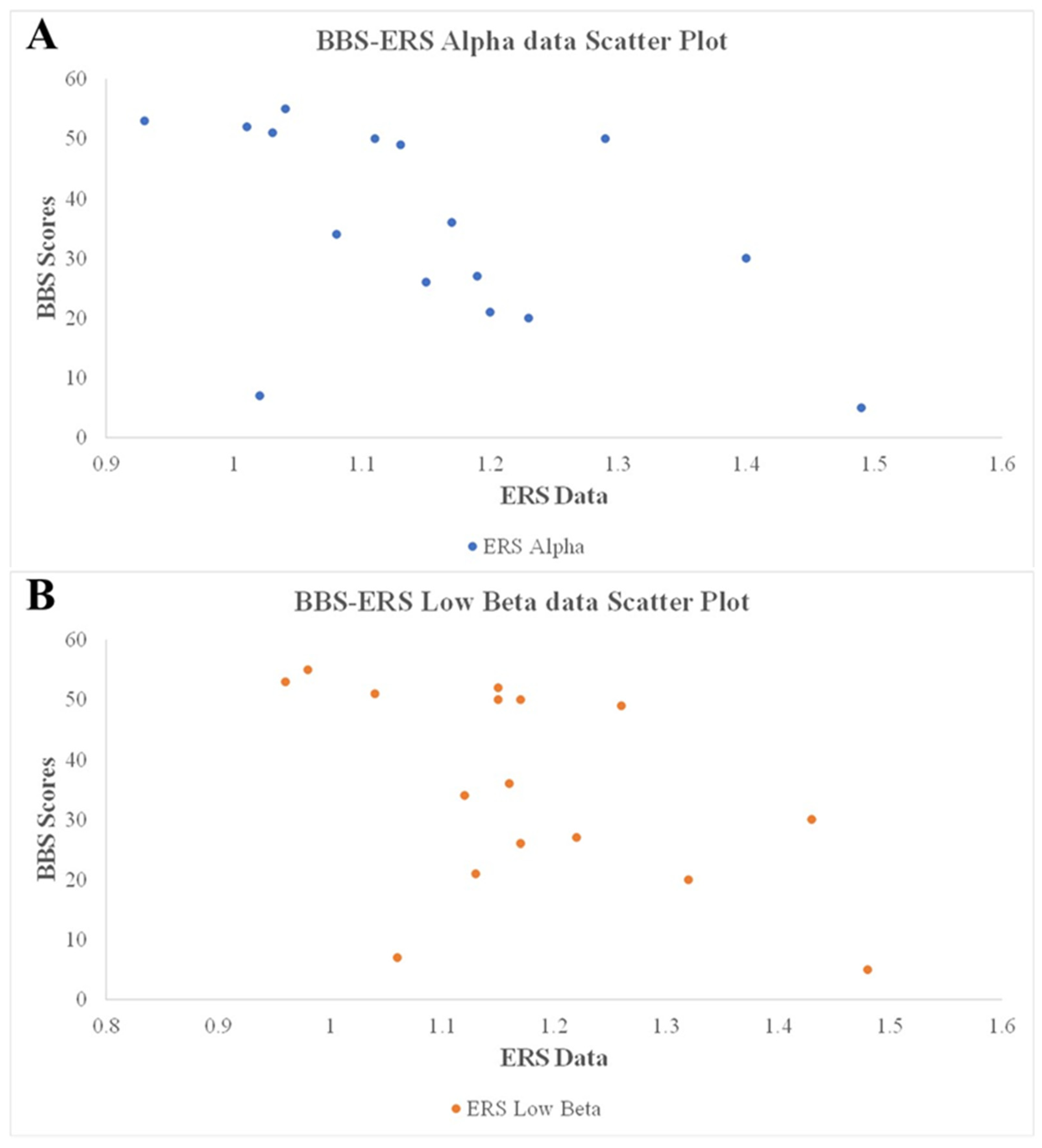
3.3. Correlations of Clinical Outcomes with ERD and ERS
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Takeuchi, N.; Izumi, S.-I. Combination of stroke neurorehabilitation to facilatate motor recovery: Perspectives on Hebbian plasticity and homeostatic metaplasticity. Front. Hum. Neurosci. 2015, 9, 349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naros, G.; Gharabaghi, A. Reinforcement learning of self-regulated β-oscillations for motor restoration in chronic stroke. Front. Hum. Neurosci. 2015, 9, 391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossiter, H.E.; Boudrias, M.-H.; Ward, N.S. Do movement-related beta oscillations change after stroke? J. Neurophysiol. 2014, 112, 2053–2058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takemi, M.; Masakado, Y.; Liu, M.; Ushiba, J. Event-related desynchronization reflects downregulation of intracortical inhibition in human primary motor cortex. J. Neurophysiol. 2013, 110, 1158–1166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, M.; Takeda, K.; Otaka, Y.; Osu, R.; Hanakawa, T.; Gouko, M.; Ito, K. Event related desynchronization-modulated functional electrical stimulation system for stroke rehabilitation: A feasibility study. J. Neuroeng. Rehabil. 2012, 9, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, Y.-H.; Kim, J.-D.; Lee, J.-H.; Cha, Y.-J. Walking and balance ability gain from two types of gait intervention in adult patients with chronic hemiplegic stroke: A pilot study. Assist. Technol. Off. J. RESNA 2019, 31, 112–115. [Google Scholar] [CrossRef] [PubMed]
- Hessam, M.; Salehi, R.; Yazdi, M.J.S.; Negahban, H.; Rafie, S.; Mehravar, M. Relationship between functional balance and walking ability in individuals with chronic stroke. J. Phys. Ther. Sci. 2018, 30, 993–996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alingh, J.F.; Groen, B.E.; Assenldonk, E.H.F.V.; Geurts, A.C.H.; Weerdesteyn, V. Effectiveness of rehabilitation interventions to improve paretic propulsion in individuals with stroke—A systematic review. Clin. Biomech. 2020, 71, 176–188. [Google Scholar] [CrossRef] [PubMed]
- Berg, K.; Wood-Dauphinee, S.; Williams, J.I. The Balance Scale: Reliability assessment with elderly residents and patients with an acute stroke. Scand. J. Rehabil. Med. 1995, 27, 27–36. [Google Scholar]
- Usuda, S.; Araya, K.; Umehara, K.; Endo, M.; Shimizu, T.; Endo, F. Construct validity of functional balance scale in stroke inpatients. J. Phys. Ther. Sci. 1998, 10, 53–56. [Google Scholar] [CrossRef] [Green Version]
- Cui, C.; Bian, G.-B.; Hou, Z.-G.; Zhao, J.; Su, G.; Zhou, H.; Peng, L.; Wang, W. Simultaneous Recognition and Assessment of Post-Stroke Hemiparetic Gait by Fusing Kinematic, Kinetic, and Electrophysiological Data. IEEE Trans. Neural Syst. Rehabil. Eng. 2018, 26, 856–864. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.-S.; Hsieh, Y.-W.; Wu, C.-Y.; Lin, Y.-T.; Lin, K.-C.; Chen, C.-L. The effects of combination of robot-assisted therapy with task-specific or impairment-oriented training on motor function and quality of life in chronic stroke. PMR 2016, 8, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Reinkensmeyer, D.J.; Emken, J.L.; Cramer, S.C. Robotics, motor learning, and neurologic recovery. Annu. Rev. Biomed. Eng. 2004, 6, 497–525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruni, M.F.; Melegari, C.; Cola, M.C.D.; Bramanti, A.; Bramanti, P.; Calabrò, R.S. What does best evidence tell us about robotic gait rehabilitation in stroke patients: A systematic review and meta-analysis. J. Clin. Neurosci. 2018, 48, 11–17. [Google Scholar] [CrossRef]
- Mehrholz, J.; Pohl, M. Electromechanical-assisted gait training after stroke: A systematic review comparing end-effector and exoskeleton devices. J. Rehabil. Med. 2012, 44, 193–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moucheboeuf, G.; Griffier, R.; Gasq, D.; Glize, B.; Bouyer, L.; Dehail, P.; Cassoudesalle, H. Effects of robotic gait training after stroke: A meta-analysis. Ann. Phys. Rehabil. Med. 2020. [Google Scholar] [CrossRef]
- Din, S.D.; Bertoldo, A.; Sawacha, Z.; Jonsdottir, J.; Rabuffetti, M.; Cobelli, C.; Ferrarin, M. Assessment of biofeedback rehabilitation in post-stroke patients combining fMRI and gait analysis: A case study. J. Neuroeng. Rehabil. 2014, 11, 1–12. [Google Scholar]
- Yang, H.E.; Kyeong, S.; Lee, S.H.; Lee, W.-J.; Ha, S.W.; Kim, S.M.; Kang, H.; Lee, W.M.; Kang, C.S.; Kim, D.H. Structural and functional improvements due to robot-assisted gait training in the stroke-injured brain. Neurosci. Lett. 2017, 637, 114–119. [Google Scholar] [CrossRef]
- Pfurtscheller, G.; Neuper, C. Future prospects of ERD/ERS in the context of brain-computer interface (BCI) developments. Prog. Brain Res. 2006, 159, 433–437. [Google Scholar]
- Wagner, J.; Solis-Escalante, T.; Grieshofer, P.; Neuper, C.; Müller-Putz, G.R.; Scherer, R. Level of participation in robotic-assisted treadmill walking modulates midline sensorimotor EEG rhythms in able-bodied subjects. NeuroImage 2012, 63, 1203–1211. [Google Scholar] [CrossRef]
- Mäkelä, J.P.; Lioumis, P.; Laaksonen, K.; Forss, N.; Tatlisumak, T.; Kaste, M.; Mustanoja, S. Cortical Excitabillity Measured with nTMS and MEG during Stroke Recovery. Neural Plast. 2015, 2015, 309546. [Google Scholar] [CrossRef] [PubMed]
- Gaetz, W.; Macdonald, M.; Cheyne, D.; Snead, O.C. Neuromagnetic imaging of movement-related cortical oscillations in children and adults: Age predicts post-movement beta rebound. Neuroimage 2010, 51, 792–807. [Google Scholar] [CrossRef]
- Calabrò, R.S.; Naro, A.; Russo, M.; Bramanti, P.; Carioti, L.; Balletta, T.; Buda, A.; Manuli, A.; Filoni, S.; Bramanti, A. Shaping neuroplasticity by using powered exoskeletons in patients with stroke: A randomized clinical trial. J. Neuroeng. Rehabil. 2018, 15, 1–16. [Google Scholar] [CrossRef]
- Müller-Putz, G.R.; Zimmermann, D.; Graimann, B.; Nestinger, K.; Korisek, G.; Pfurtscheller, G. Event-related beta EEG-changes during passive and attempted foot movements in paraplegic patients. Brain Res. 2007, 1137, 84–91. [Google Scholar] [CrossRef]
- Neuper, C.; Wörtz, M.; Pfurtscheller, G. ERD/ERS patterns reflecting sensorimotor activation and deactivation. Prog. Brain Res. 2006, 159, 211–222. [Google Scholar] [PubMed]
- Pfurtscheller, G. The cortical activation model (CAM). Prog. Brain Res. 2006, 159, 19–27. [Google Scholar]
- Formaggio, E.; Storti, S.F.; Galazzo, I.B.; Gandolfi, M.; Geroin, C.; Smania, N.; Fiaschi, A.; Manganotti, P. Time–frequency modulation of ERD and EEG coherence in robot-assisted hand performance. Brain Topogr. 2015, 28, 352–363. [Google Scholar] [CrossRef] [PubMed]
- Klem, G.H.; Lüders, H.O.; Jasper, H.H.; Elger, C. The ten-twenty electrode system of the International Federation. The International Federation of Clinical Neurophysiology. Electroencephalogr. Clin. Neurophysiol. Suppl. 1999, 52, 3–6. [Google Scholar] [PubMed]
- Delorme, A.; Makeig, S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfurtscheller, G.; Aranibar, A. Event-related cortical desynchronization detected by power measurements of scalp EEG. Electroencephalogr. Clin. Neurophysiol. 1977, 42, 817–826. [Google Scholar] [CrossRef]
- Aoh, Y.; Hsiao, H.-J.; Lu, M.-K.; Macerollo, A.; Huang, H.-C.; Hamada, M.; Tsai, C.-H.; Chen, J.-C. Event-Related Desynchronization/Synchronization in Spinocerebellar Ataxia Type 3. Front. Neurol. 2019, 10, 822. [Google Scholar] [CrossRef] [Green Version]
- Podsiadlo, D.; Richardson, S. The Timed “Up and Go” Test: A Test of Basic Functional Mobility for Frail Elderly Persons. J. Am. Geriatr. Soc. 1991, 39, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Berg, K.O.; Wood-Dauphinee, S.L.; Williams, J.I.; Maki, B. Measuring balance in the elderly: Validation of an instrument. Can. J. Public Health 1992, 83, S7–S11. [Google Scholar] [PubMed]
- Donoghue, D.; Stokes, E.K. How much change is true change? The minimum detectable change of the Berg Balance Scale in elderly people. J. Rehabil. Med. 2009, 41, 343–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Middleton, A.; Braun, C.H.; Lewek, M.D.; Fritz, S.L. Balance impairment limits ability to increase walking speed in individuals with chronic stroke. Disabil. Rehabil. 2016, 1, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Patterson, K.K.; Parafianowicz, I.; Danells, C.J.; Closson, V.; Verrier, M.C.; Staines, W.R.; Black, S.E.; Mcllroy, W.E. Gait asymmetry in community-ambulating stroke survivors. Achieves Phys. Med. Rehabil. 2008, 89, 304–310. [Google Scholar] [CrossRef]
- Whitney, S.; Wrisley, D.; Furman, J. Concurrent validity of the Berg Balance Scale and the Dynamic Gait Index in people with vestibular dysfunction. Physiother. Res. Int. 2003, 8, 178–186. [Google Scholar] [CrossRef]
- Louie, D.R.; Eng, J.J. Berg Balance Scale score at admission can predict walking suitable for community ambulation at discharge from inpatient stroke rehabilitation. J. Rehabil. Med. 2018, 50, 37–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfurtscheller, G. Functional brain imaging based on ERD/ERS. Vis. Res. 2001, 41, 1257–1260. [Google Scholar] [CrossRef] [Green Version]
- Pavlov, I.; Walker, M.C. Tonic GABAA receptor-mediated signalling in temporal lobe epilepsy. Neuropharmacology 2013, 69, 55–61. [Google Scholar] [CrossRef] [Green Version]
- Mann, E.O.; Mody, I. Control of hippocampal gamma oscillation frequency by tonic inhibition and excitation of interneurons. Nat. Neurosci. 2010, 13, 205–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clarkson, A.N.; Huang, B.S.; Macisaac, S.E.; Mody, I.; Carmichael, S.T. Reducing excessive GABA-mediated tonic inhibition promotes functional recovery after stroke. Nature 2010, 468, 305–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Stroke-Traditional | Stroke-RAGT | p | Statistic | |
|---|---|---|---|---|
| n = 22 | 11 | 11 | ||
| Gender, n (%) | 0.666 | t (20) = 0.439 | ||
| Male | 8 (72.7) | 7 (63.6) | ||
| Female | 3 (27.3) | 4 (36.4) | ||
| Age (years) | 61.27 ± 9.79 | 61.82 ± 7.97 | 0.887 | t (20) = 0.143 |
| Type of injury | 0.400 | t (20) = 0.861 | ||
| Ischemia | 6 (54.5) | 8 (72.7) | ||
| Hemorrhage | 5 (45.5) | 3 (27.3) | ||
| Affected Limb | 0.682 | t (20) = 0.415 | ||
| Left | 7 (63.6) | 6 (54.5) | ||
| Right | 4 (36.4) | 5 (45.5) | ||
| Time post-stroke (month) | 18.09 ± 19.58 | 25.36 ± 17.17 | 0.365 | t (20) = 0.926 |
| BBS Score | ||||
| Pre-rehabilitation | 32.18 ± 15.14 | 26.73 ± 15.38 | 0.011 ** | F (1,20) = 7.97 |
| Post-rehabilitation | 35.64 ± 22.11 | 42.64 ± 11.99 |
| Healthy Control | Stroke Pre | Stroke Post | Control-Pre p-Value | Pre-Post p-Value | |
|---|---|---|---|---|---|
| Gender | 7M5F | 7M5F | N/A | N/A | |
| Age | 61.25 ± 6.75 | 62.83 ± 6.88 | N/A | N/A | |
| ERD Ipsilesion | |||||
| Alpha | 0.64 ± 0.14 | 0.73 ± 0.21 | 0.74 ± 0.20 | 0.314 | 0.630 |
| Low Beta | 0.64 ± 0.14 | 0.74 ± 0.18 | 0.74 ± 0.20 | 0.179 | 0.541 |
| High Beta | 0.67 ± 0.14 | 0.77 ± 0.16 | 0.78 ± 0.18 | 0.165 | 0.804 |
| ERS Ipsilesion | |||||
| Alpha | 1.14 ± 0.182 | 1.18 ± 0.19 | 1.12 ± 0.096 | 0.647 | 0.054 |
| Low Beta | 1.20 ± 0.205 | 1.23 ± 0.18 | 1.12 ± 0.069 | 0.730 | 0.033 ** |
| High Beta | 1.23 ± 0.179 | 1.26 ± 0.17 | 1.11 ± 0.097 | 0.779 | 0.034 ** |
| GAIT analysis | |||||
| Walking speed (cm/s) | 101.29 ± 15.15 | 26.61 ± 15.17 | 35.52 ± 15.18 | 0.000 ** | 0.096 |
| Walking cadence (steps/min) | 108.81 ± 8.42 | 61.13 ± 13.51 | 72.56 ± 18.84 | 0.000 ** | 0.056 |
| Step Length Mean (cm) | 55.36 ± 8.03 | 24.45 ± 10.18 | 27.65 ± 9.30 | 0.000 ** | 0.195 |
| Step Length Sub (cm) | 1.75 ± 1.68 | 11.65 ± 6.76 | 11.59 ± 6.54 | 0.008 ** | 0.977 |
| Stride Length Mean (cm) | 110.93 ± 15.87 | 48.85 ± 20.42 | 55.31 ± 18.92 | 0.000 ** | 0.190 |
| Stride Length Sub (cm) | 1.21 ± 1.40 | 0.471 ± 0.352 | 0.772 ± 0.764 | 0.108 | 0.294 |
| Stride Width Mean (cm) | 11.74 ± 2.19 | 16.44 ± 3.57 | 17.60 ± 3.98 | 0.012 ** | 0.322 |
| Stride Width Sub (cm) | 0.377 ± 0.399 | 0.113 ± 0.133 | 0.136 ± 0.087 | 0.111 | 0.747 |
| Gait Cycle Dur Mean (s) | 1.10 ± 0.084 | 2.05 ± 0.507 | 1.74 ± 0.539 | 0.002 ** | 0.015 ** |
| Gait Cycle Dur Sub (s) | 0.018 ± 0.020 | 0.041 ± 0.053 | 0.029 ± 0.056 | 0.181 | 0.034 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heng, H.-M.; Lu, M.-K.; Chou, L.-W.; Meng, N.-H.; Huang, H.-C.; Hamada, M.; Tsai, C.-H.; Chen, J.-C. Changes in Balance, Gait and Electroencephalography Oscillations after Robot-Assisted Gait Training: An Exploratory Study in People with Chronic Stroke. Brain Sci. 2020, 10, 821. https://doi.org/10.3390/brainsci10110821
Heng H-M, Lu M-K, Chou L-W, Meng N-H, Huang H-C, Hamada M, Tsai C-H, Chen J-C. Changes in Balance, Gait and Electroencephalography Oscillations after Robot-Assisted Gait Training: An Exploratory Study in People with Chronic Stroke. Brain Sciences. 2020; 10(11):821. https://doi.org/10.3390/brainsci10110821
Chicago/Turabian StyleHeng, Hoon-Ming, Ming-Kuei Lu, Li-Wei Chou, Nai-Hsin Meng, Hui-Chun Huang, Masashi Hamada, Chon-Haw Tsai, and Jui-Cheng Chen. 2020. "Changes in Balance, Gait and Electroencephalography Oscillations after Robot-Assisted Gait Training: An Exploratory Study in People with Chronic Stroke" Brain Sciences 10, no. 11: 821. https://doi.org/10.3390/brainsci10110821
APA StyleHeng, H.-M., Lu, M.-K., Chou, L.-W., Meng, N.-H., Huang, H.-C., Hamada, M., Tsai, C.-H., & Chen, J.-C. (2020). Changes in Balance, Gait and Electroencephalography Oscillations after Robot-Assisted Gait Training: An Exploratory Study in People with Chronic Stroke. Brain Sciences, 10(11), 821. https://doi.org/10.3390/brainsci10110821






