Combining Magnetization Transfer Ratio MRI and Quantitative Measures of Walking Improves the Identification of Fallers in MS
Abstract
:1. Introduction
2. Methods
2.1. Walking Measures
2.2. Statistical Analyses
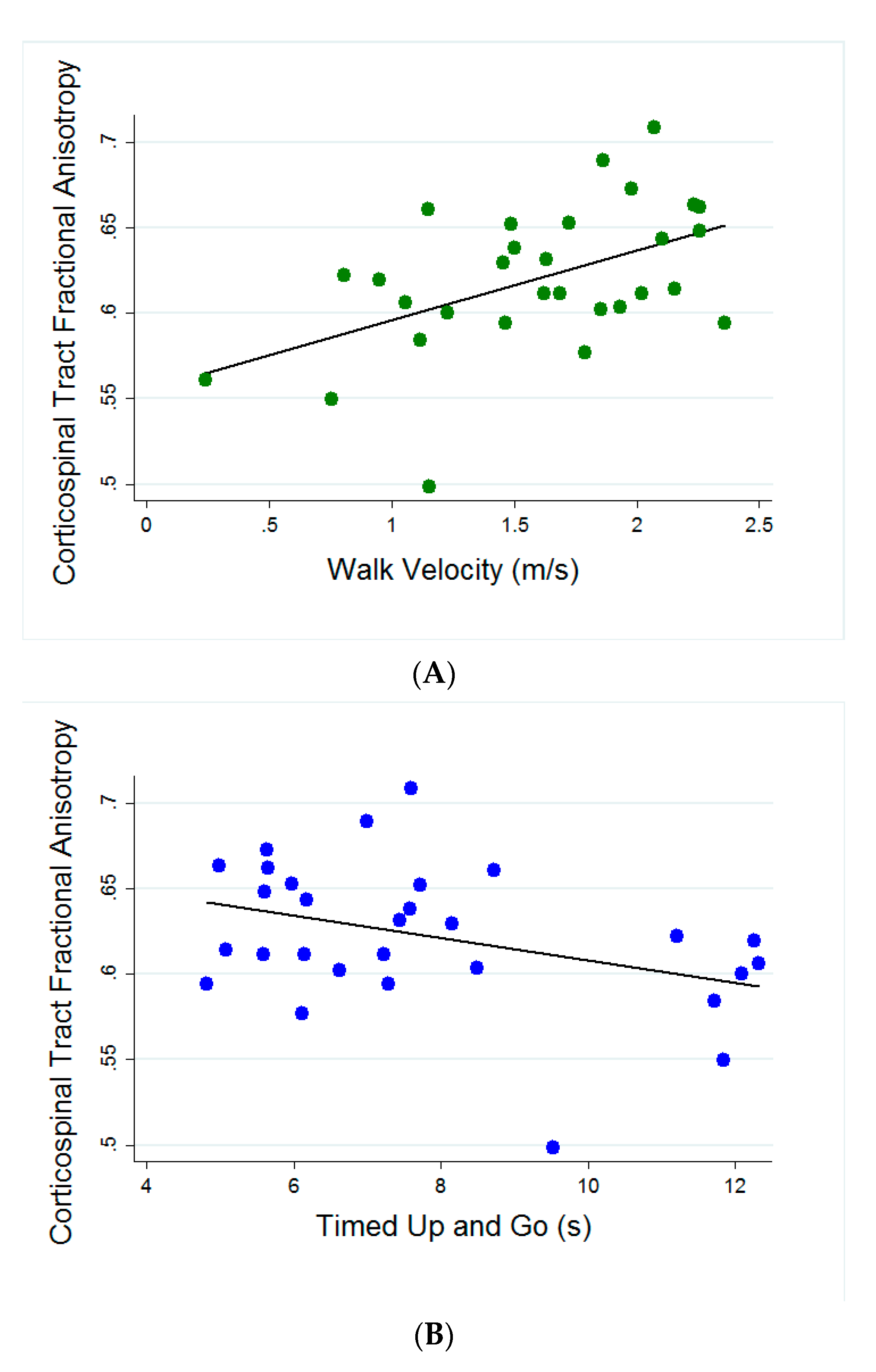
3. Results
Predicting Fall Status
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Huang, W.J.; Chen, W.W.; Zhang, X. Multiple sclerosis: Pathology, diagnosis and treatments. Exp. Ther. Med. 2017, 13, 3163–3166. [Google Scholar] [CrossRef] [Green Version]
- Bonetto, G.; Kamen, Y.; Evans, K.A.; Káradóttir, R.T. Unraveling Myelin Plasticity. Front. Cell. Neurosci. 2020. [Google Scholar] [CrossRef]
- Pérez-Cerdá, F.; Sánchez-Gómez, M.V.; Matute, C. The link of inflammation and neurodegeneration in progressive multiple sclerosis. Mult. Scler. Demyelinating Disord. 2016, 1. [Google Scholar] [CrossRef]
- Motl, R.W.; Hubbard, E.A.; Sreekumar, N.; Wetter, N.C.; Sutton, B.P.; Pilutti, L.A.; Benedict, R.H.B. Pallidal and caudate volumes correlate with walking function in multiple sclerosis. J. Neurol. Sci. 2015, 354, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Carling, A.; Forsberg, A.; Nilsagård, Y. Falls in people with multiple sclerosis: Experiences of 115 fall situations. Clin. Rehabil. 2018, 32, 526–535. [Google Scholar] [CrossRef] [Green Version]
- Cameron, M.H.; Asano, M.; Bourdette, D.; Finlayson, M.L. People with multiple sclerosis use many fall prevention strategies but still fall frequently. Arch. Phys. Med. Rehabil. 2013, 94, 1562–1566. [Google Scholar] [CrossRef]
- Sosnoff, J.J.; Sandroff, B.M.; Pula, J.H.; Morrison, S.M.; Motl, R.W. Falls and Physical Activity in Persons with Multiple Sclerosis. Mult. Scler. Int. 2012, 2012, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Peterson, E.W.; Cho, C.C.; Finlayson, M.L. Fear of falling and associated activity curtailment among middle aged and older adults with multiple sclerosis. Mult. Scler. J. 2007, 13, 1168–1175. [Google Scholar] [CrossRef]
- Coote, S.; Sosnoff, J.J.; Gunn, H. Fall incidence as the primary outcome in multiple sclerosis falls-prevention trials: Recommendation from the international MS falls prevention research network. Int. J. MS Care 2014, 16, 178–184. [Google Scholar] [CrossRef] [Green Version]
- Matsuda, P.N.; Shumway-Cook, A.; Ciol, M.A.; Bombardier, C.H.; Kartin, D.A. Understanding falls in multiple sclerosis: Association of mobility status, concerns about falling, and accumulated impairments. Phys. Ther. 2012, 92, 407–415. [Google Scholar] [CrossRef] [Green Version]
- Williams, R.M.; Turner, A.P.; Hatzakis, M.; Bowen, J.D.; Rodriquez, A.A.; Haselkorn, J.K. Prevalence and correlates of depression among veterans with multiple sclerosis. Neurology 2005, 64, 75–80. [Google Scholar] [CrossRef]
- Peterson, E.W.; Cho, C.C.; von Koch, L.; Finlayson, M.L. Injurious Falls among Middle Aged and Older Adults With Multiple Sclerosis. Arch. Phys. Med. Rehabil. 2008, 89, 1031–1037. [Google Scholar] [CrossRef]
- Matsuda, P.N.; Shumway-Cook, A.; Bamer, A.M.; Johnson, S.L.; Amtmann, D.; Kraft, G.H. Falls in multiple sclerosis. PM&R 2011, 3, 624–632. [Google Scholar] [CrossRef]
- Cameron, M.H.; Poel, A.J.; Haselkorn, J.K.; Linke, A.; Bourdette, D. Falls requiring medical attention among veterans with multiple sclerosis: A cohort study. J. Rehabil. Res. Dev. 2011, 48, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Coote, S.; Comber, L.; Quinn, G.; Santoyo-Medina, C.; Kalron, A.; Gunn, H. Falls in People with Multiple Sclerosis: Risk Identification, Intervention, and Future Directions. Int. J. MS Care 2020. [Google Scholar] [CrossRef]
- Cameron, M.H.; Thielman, E.; Mazumder, R.; Bourdette, D. Predicting falls in people with multiple sclerosis: Fall history is as accurate as more complex measures. Mult. Scler. Int. 2013, 2013, 496325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cattaneo, D.; Regola, A.; Meotti, M. Validity of six balance disorders scales in persons with multiple sclerosis. Disabil. Rehabil. 2006, 28, 789–795. [Google Scholar] [CrossRef]
- Nilsagard, Y.; Lundholm, C.; Gunnarsson, L.-G.; Dcnison, E. Clinical relevance using timed walk tests and “timed up and go” testing in persons with multiple sclerosis. Physiother. Res. Int. J. Res. Clin. Phys. Ther. 2007, 12, 105–114. [Google Scholar] [CrossRef]
- Giannì, C.; Prosperini, L.; Jonsdottir, J.; Cattaneo, D. A systematic review of factors associated with accidental falls in people with multiple sclerosis: A meta-analytic approach. Clin. Rehabil. 2014, 28, 704–716. [Google Scholar] [CrossRef]
- Nilsagård, Y.; Gunn, H.; Freeman, J.; Hoang, P.; Lord, S.; Mazumder, R.; Cameron, M. Falls in people with MS--an individual data meta-analysis from studies from Australia, Sweden, United Kingdom and the United States. Mult. Scler. 2015, 21, 92–100. [Google Scholar] [CrossRef] [Green Version]
- Gunn, H.J.; Newell, P.; Haas, B.; Marsden, J.F.; Freeman, J.A. Identification of risk factors for falls in multiple sclerosis: A systematic review and meta-analysis. Phys. Ther. 2013, 93, 504–513. [Google Scholar] [CrossRef]
- Fritz, N.E.; Eloyan, A.; Baynes, M.; Newsome, S.D.; Calabresi, P.A.; Zackowski, K.M. Distinguishing among multiple sclerosis fallers, near-fallers and non-fallers. Mult. Scler. Relat. Disord. 2018, 19, 99–104. [Google Scholar] [CrossRef]
- Fritz, N.E.; Keller, J.; Calabresi, P.A.; Zackowski, K.M. Quantitative measures of walking and strength provide insight into brain corticospinal tract pathology in multiple sclerosis. Neuroimage Clin. 2017, 14, 490–498. [Google Scholar] [CrossRef]
- Prosperini, L.; Sbardella, E.; Raz, E.; Cercignani, M.; Tona, F.; Bozzali, M.; Petsas, N.; Pozzilli, C.; Pantano, P. Multiple sclerosis: White and gray matter damage associated with balance deficit detected at static posturography. Radiology 2013, 268, 181–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibrahim, I.; Tintera, J.; Skoch, A.; Jirů, F.; Hlustik, P.; Martinkova, P.; Zvara, K.; Rasova, K. Fractional anisotropy and mean diffusivity in the corpus callosum of patients with multiple sclerosis: The effect of physiotherapy. Neuroradiology 2011, 53, 917–926. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, L.; Xie, R.; Peng, Y.; Wang, H.; Chen, Z.; Wu, S.; Ni, C.; Zheng, J.; Li, X.; et al. Value of using the international classification of functioning, disability, and health for stroke rehabilitation assessment. Medicine (Baltimore) 2018, 97, e12802. [Google Scholar]
- Newsome, S.D.; Wang, J.I.; Kang, J.Y.; Calabresi, P.A.; Zackowski, K.M. Quantitative measures detect sensory and motor impairments in multiple sclerosis. J. Neurol. Sci. 2011, 305, 103–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keller, J.L.; Fritz, N.; Chiang, C.C.; Jiang, A.; Thompson, T.; Cornet, N.; Newsome, S.D.; Calabresi, P.A.; Zackowski, K. Adapted Resistance Training Improves Strength in Eight Weeks in Individuals with Multiple Sclerosis. J. Vis. Exp. JoVE 2016, 107, e53449. [Google Scholar] [CrossRef] [Green Version]
- Fritz, N.E.; Newsome, S.D.; Eloyan, A.; Marasigan, R.E.R.; Calabresi, P.A.; Zackowski, K.M. Longitudinal relationships among posturography and gait measures in multiple sclerosis. Neurology 2015, 84, 2048–2056. [Google Scholar]
- Zackowski, K.M.; Wang, J.I.; McGready, J.; Calabresi, P.A.; Newsome, S.D. Quantitative sensory and motor measures detect change overtime and correlate with walking speed in individuals with multiple sclerosis. Mult. Scler. Relat. Disord. 2015, 4, 67–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arezzo, J.C.; Schaumburg, H.H.; Laudadio, C. The Vibraton: A simple device for quantitative evaluation of tactile/vibratory sense. Neurology 1985, 35 (Suppl. 1), 169. [Google Scholar]
- Rudick, R.; Antel, J.; Confavreux, C.; Cutter, G.; Ellison, G.; Fischer, J.; Lublin, F.; Miller, A.; Petkau, J.; Rao, S.; et al. Recommendations from the National Multiple Sclerosis Society Clinical Outcomes Assessment Task Force. Ann. Neurol. 1997, 42, 379–382. [Google Scholar] [CrossRef]
- Rosti-Otajärvi, E.; Hämäläinen, P.; Koivisto, K.; Hokkanen, L. The reliability of the MSFC and its components. Acta Neurol. Scand. 2008, 117, 421–427. [Google Scholar] [CrossRef]
- Kieseier, B.; Pozzilli, C. Assessing walking disability in multiple sclerosis. Mult. Scler. J. 2012, 18, 914–924. [Google Scholar]
- Polman, C.H.; Rudick, R.A. The multiple sclerosis functional composite: A clinically meaningful measure of disability. Neurology 2010, 74 (Suppl. 3), S8–S15. [Google Scholar] [CrossRef]
- MS-EDGE Outcome Measures for In- and Out-Patient Rehabilitation. Academy of Neuologic Physical Therapy Website. 2012. Available online: https://www.neuropt.org/docs/ms-edge-documents/ms-edge_rehab_recs5417E23D4B53.pdf (accessed on 26 October 2020).
- Podsiadlo, D.; Richardson, S. The Timed “Up & Go”: A Test of Basic Functional Mobility for Frail Elderly Persons. J. Am. Geriatr. Soc. 1991, 39, 142–148. [Google Scholar] [CrossRef]
- Valet, M.; Lejeune, T.; Devis, M.; van Pesch, V.; El Sankari, S.; Stoquart, G. Timed Up-and-Go and 2-Minute Walk Test in patients with multiple sclerosis with mild disability: Reliability, responsiveness and link with perceived fatigue. Eur. J. Phys. Rehabil. Med. 2019, 55, 450–455. [Google Scholar] [CrossRef]
- Gijbels, D.; Eijnde, B.O.; Feys, P. Comparison of the 2- and 6-minute walk test in multiple sclerosis. Mult. Scler. 2011, 17, 1269–1272. [Google Scholar] [CrossRef]
- Goldman, M.D.; Marrie, R.A.; Cohen, J.A. Evaluation of the six-minute walk in multiple sclerosis subjects and healthy controls. Mult. Scler. 2008, 14, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.L.; Potter, K.; Blankshain, K.; Kaplan, S.L.; O’Dwyer, L.C.; Sullivan, J.E. A core set of outcome measures for adults with neurologic conditions undergoing rehabilitation: A clinical practice guideline. J. Neurol. Phys. Ther. 2018, 42, 174–220. [Google Scholar]
- Reich, D.S.; Smith, S.A.; Jones, C.K.; Zackowski, K.M.; van Zijl, P.C.; Calabresi, P.A.; Mori, S. Quantitative Characterization of the Corticospinal Tract at 3T. Am. J. Neuroradiol. 2006, 27, 2168–2178. [Google Scholar]
- Reich, D.S.; Smith, S.A.; Zackowski, K.M.; Gordon-Lipkin, E.M.; Jones, C.K.; Farrell, J.A.D.; Mori, S.; van Zijl, P.C.M.; Calabresi, P.A. Multiparametric magnetic resonance imaging analysis of the corticospinal tract in multiple sclerosis. NeuroImage 2007, 38, 271–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reich, D.S.; Ozturk, A.; Calabresi, P.A.; Mori, S. Automated vs. conventional tractography in multiple sclerosis: Variability and correlation with disability. NeuroImage 2010, 49, 3047–3056. [Google Scholar] [CrossRef] [Green Version]
- Mori, S.; Crain, B.J.; Chacko, V.P.; van Zijl, P.C. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann. Neurol. 1999, 45, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; van Zijl, P.C.M.; Kim, J.; Pearlson, G.D.; Mori, S. DtiStudio: Resource program for diffusion tensor computation and fiber bundle tracking. Comput. Methods Programs Biomed. 2006, 81, 106–116. [Google Scholar] [CrossRef]
- Akaike, H. A New Look at the Statistical Model Identification. IEEE Trans. Autom. Control 1974, 19, 716–723. [Google Scholar] [CrossRef]
- Kister, I.; Bacon, T.E.; Chamot, E.; Salter, A.R.; Cutter, G.R.; Kalina, J.T.; Herbert, J. Natural history of multiple sclerosis symptoms. Int. J. MS Care 2013, 15, 146–158. [Google Scholar] [CrossRef] [Green Version]
- Coote, S.; Garrett, M.; Hogan, N.; Larkin, A.; Saunders, J. Getting the balance right: A randomised controlled trial of physiotherapy and Exercise Interventions for ambulatory people with multiple sclerosis. BMC Neurol. 2009, 9, 34. [Google Scholar] [CrossRef] [Green Version]
- Citaker, S.; Gunduz, A.G.; Guclu, M.B.; Nazliel, B.; Irkec, C.; Kaya, D. Relationship between foot sensation and standing balance in patients with multiple sclerosis. Gait Posture 2011, 34, 275–278. [Google Scholar] [CrossRef]
- Uszynski, M.; Purtill, H.; Coote, S. Relationship between foot vibration threshold and walking and balance functions in people with Multiple Sclerosis (PwMS). Gait Posture 2015, 41, 228–232. [Google Scholar] [CrossRef]
- Schwid, S.R.; Goodman, A.D.; Apatoff, B.R.; Coyle, P.K.; Jacobs, L.D.; Krupp, L.B.; Miller, A.E.; Wende, K.E.; Brownscheidle, C.M.; Sclero, N.Y.S.M. Are quantitative functional measures more sensitive to worsening MS than traditional measures? Neurology 2000, 55, 1901–1903. [Google Scholar] [CrossRef]
- Fritz, N.E.; Eloyan, A.; Glaister, J.; Dewey, B.E.; Al-Louzi, O.; Costello, M.G.; Chen, M.; Prince, J.L.; Calabresi, P.A.; Zackowski, K.M. Quantitative vibratory sensation measurement is related to sensory cortical thickness in MS. Ann. Clin. Transl. Neurol. 2019, 6, 586–595. [Google Scholar] [CrossRef]
- Nilsagård, Y.; Lundholm, C.; Denison, E.; Gunnarsson, L.-G. Predicting accidental falls in people with multiple sclerosis—A longitudinal study. Clin. Rehabil. 2009, 23, 259–269. [Google Scholar] [CrossRef]
- Dibble, L.E.; Lopez-Lennon, C.; Lake, W.; Hoffmeister, C.; Gappmaier, E. Utility of disease-specific measures and clinical balance tests in prediction of falls in persons with multiple sclerosis. J. Neurol. Phys. Ther. JNPT 2013, 37, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Benedict, R.H.B.; Zivadinov, R. Risk factors for and management of cognitive dysfunction in multiple sclerosis. Nat. Rev. Neurol. 2011, 7, 332–342. [Google Scholar] [CrossRef]
- Zackowski, K.M.; Smith, S.A.; Reich, D.S.; Gordon-Lipkin, E.; Chodkowski, B.A.; Sambandan, D.R.; Shteyman, M.; Bastian, A.J.; van Zijl, P.C.; Calabresi, P.A. Sensorimotor dysfunction in multiple sclerosis and column-specific magnetization transfer-imaging abnormalities in the spinal cord. Brain 2009, 132 Pt. 5, 1200–1209. [Google Scholar] [CrossRef] [Green Version]
- Hubbard, E.A.; Wetter, N.C.; Sutton, B.P.; Pilutti, L.A.; Motl, R.W. Diffusion tensor imaging of the corticospinal tract and walking performance in multiple sclerosis. J. Neurol. Sci. 2016, 363, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Baird, J.F.; Hubbard, E.A.; Sutton, B.P.; Motl, R.W. The relationship between corticospinal tract integrity and lower-extremity strength is attenuated when controlling for age and sex in multiple sclerosis. Brain Res. 2018, 1701, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Klineova, S.; Farber, R.; Saiote, C.; Farrell, C.; Delman, B.N.; Tanenbaum, L.N.; Friedman, J.; Inglese, M.; Lublin, F.D.; Krieger, S. Relationship between timed 25-foot walk and diffusion tensor imaging in multiple sclerosis. Mult. Scler. J. Exp. Transl. Clin. 2016, 2, 2055217316655365. [Google Scholar] [CrossRef]
- Beaulieu, C. The basis of anisotropic water diffusion in the nervous system—A technical review. NMR Biomed. 2002, 15, 435–455. [Google Scholar] [CrossRef]
- Harsan, L.A.; Poulet, P.; Guignard, B.; Steibel, J.; Parizel, N.; de Sousa, P.L.; Boehm, N.; Grucker, D.; Ghandour, M.S. Brain dysmyelination and recovery assessment by noninvasive in vivo diffusion tensor magnetic resonance imaging. J. Neurosci. Res. 2006, 83, 392–402. [Google Scholar] [CrossRef]
- McCreary, C.R.; Bjarnason, T.A.; Skihar, V.; Mitchell, J.R.; Yong, V.W.; Dunn, J.F. Multiexponential T2 and magnetization transfer MRI of demyelination and remyelination in murine spinal cord. NeuroImage 2009, 45, 1173–1182. [Google Scholar] [CrossRef] [Green Version]


| All MS (n = 29) | Fallers (n = 14) | Non-Fallers (n = 15) | p-value | |
|---|---|---|---|---|
| Age (years) | 48.7 (11.5) | 49.1 (12.1) | 48.3 (11.2) | 0.841 |
| Sex | 17 F; 12 M | 8 F; 6 M | 9 F; 6 M | 0.878 |
| Symptom Duration (years) | 11.9 (8.68) | 12.3 (9.47) | 11.6 (8.20) | 0.839 |
| Expanded Disability Status Scale (EDSS) | 4.0 [1–6.5] | 4.0 [1–6.5] | 3.5 [1,2,3,4,5,6] | 0.123 |
| Walk Velocity (m/s) | 1.64 (0.47) | 1.49 (0.44) | 1.79 (0.46) | 0.0842 |
| Timed Up and Go (s) | 7.74 (2.33) | 8.35 (2.60) | 7.18 (1.97) | 0.180 |
| Timed 25 Foot Walk (s) | 5.42 (1.99) | 6.02 (2.36) | 4.86 (1.45) | 0.1161 |
| Two Minute Walk Test (m) | 161.2 (46.4) | 147.2 (44.6) | 173.19 (46.0) | 0.157 |
| Summed Strength (lbs) | 240.1 (84.1) | 236.2 (87.1) | 243.6 (84.2) | 0.817 |
| Vibration Sensation (vu) | 5.85 (3.11) | 6.06 (3.43) | 5.66 (2.89) | 0.737 |
| Corticospinal Tract Fractional Anisotropy (CST FA) | 0.62 (0.04) | 0.62 (0.05) | 0.64 (0.03) | 0.541 |
| Corticospinal Tract Magnetization Transfer Ratio (CST MTR) | 0.46 (0.02) | 0.46 (0.02) | 0.46 (0.02) | 0.275 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fritz, N.E.; Edwards, E.M.; Keller, J.; Eloyan, A.; Calabresi, P.A.; Zackowski, K.M. Combining Magnetization Transfer Ratio MRI and Quantitative Measures of Walking Improves the Identification of Fallers in MS. Brain Sci. 2020, 10, 822. https://doi.org/10.3390/brainsci10110822
Fritz NE, Edwards EM, Keller J, Eloyan A, Calabresi PA, Zackowski KM. Combining Magnetization Transfer Ratio MRI and Quantitative Measures of Walking Improves the Identification of Fallers in MS. Brain Sciences. 2020; 10(11):822. https://doi.org/10.3390/brainsci10110822
Chicago/Turabian StyleFritz, Nora E., Erin M. Edwards, Jennifer Keller, Ani Eloyan, Peter A. Calabresi, and Kathleen M. Zackowski. 2020. "Combining Magnetization Transfer Ratio MRI and Quantitative Measures of Walking Improves the Identification of Fallers in MS" Brain Sciences 10, no. 11: 822. https://doi.org/10.3390/brainsci10110822





