Individual Cerebral Blood Flow Responses to Transcranial Direct Current Stimulation at Various Intensities
Abstract
:1. Introduction
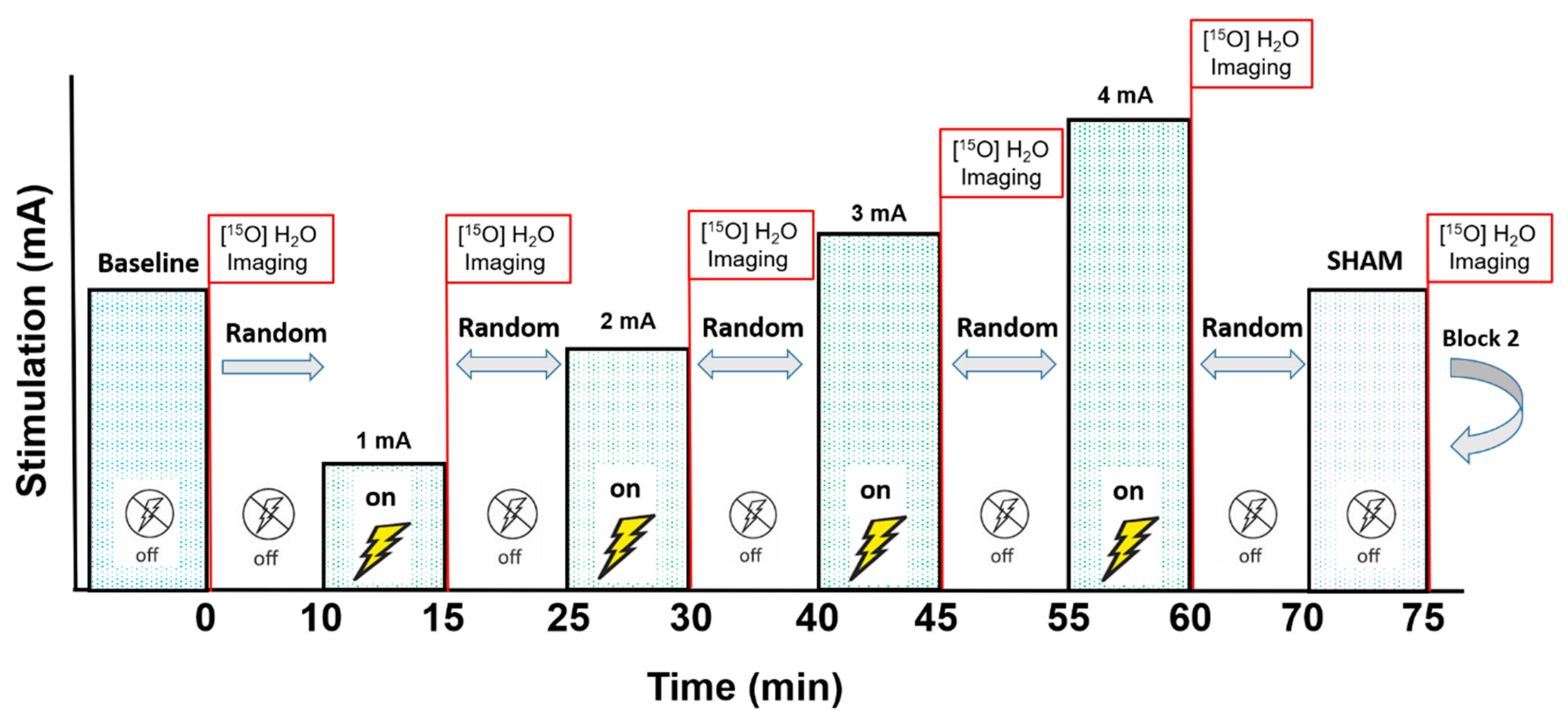
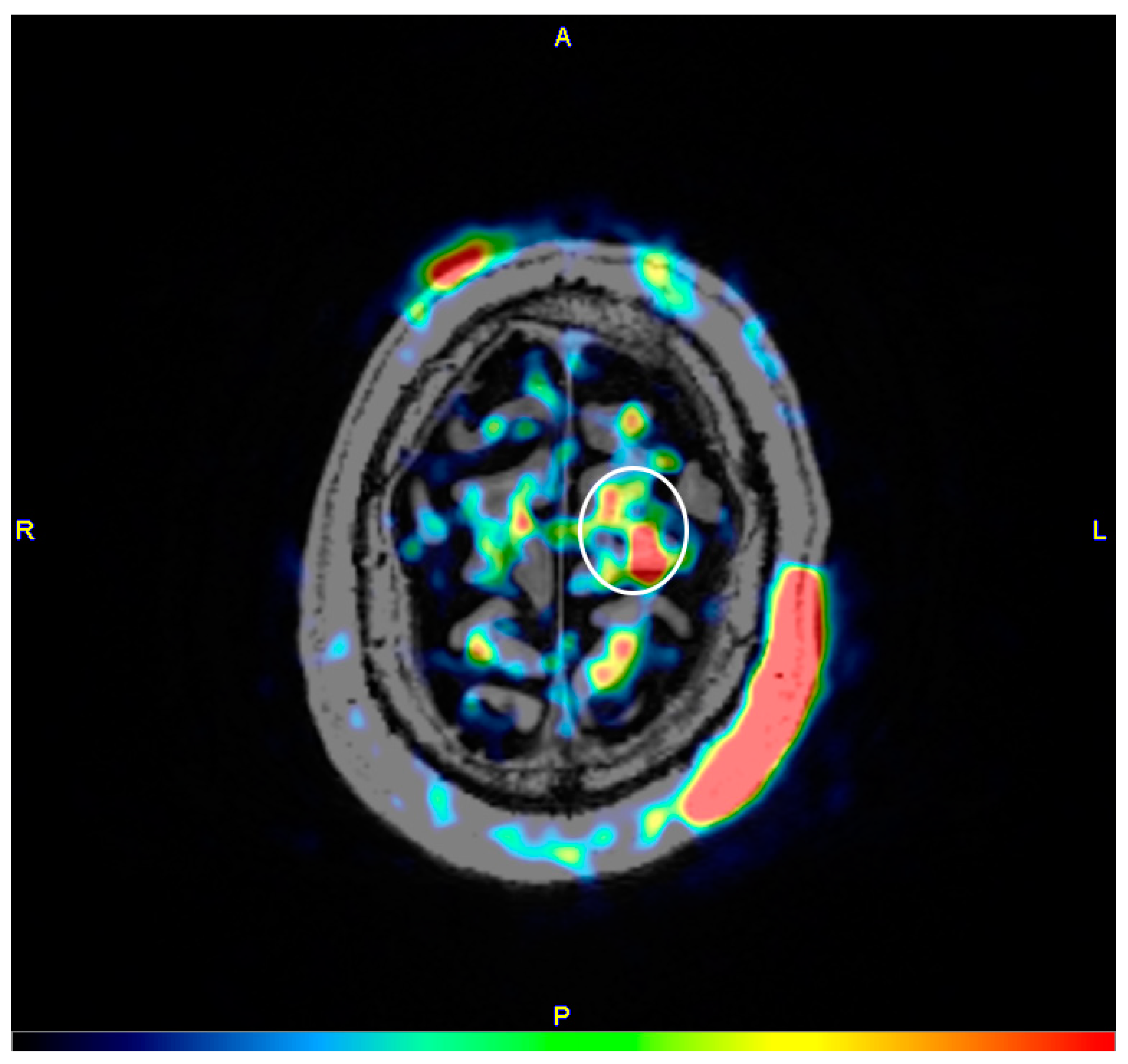
2. Materials and Methods
3. Results
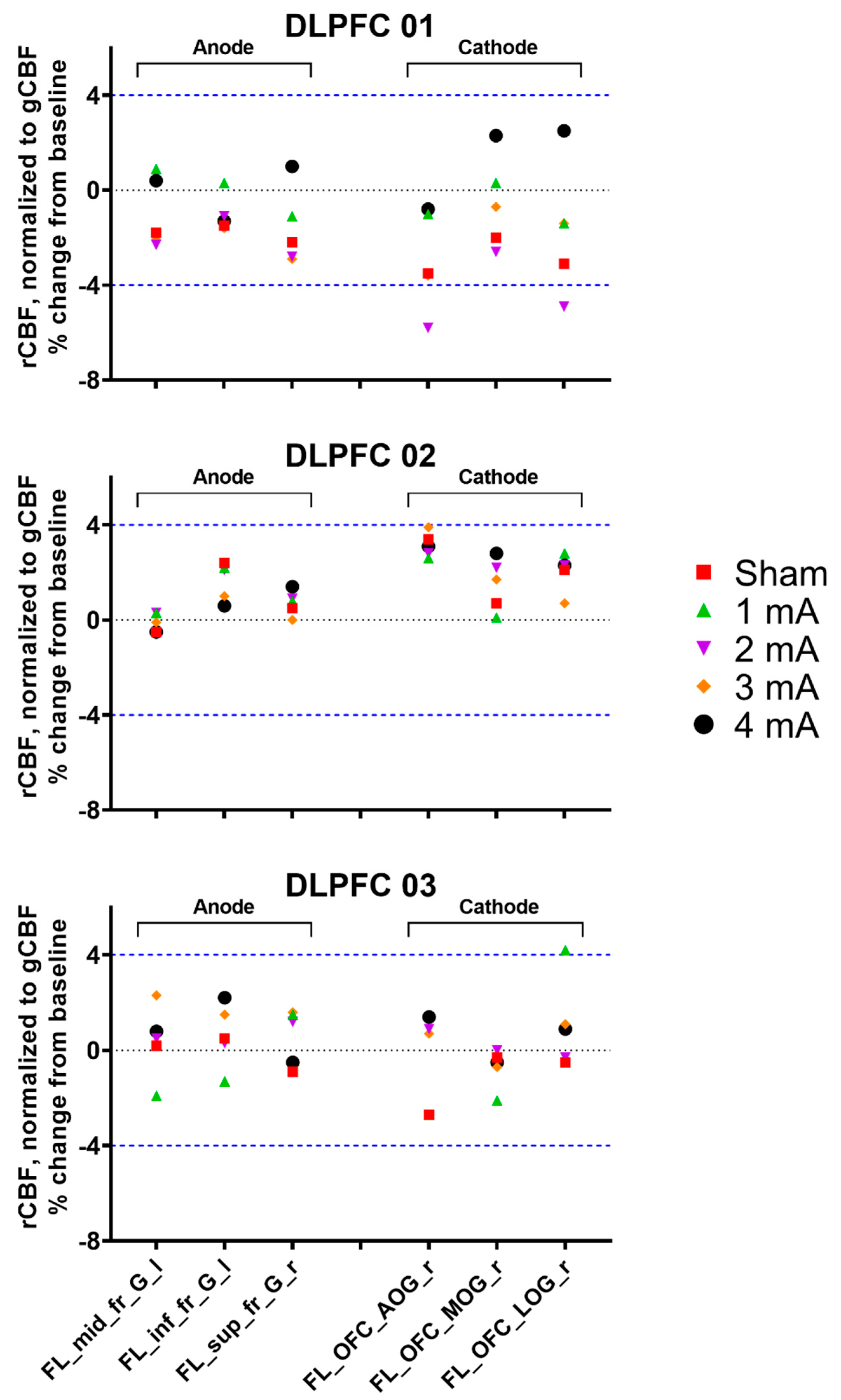
3.1. Experiment 1, Dorsolateral Prefrontal Cortex
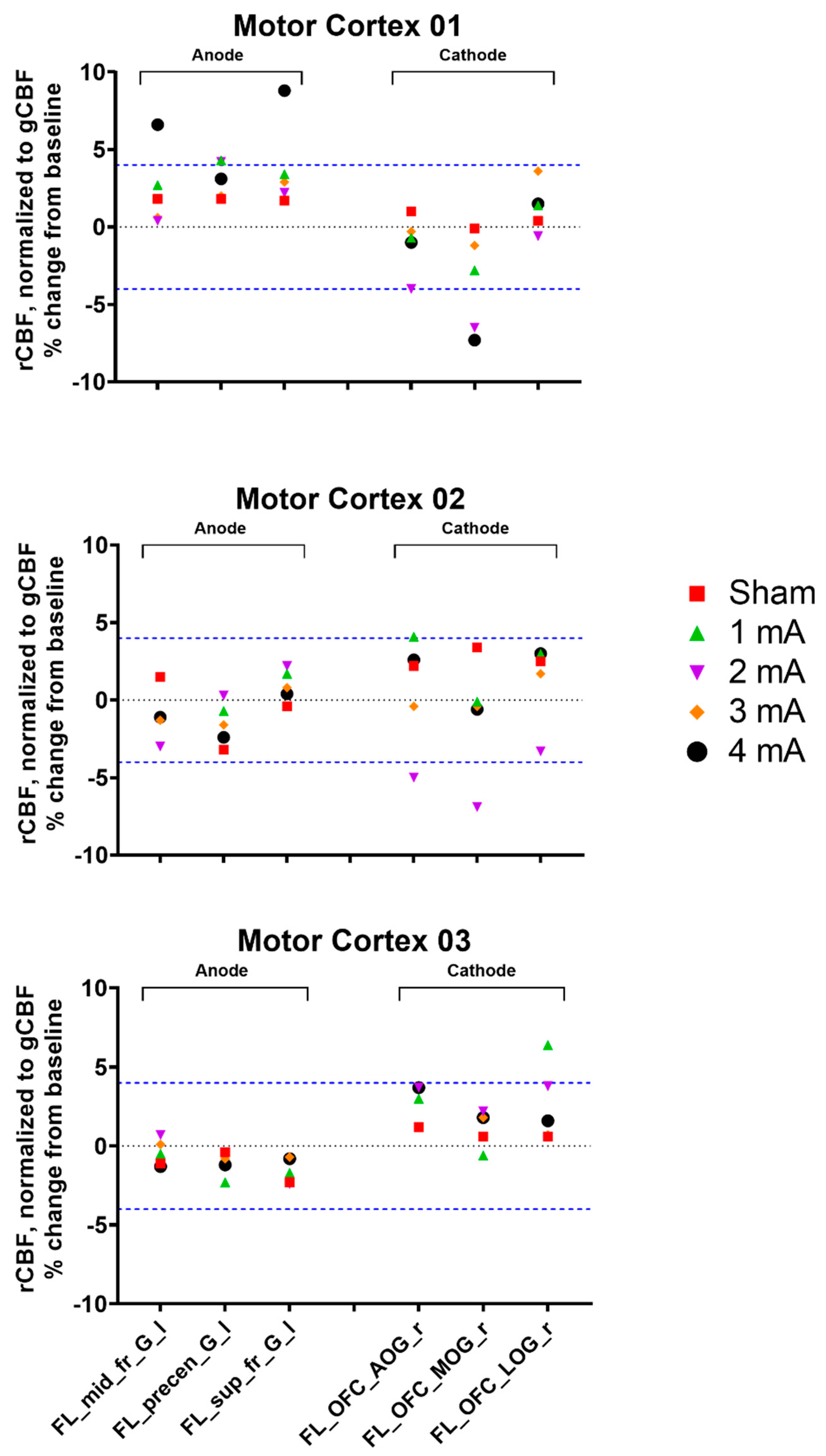
3.2. Experiment 2, Primary Motor Cortex
3.3. Tolerability and Blinding Maintenance
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bishop, M.; Rumrill, P.D. Multiple sclerosis: Etiology, symptoms, incidence and prevalence, and implications for community living and employment. Work 2015, 52, 725–734. [Google Scholar] [CrossRef]
- Confavreux, C.; Vukusic, S.; Moreau, T.; Adeleine, P. Relapses and progression of disability in multiple sclerosis. N. Engl. J. Med. 2000, 343, 1430–1438. [Google Scholar] [CrossRef]
- Solaro, C.; Tanganelli, P.; Messmer Uccelli, M. Pharmacological treatment of pain in multiple sclerosis. Expert Rev. Neurother. 2007, 7, 1165–1174. [Google Scholar] [CrossRef]
- Delgado-Mendilívar, J.M.; Cadenas-Díaz, J.C.; Fernández-Torrico, J.M.; Navarro-Mascarell, G.; Izquierdo, G. A study of the quality of life in cases of multiple sclerosis. Rev. Neurol. 2005, 41, 257–262. [Google Scholar]
- Nitsche, M.A.; Paulus, W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J. Physiol. 2000, 527, 633–639. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Paulus, W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology 2001, 57, 1899–1901. [Google Scholar] [CrossRef]
- Gough, N.; Brkan, L.; Subramaniam, P.; Chiuccariello, L.; De Petrillo, A.; Mulsant, B.H.; Bowie, C.R.; Rajji, T.K. Feasibility of remotely supervised transcranial direct current stimulation and cognitive remediation: A systematic review. PLoS ONE 2020, 15, e0223029. [Google Scholar] [CrossRef]
- Benussi, A.; Pascual-Leone, A.; Borroni, B. Non-invasive cerebellar stimulation in neurodegenerative ataxia: A literature review. Int. J. Mol. Sci. 2020, 21, 1948. [Google Scholar] [CrossRef] [Green Version]
- Beretta, V.S.; Conceição, N.R.; Nóbrega-Sousa, P.; Orcioli-Silva, D.; Dantas, L.; Gobbi, L.T.B.; Vitório, R. Transcranial direct current stimulation combined with physical or cognitive training in people with Parkinson’s disease: A systematic review. J. Neuroeng. Rehabil. 2020, 17, 74. [Google Scholar] [CrossRef]
- de Araújo, A.V.L.; Ribeiro, F.P.G.; Massetti, T.; Potter-Baker, K.A.; Cortes, M.; Plow, E.B.; da Silva, T.D.; Tonks, J.; Anghinah, R.; Magalhães, F.H.; et al. Effectiveness of anodal transcranial direct current stimulation to improve muscle strength and motor functionality after incomplete spinal cord injury: A systematic review and meta-analysis. Spinal Cord 2020. [Google Scholar] [CrossRef]
- Horvath, J.C.; Forte, J.D.; Carter, O. Evidence that transcranial direct current stimulation (tDCS) generates little-to-no reliable neurophysiologic effect beyond MEP amplitude modulation in healthy human subjects: A systematic review. Neuropsychologia 2015, 66, 213–236. [Google Scholar] [CrossRef]
- Medina, J.; Cason, S. No evidential value in samples of transcranial direct current stimulation (tDCS) studies of cognition and working memory in healthy populations. Cortex 2017, 94, 131–141. [Google Scholar] [CrossRef]
- Santos, A.; Santos, A.P.S.; Santos, H.S.; Silva, A.C.D. The use of tDCS as a therapeutic option for tinnitus: A systematic review. Braz. J. Otorhinolaryngol. 2018, 84, 653–659. [Google Scholar] [CrossRef]
- Antal, A.; Keeser, D.; Priori, A.; Padberg, F.; Nitsche, M.A. Conceptual and procedural shortcomings of the systematic review “evidence that transcranial direct current stimulation (tDCS) generates little-to-no reliable neurophysiologic effect beyond MEP amplitude modulation in healthy human subjects: A systematic review” by Horvath and co-workers. Brain Stimul. 2015, 8, 846–849. [Google Scholar] [CrossRef]
- López-Alonso, V.; Cheeran, B.; Río-Rodríguez, D.; Fernández-Del-Olmo, M. Inter-individual variability in response to non-invasive brain stimulation paradigms. Brain Stimul. 2014, 7, 372–380. [Google Scholar] [CrossRef]
- Wiethoff, S.; Hamada, M.; Rothwell, J.C. Variability in response to transcranial direct current stimulation of the motor cortex. Brain Stimul. 2014, 7, 468–475. [Google Scholar] [CrossRef]
- Vöröslakos, M.; Takeuchi, Y.; Brinyiczki, K.; Zombori, T.; Oliva, A.; Fernández-Ruiz, A.; Kozák, G.; Kincses, Z.T.; Iványi, B.; Buzsáki, G.; et al. Direct effects of transcranial electric stimulation on brain circuits in rats and humans. Nat. Commun. 2018, 9, 483. [Google Scholar] [CrossRef] [Green Version]
- Asamoah, B.; Khatoun, A.; Mc Laughlin, M. tACS motor system effects can be caused by transcutaneous stimulation of peripheral nerves. Nat. Commun. 2019, 10, 266. [Google Scholar] [CrossRef] [Green Version]
- Rudroff, T.; Workman, C.D.; Fietsam, A.C.; Ponto, L.L.B. Imaging transcranial direct current stimulation (tDCS) with positron emission tomography (PET). Brain Sci. 2020, 10, 236. [Google Scholar] [CrossRef] [Green Version]
- Iadecola, C. Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat. Rev. Neurosci. 2004, 5, 347–360. [Google Scholar] [CrossRef]
- Sun, X.; Tanaka, M.; Kondo, S.; Okamoto, K.; Hirai, S. Clinical significance of reduced cerebral metabolism in multiple sclerosis: A combined PET and MRI study. Ann. Nucl. Med. 1998, 12, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Workman, C.D.; Ponto, L.L.B.; Kamholz, J.; Rudroff, T. No immediate effects of transcranial direct current stimulation at various intensities on cerebral blood flow in people with multiple sclerosis. Brain Sci. 2020, 10, 82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giovannella, M.; Andresen, B.; Andersen, J.B.; El-Mahdaoui, S.; Contini, D.; Spinelli, L.; Torricelli, A.; Greisen, G.; Durduran, T.; Weigel, U.M.; et al. Validation of diffuse correlation spectroscopy against (15)o-water PET for regional cerebral blood flow measurement in neonatal piglets. J. Cereb. Blood Flow Metab. 2019, 40, 2055–2065. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Gong, E.; Fan, A.P.; Goubran, M.; Khalighi, M.M.; Zaharchuk, G. Predicting (15)o-water PET cerebral blood flow maps from multi-contrast MRI using a deep convolutional neural network with evaluation of training cohort bias. J. Cereb. Blood Flow Metab. 2019. [Google Scholar] [CrossRef]
- Herscovitch, P.; Markham, J.; Raichle, M.E. Brain blood flow measured with intravenous H2(15)O. I. Theory and error analysis. J. Nucl. Med. 1983, 24, 782–789. [Google Scholar]
- Puig, O.; Henriksen, O.M.; Vestergaard, M.B.; Hansen, A.E.; Andersen, F.L.; Ladefoged, C.N.; Rostrup, E.; Larsson, H.B.; Lindberg, U.; Law, I. Comparison of simultaneous arterial spin labeling MRI and (15)o-h2o PET measurements of regional cerebral blood flow in rest and altered perfusion states. J. Cereb. Blood Flow Metab. 2019, 40, 1621–1633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raichle, M.E.; Martin, W.R.; Herscovitch, P.; Mintun, M.A.; Markham, J. Brain blood flow measured with intravenous H2(15)O. II. Implementation and validation. J. Nucl. Med. 1983, 24, 790–798. [Google Scholar]
- Stark, C.E.; Squire, L.R. When zero is not zero: The problem of ambiguous baseline conditions in fMRI. Proc. Natl. Acad. Sci. USA 2001, 98, 12760–12766. [Google Scholar] [CrossRef] [Green Version]
- Paquette, C.; Sidel, M.; Radinska, B.A.; Soucy, J.P.; Thiel, A. Bilateral transcranial direct current stimulation modulates activation-induced regional blood flow changes during voluntary movement. J. Cereb. Blood Flow Metab. 2011, 31, 2086–2095. [Google Scholar] [CrossRef] [Green Version]
- Foerster, Á.; Yavari, F.; Farnad, L.; Jamil, A.; Paulus, W.; Nitsche, M.A.; Kuo, M.F. Effects of electrode angle-orientation on the impact of transcranial direct current stimulation on motor cortex excitability. Brain Stimul. 2019, 12, 263–266. [Google Scholar] [CrossRef]
- Workman, C.D.; Kamholz, J.; Rudroff, T. The tolerability and efficacy of 4 mA transcranial direct current stimulation on leg muscle fatigability. Brain Sci. 2020, 10, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petersen, T.H.; Puthusserypady, S. Assessing tDCS placebo effects on EEG and cognitive tasks. In Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 23–27 July 2019; pp. 4509–4512. [Google Scholar]
- Jamil, A.; Batsikadze, G.; Kuo, H.I.; Meesen, R.L.J.; Dechent, P.; Paulus, W.; Nitsche, M.A. Current intensity- and polarity-specific online and aftereffects of transcranial direct current stimulation: An fMRI study. Hum. Brain Mapp. 2019, 41, 1644–1666. [Google Scholar] [CrossRef] [PubMed]
- Stagg, C.J.; Best, J.G.; Stephenson, M.C.; O’Shea, J.; Wylezinska, M.; Kincses, Z.T.; Morris, P.G.; Matthews, P.M.; Johansen-Berg, H. Polarity-sensitive modulation of cortical neurotransmitters by transcranial stimulation. J. Neurosci. 2009, 29, 5202–5206. [Google Scholar] [CrossRef] [PubMed]
- Pellerin, L.; Magistretti, P.J. Glutamate uptake into astrocytes stimulates aerobic glycolysis: A mechanism coupling neuronal activity to glucose utilization. Proc. Natl. Acad. Sci. USA 1994, 91, 10625–10629. [Google Scholar] [CrossRef] [Green Version]
- Girouard, H.; Iadecola, C. Neurovascular coupling in the normal brain and in hypertension, stroke, and Alzheimer disease. J. Appl. Physiol. 2006, 100, 328–335. [Google Scholar] [CrossRef]
- Lang, N.; Siebner, H.R.; Ward, N.S.; Lee, L.; Nitsche, M.A.; Paulus, W.; Rothwell, J.C.; Lemon, R.N.; Frackowiak, R.S. How does transcranial DC stimulation of the primary motor cortex alter regional neuronal activity in the human brain? Eur. J. Neurosci. 2005, 22, 495–504. [Google Scholar] [CrossRef]
- Fox, P.T.; Narayana, S.; Tandon, N.; Fox, S.P.; Sandoval, H.; Kochunov, P.; Capaday, C.; Lancaster, J.L. Intensity modulation of TMS-induced cortical excitation: Primary motor cortex. Hum. Brain Mapp. 2006, 27, 478–487. [Google Scholar] [CrossRef]
- Speer, A.M.; Willis, M.W.; Herscovitch, P.; Daube-Witherspoon, M.; Shelton, J.R.; Benson, B.E.; Post, R.M.; Wassermann, E.M. Intensity-dependent regional cerebral blood flow during 1-hz repetitive transcranial magnetic stimulation (rTMS) in healthy volunteers studied with h215o positron emission tomography: I. Effects of primary motor cortex rTMS. Biol. Psychiatry 2003, 54, 818–825. [Google Scholar] [CrossRef]
- Lee, J.; Lee, A.; Kim, H.; Shin, M.; Yun, S.M.; Jung, Y.; Chang, W.H.; Kim, Y.H. Different brain connectivity between responders and nonresponders to dual-mode noninvasive brain stimulation over bilateral primary motor cortices in stroke patients. Neural. Plast 2019, 2019, 3826495. [Google Scholar] [CrossRef]
- Rawji, V.; Ciocca, M.; Zacharia, A.; Soares, D.; Truong, D.; Bikson, M.; Rothwell, J.; Bestmann, S. tDCS changes in motor excitability are specific to orientation of current flow. Brain Stimul. 2018, 11, 289–298. [Google Scholar] [CrossRef]
- Liu, A.; Voroslakos, M.; Kronberg, G.; Henin, S.; Krause, M.R.; Huang, Y.; Opitz, A.; Mehta, A.; Pack, C.C.; Krekelberg, B.; et al. Immediate neurophysiological effects of transcranial electrical stimulation. Nat. Commun. 2018, 9, 5092. [Google Scholar] [CrossRef]
- Workman, C.D.; Fietsam, A.C.; Uc, E.Y.; Rudroff, T. Cerebellar transcranial direct current stimulation in people with Parkinson’s disease: A pilot study. Brain Sci. 2020, 10, 96. [Google Scholar] [CrossRef] [Green Version]
- Kuo, M.F.; Paulus, W.; Nitsche, M.A. Sex differences in cortical neuroplasticity in humans. Neuroreport 2006, 17, 1703–1707. [Google Scholar] [CrossRef]
- Rudroff, T.; Workman, C.D.; Fietsam, A.C.; Kamholz, J. Response variability in transcranial direct current stimulation: Why sex matters. Front. Psychiatry 2020, 11, 585. [Google Scholar] [CrossRef]
- Fertonani, A.; Ferrari, C.; Miniussi, C. What do you feel if I apply transcranial electric stimulation? Safety, sensations and secondary induced effects. Clin. Neurophysiol. 2015, 126, 2181–2188. [Google Scholar] [CrossRef]
- Turi, Z.; Csifcsák, G.; Boayue, N.M.; Aslaksen, P.; Antal, A.; Paulus, W.; Groot, J.; Hawkins, G.E.; Forstmann, B.; Opitz, A.; et al. Blinding is compromised for transcranial direct current stimulation at 1 mA for 20 min in young healthy adults. Eur. J. Neurosci. 2019, 50, 3261–3268. [Google Scholar] [CrossRef] [Green Version]
- O’Connell, N.E.; Cossar, J.; Marston, L.; Wand, B.M.; Bunce, D.; Moseley, G.L.; De Souza, L.H. Rethinking clinical trials of transcranial direct current stimulation: Participant and assessor blinding is inadequate at intensities of 2 mA. PLoS ONE 2012, 7, e47514. [Google Scholar] [CrossRef] [Green Version]
- Aslaksen, P.M.; Vasylenko, O.; Fagerlund, A.J. The effect of transcranial direct current stimulation on experimentally induced heat pain. Exp. Brain Res. 2014, 232, 1865–1873. [Google Scholar] [CrossRef]
- Bin Dawood, A.; Dickinson, A.; Aytemur, A.; Howarth, C.; Milne, E.; Jones, M. Investigating the effects of tDCS on visual orientation discrimination task performance: ‘The possible influence of placebo’. J. Cogn. Enhanc. 2020, 4, 235–249. [Google Scholar] [CrossRef] [Green Version]
- Andreasen, N.C.; Arndt, S.; Cizadlo, T.; O’Leary, D.S.; Watkins, G.L.; Ponto, L.L.; Hichwa, R.D. Sample size and statistical power in [15o]h2o studies of human cognition. J. Cereb. Blood Flow Metab. 1996, 16, 804–816. [Google Scholar] [CrossRef] [Green Version]
- Santarnecchi, E.; Feurra, M.; Barneschi, F.; Acampa, M.; Bianco, G.; Cioncoloni, D.; Rossi, A.; Rossi, S. Time course of corticospinal excitability and autonomic function interplay during and following monopolar tDCS. Front. Psychiatry 2014, 5, 86. [Google Scholar] [CrossRef] [Green Version]
- Fonteneau, C.; Mondino, M.; Arns, M.; Baeken, C.; Bikson, M.; Brunoni, A.R.; Burke, M.J.; Neuvonen, T.; Padberg, F.; Pascual-Leone, A.; et al. Sham tDCS: A hidden source of variability? Reflections for further blinded, controlled trials. Brain Stimul. 2019, 12, 668–673. [Google Scholar] [CrossRef]
- McFadden, J.L.; Borckardt, J.J.; George, M.S.; Beam, W. Reducing procedural pain and discomfort associated with transcranial direct current stimulation. Brain Stimul. 2011, 4, 38–42. [Google Scholar] [CrossRef] [Green Version]
| Demographic | Experiment 1 | Experiment 2 |
|---|---|---|
| Sex (M/F) | 2/1 | 3/0 |
| Age (years) | 45.3 ± 19.0 | 61.0 ± 14.0 |
| Height (cm) | 171.9 ± 18.7 | 177.8 ± 2.5 |
| Weight (kg) | 83.5 ± 19.9 | 92.4 ± 9.1 |
| Time since MS diagnosis (years) | 8.0 ± 5.3 | n/a |
| Patient-Determined Disease Steps * | 2.3 ± 2.1 | n/a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Workman, C.D.; Fietsam, A.C.; Ponto, L.L.B.; Kamholz, J.; Rudroff, T. Individual Cerebral Blood Flow Responses to Transcranial Direct Current Stimulation at Various Intensities. Brain Sci. 2020, 10, 855. https://doi.org/10.3390/brainsci10110855
Workman CD, Fietsam AC, Ponto LLB, Kamholz J, Rudroff T. Individual Cerebral Blood Flow Responses to Transcranial Direct Current Stimulation at Various Intensities. Brain Sciences. 2020; 10(11):855. https://doi.org/10.3390/brainsci10110855
Chicago/Turabian StyleWorkman, Craig D., Alexandra C. Fietsam, Laura L. Boles Ponto, John Kamholz, and Thorsten Rudroff. 2020. "Individual Cerebral Blood Flow Responses to Transcranial Direct Current Stimulation at Various Intensities" Brain Sciences 10, no. 11: 855. https://doi.org/10.3390/brainsci10110855






