Cortical Beta Oscillatory Activity Evoked during Reactive Balance Recovery Scales with Perturbation Difficulty and Individual Balance Ability
Abstract
:1. Introduction
2. Methods
2.1. Participants
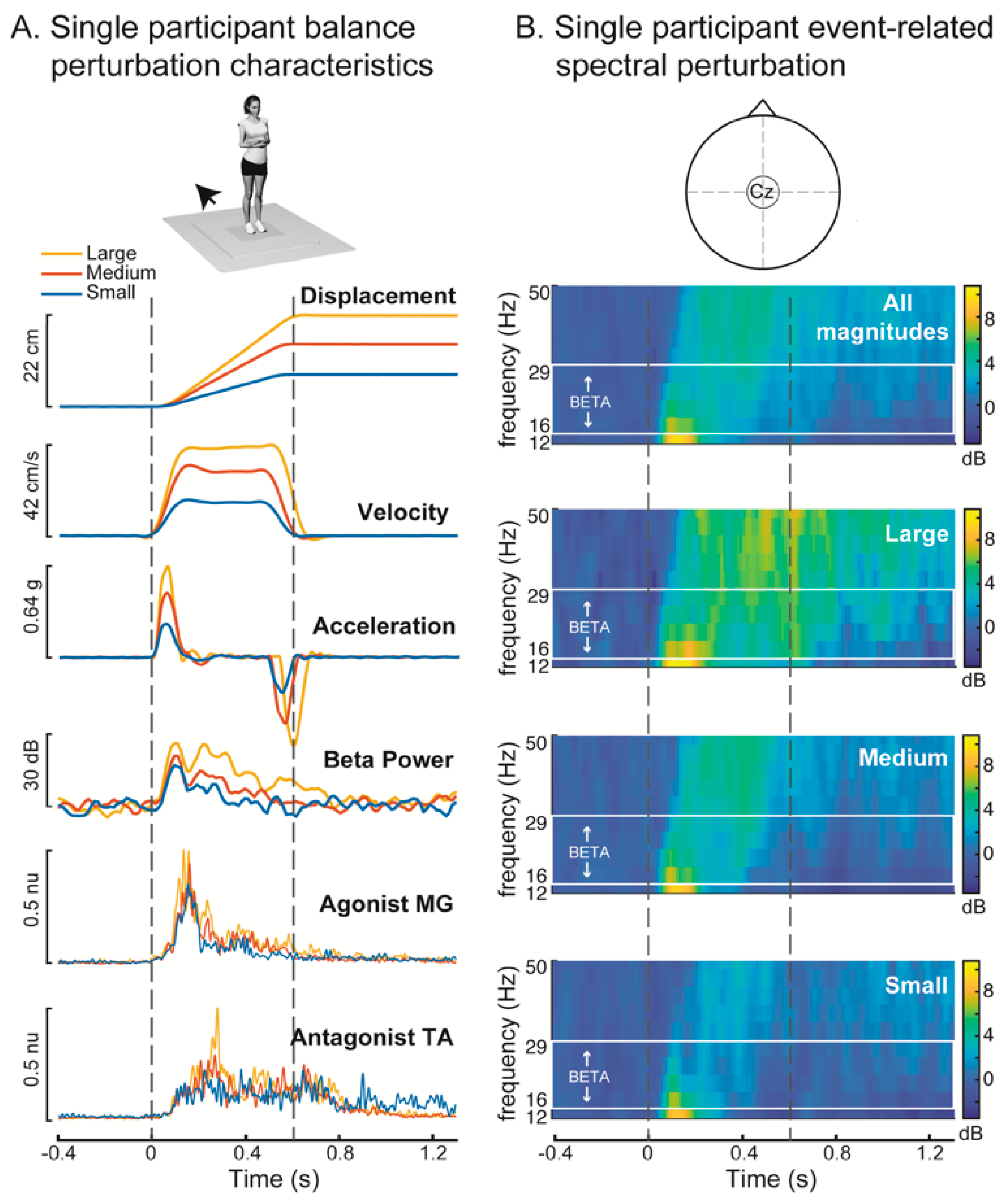
2.2. Balance Perturbations
2.3. Defining Successful Feet-in-Place Trials
2.4. Balance Ability
2.5. Electroencephalography (EEG) Data Collection and Analysis
2.6. Electromyography (EMG) Data Collection and Analysis
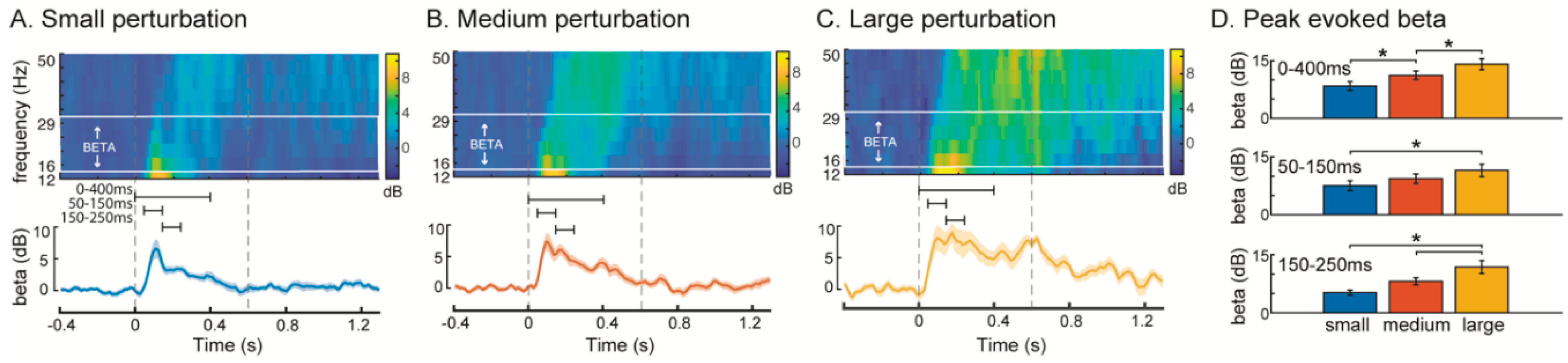
2.7. Quantification of Early- and Late-Phase-Evoked Beta Power within Discrete Time Windows
2.8. Differences in Beta Power across Perturbation Magnitudes
2.9. Relationship between Balance Ability and Peak Beta Power
2.10. Influence of Different Numbers of Nonstepping Trials as a Potential Confound
2.11. Temporal Characterization of Evoked Cortical and Muscle Activity Using Wavelet Decomposition
2.12. Statistical Analyses Using Wavelet t-Tests
2.13. Identification of Activity Onset
2.14. Statistical Analyses Using Wavelet ANOVA
2.15. Data Normality
2.16. Data Availability Statement
3. Results
4. Discussion
4.1. Increased Cortical Recruitment with Increasing Balance Difficulty
4.2. Role of Sensorimotor Cortical Processing in Standing Balance Control
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Deliagina, T.G.; Orlovsky, G.N.; Zelenin, P.V.; Beloozerova, I.N. Neural Bases of Postural Control. Physiology 2006, 21, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Deliagina, T.G.; Zelenin, P.V.; Beloozerova, I.N.; Orlovsky, G.N. Nervous mechanisms controlling body posture. Physiol. Behav. 2007, 92, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Horak, F.B.; MacPherson, J.M. Postural Orientation and Equilibrium. In Handbook of Physiology, Section 12; American Physiological Society: Rockville, MD, USA, 2011; pp. 255–292. [Google Scholar]
- Welch, T.D.J.; Ting, L.H. A Feedback Model Reproduces Muscle Activity During Human Postural Responses to Support-Surface Translations. J. Neurophysiol. 2008, 99, 1032–1038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Welch, T.D.J.; Ting, L.H. A feedback model explains the differential scaling of human postural responses to perturbation acceleration and velocity. J. Neurophysiol. 2009, 101, 3294–3309. [Google Scholar] [CrossRef] [Green Version]
- Jacobs, J.V.; Horak, F.B. Cortical control of postural responses. J. Neural Transm. 2007, 114, 1339–1348. [Google Scholar] [CrossRef] [Green Version]
- Maki, B.E.; McIlroy, W.E. Cognitive demands and cortical control of human balance-recovery reactions. J. Neural Transm. 2007, 114, 1279–1296. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.-H.; Tang, P.-F.; Wang, Y.-H.; Eng, J.J.; Lin, K.-C.; Lu, L.; Jeng, J.-S.; Chen, S.-C. Reactive Postural Control Deficits in Patients with Posterior Parietal Cortex Lesions after Stroke and the Influence of Auditory Cueing. Am. J. Phys. Med. Rehabil. 2014, 93, 849–859. [Google Scholar] [CrossRef]
- Patel, P.J.; Bhatt, T. Does aging with a cortical lesion increase fall-risk: Examining effect of age versus stroke on intensity modulation of reactive balance responses from slip-like perturbations. Neuroscience 2016, 333, 252–263. [Google Scholar] [CrossRef]
- Pérennou, D.A.; Leblond, C.; Amblard, B.; Micallef, J.P.; Rouget, E.; Pélissier, J. The polymodal sensory cortex is crucial for controlling lateral postural stability: Evidence from stroke patients. Brain Res. Bull. 2000, 53, 359–365. [Google Scholar] [CrossRef]
- Bolton, D.A.; McIlroy, W.E.; Staines, W.R. The impact of light fingertip touch on haptic cortical processing during a standing balance task. Exp. Brain Res. 2011, 212, 279–291. [Google Scholar] [CrossRef]
- Shumway-Cook, A.; Woollacott, M.; Kerns, K.A.; Baldwin, M. The Effects of Two Types of Cognitive Tasks on Postural Stability in Older Adults with and Without a History of Falls. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 1997, 52, M232–M240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rankin, J.K.; Woollacott, M.H.; Shumway-Cook, A.; Brown, L.A. Cognitive Influence on Postural Stability: A Neuromuscular Analysis in Young and Older Adults. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2000, 55, M112–M119. [Google Scholar] [CrossRef] [PubMed]
- Stuart, S.; Vitorio, R.; Morris, R.; Martini, D.N.; Fino, P.C.; Mancini, M. Cortical activity during walking and balance tasks in older adults and in people with Parkinson’s disease: A structured review. Maturitas 2018, 113, 53–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Little, C.E.; Woollacott, M. EEG measures reveal dual-task interference in postural performance in young adults. Exp. Brain Res. 2015, 233, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Quant, S.; Adkin, A.L.; Staines, W.R.; Maki, B.E.; McIlroy, W.E. The effect of a concurrent cognitive task on cortical potentials evoked by unpredictable balance perturbations. BMC Neurosci. 2004, 5, 18. [Google Scholar] [CrossRef] [Green Version]
- Adkin, A.L.; Campbell, A.D.; Chua, R.; Carpenter, M.G. The influence of postural threat on the cortical response to unpredictable and predictable postural perturbations. Neurosci. Lett. 2008, 435, 120–125. [Google Scholar] [CrossRef]
- Payne, A.M.; Hajcak, G.; Ting, L.H. Dissociation of muscle and cortical response scaling to balance perturbation acceleration. J. Neurophysiol. 2019, 121, 867–880. [Google Scholar] [CrossRef]
- Mochizuki, G.; Boe, S.; Marlin, A.; McIlroy, W. Perturbation-evoked cortical activity reflects both the context and consequence of postural instability. Neuroscience 2010, 170, 599–609. [Google Scholar] [CrossRef]
- Payne, A.M.; Ting, L.H. Worse balance is associated with larger perturbation-evoked cortical responses in healthy young adults. Gait Posture 2020, 80, 324–330. [Google Scholar] [CrossRef]
- Van Wijk, B.C.; Beek, P.; Daffertshofer, A. Neural synchrony within the motor system: What have we learned so far? Front. Hum. Neurosci. 2012, 6, 252. [Google Scholar] [CrossRef] [Green Version]
- Zaepffel, M.; Trachel, R.; Kilavik, B.E.; Brochier, T. Modulations of EEG Beta Power during Planning and Execution of Grasping Movements. PLoS ONE 2013, 8, e60060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joundi, R.A.; Jenkinson, N.; Brittain, J.-S.; Aziz, T.Z.; Brown, P. Driving Oscillatory Activity in the Human Cortex Enhances Motor Performance. Curr. Biol. 2012, 22, 403–407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pogosyan, A.; Gaynor, L.D.; Eusebio, A.; Brown, P. Boosting Cortical Activity at Beta-Band Frequencies Slows Movement in Humans. Curr. Biol. 2009, 19, 1637–1641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guerra, A.; Bologna, M.; Paparella, G.; Suppa, A.; Colella, D.; Di Lazzaro, V.; Brown, P.; Berardelli, A. Effects of Transcranial Alternating Current Stimulation on Repetitive Finger Movements in Healthy Humans. Neural Plast. 2018, 2018, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Engel, A.K.; Fries, P. Beta-band oscillations—Signalling the status quo? Curr. Opin. Neurobiol. 2010, 20, 156–165. [Google Scholar] [CrossRef]
- Baker, S.N. Oscillatory interactions between sensorimotor cortex and the periphery. Curr. Opin. Neurobiol. 2007, 17, 649–655. [Google Scholar] [CrossRef] [Green Version]
- Muthukumaraswamy, S.; Myers, J.; Wilson, S.; Nutt, D.; Lingford-Hughes, A.; Singh, K.; Hamandi, K. The effects of elevated endogenous GABA levels on movement-related network oscillations. NeuroImage 2013, 66, 36–41. [Google Scholar] [CrossRef]
- Rossiter, H.E.; Boudrias, M.-H.; Ward, N.S. Do movement-related beta oscillations change after stroke? J. Neurophysiol. 2014, 112, 2053–2058. [Google Scholar] [CrossRef] [Green Version]
- Rossiter, H.E.; Davis, E.M.; Clark, E.V.; Boudrias, M.-H.; Ward, N.S. Beta oscillations reflect changes in motor cortex inhibition in healthy ageing. NeuroImage 2014, 91, 360–365. [Google Scholar] [CrossRef] [Green Version]
- Heise, K.-F.; Zimerman, M.; Hoppe, J.; Gerloff, C.; Wegscheider, K.; Hummel, F.C. The Aging Motor System as a Model for Plastic Changes of GABA-Mediated Intracortical Inhibition and Their Behavioral Relevance. J. Neurosci. 2013, 33, 9039–9049. [Google Scholar] [CrossRef]
- Brown, P. Oscillatory nature of human basal ganglia activity: Relationship to the pathophysiology of Parkinson’s disease. Mov. Disord. Off. J. Mov. Disord. Soc. 2003, 18, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Kühn, A.A.; Kupsch, A.; Schneider, G.-H.; Brown, P. Reduction in subthalamic 8-35 Hz oscillatory activity correlates with clinical improvement in Parkinson’s disease. Eur. J. Neurosci. 2006, 23, 1956–1960. [Google Scholar] [CrossRef] [PubMed]
- Kühn, A.A.; Tsui, A.; Aziz, T.; Ray, N.; Brücke, C.; Kupsch, A.; Schneider, G.-H.; Brown, P. Pathological synchronisation in the subthalamic nucleus of patients with Parkinson’s disease relates to both bradykinesia and rigidity. Exp. Neurol. 2009, 215, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, A.; Suzuki, Y.; Milosevic, M.; Nomura, T. Long-lasting event-related beta synchronization and theta desynchronization of electroencephalographic activity in response to support-surface perturbations during upright stance: Novel findings from experimental and model-based results. Neuroscience 2020, preprint. [Google Scholar] [CrossRef]
- Peterson, S.M.; Ferris, D.P. Differentiation in Theta and Beta Electrocortical Activity between Visual and Physical Perturbations to Walking and Standing Balance. Eneuro 2018, 5. [Google Scholar] [CrossRef] [Green Version]
- Peterson, S.M.; Ferris, D.P. Group-level cortical and muscular connectivity during perturbations to walking and standing balance. NeuroImage 2019, 198, 93–103. [Google Scholar] [CrossRef]
- Solis-Escalante, T.; Van Der Cruijsen, J.; De Kam, D.; Van Kordelaar, J.; Weerdesteyn, V.; Schouten, A.C. Cortical dynamics during preparation and execution of reactive balance responses with distinct postural demands. NeuroImage 2019, 188, 557–571. [Google Scholar] [CrossRef]
- Varghese, J.P.; Marlin, A.; Beyer, K.B.; Staines, W.R.; Mochizuki, G.; McIlroy, W.E. Frequency characteristics of cortical activity associated with perturbations to upright stability. Neurosci. Lett. 2014, 578, 33–38. [Google Scholar] [CrossRef]
- Varghese, J.P.; Staines, W.R.; McIlroy, W.E. Activity in Functional Cortical Networks Temporally Associated with Postural Instability. Neuroscience 2019, 401, 43–58. [Google Scholar] [CrossRef]
- Lajoie, Y.; Teasdale, N.; Bard, C.; Fleury, M. Attentional demands for static and dynamic equilibrium. Exp. Brain Res. 1993, 97, 139–144. [Google Scholar] [CrossRef]
- Woollacott, M.; Shumway-Cook, A. Attention and the control of posture and gait: A review of an emerging area of research. Gait Posture 2002, 16, 1–14. [Google Scholar] [CrossRef]
- Sawers, A.; Ting, L.H. Beam walking can detect differences in walking balance proficiency across a range of sensorimotor abilities. Gait Posture 2015, 41, 619–623. [Google Scholar] [CrossRef] [PubMed]
- Delorme, A.; Makeig, S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makeig, S. Auditory event-related dynamics of the EEG spectrum and effects of exposure to tones. Electroencephalogr. Clin. Neurophysiol. 1993, 86, 283–293. [Google Scholar] [CrossRef]
- Pfurtscheller, G.; Da Silva, F.L. Event-related EEG/MEG synchronization and desynchronization: Basic principles. Clin. Neurophysiol. 1999, 110, 1842–1857. [Google Scholar] [CrossRef]
- Hanslmayr, S.; Volberg, G.; Wimber, M.; Raabe, M.; Greenlee, M.W.; Bauml, K.-H.T. The Relationship between Brain Oscillations and BOLD Signal during Memory Formation: A Combined EEG-FMRI Study. J. Neurosci. 2011, 31, 15674–15680. [Google Scholar] [CrossRef]
- Basmajian, J.V.; Blumenstein, R. Electrode Placement in EMG Biofeedback; Williams & Wilkins: Baltimore, MD, USA, 1980. [Google Scholar]
- McKay, J.L.; Welch, T.D.J.; Vidakovic, B.; Ting, L.H. Statistically significant contrasts between EMG waveforms revealed using wavelet-based functional ANOVA. J. Neurophysiol. 2013, 109, 591–602. [Google Scholar] [CrossRef] [Green Version]
- Schucany, W.R.; Ng, H.K.T. Preliminary Goodness-of-Fit Tests for Normality Do Not Validate the One-Sample Student. Commun. Stat. Theory Methods 2006, 35, 2275–2286. [Google Scholar] [CrossRef]
- McIlroy, W.E.; Maki, B.E. The ‘deceleration response’ to transient perturbation of upright stance. Neurosci. Lett. 1994, 175, 13–16. [Google Scholar] [CrossRef]
- Makeig, S.; Onton, J. ERP Features and EEG Dynamics: An ICA Perspective. Oxf. Handb. Event-Relat. Potential Compon. 2012. [Google Scholar] [CrossRef]
- Hülsdünker, T.; Mierau, A.; Neeb, C.; Kleinöder, H.; Strüder, H. Cortical processes associated with continuous balance control as revealed by EEG spectral power. Neurosci. Lett. 2015, 592, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Hülsdünker, T.; Mierau, A.; Strüder, H.K. Higher Balance Task Demands are Associated with an Increase in Individual Alpha Peak Frequency. Front. Hum. Neurosci. 2016, 9, 695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sipp, A.R.; Gwin, J.T.; Makeig, S.; Ferris, D.P. Loss of balance during balance beam walking elicits a multifocal theta band electrocortical response. J. Neurophysiol. 2013, 110, 2050–2060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Espenhahn, S.; Van Wijk, B.C.; Rossiter, H.E.; De Berker, A.O.; Redman, N.D.; Rondina, J.; Diedrichsen, J.; Ward, N.S. Cortical beta oscillations are associated with motor performance following visuomotor learning. NeuroImage 2019, 195, 340–353. [Google Scholar] [CrossRef]
- Guadagnoli, M.A.; Lee, T.D. Challenge Point: A Framework for Conceptualizing the Effects of Various Practice Conditions in Motor Learning. J. Mot. Behav. 2004, 36, 212–224. [Google Scholar] [CrossRef]
- Maki, B.E.; McIlroy, W.E. The Role of Limb Movements in Maintaining Upright Stance: The “Change-in-Support” Strategy. Phys. Ther. 1997, 77, 488–507. [Google Scholar] [CrossRef]
- Horak, F.B.; Nashner, L.M. Central programming of postural movements: Adaptation to altered support-surface configurations. J. Neurophysiol. 1986, 55, 1369–1381. [Google Scholar] [CrossRef]
- Welch, T.D.J.; Ting, L.H. Mechanisms of Motor Adaptation in Reactive Balance Control. PLoS ONE 2014, 9, e96440. [Google Scholar] [CrossRef] [Green Version]
- Heideman, S.G.; Quinn, A.J.; Woolrich, M.W.; Van Ede, F.; Nobre, A.C. Dissecting beta-state changes during timed movement preparation in Parkinson’s disease. Prog. Neurobiol. 2020, 184, 101731. [Google Scholar] [CrossRef]
- Jones, S.R.; Kerr, C.E.; Wan, Q.; Pritchett, D.L.; Hamalainen, M.S.; Moore, C.I. Cued Spatial Attention Drives Functionally Relevant Modulation of the Mu Rhythm in Primary Somatosensory Cortex. J. Neurosci. 2010, 30, 13760–13765. [Google Scholar] [CrossRef] [Green Version]
- Little, S.; Bonaiuto, J.; Barnes, G.; Bestmann, S. Human motor cortical beta bursts relate to movement planning and response errors. PLoS Biol. 2019, 17, e3000479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, H.; Law, R.; Tsutsui, S.; Moore, C.I.; Jones, S.R. The rate of transient beta frequency events predicts behavior across tasks and species. eLife 2017, 6, e29086. [Google Scholar] [CrossRef] [PubMed]
- McKay, J.L.; Lang, K.C.; Bong, S.M.; Hackney, M.E.; Factor, S.A.; Ting, L.H. Abnormal center of mass control during balance is associated with falls in Parkinson’s disease. Neuroscience 2020, preprint. [Google Scholar] [CrossRef] [Green Version]
- He, J.; Levine, W.; Loeb, G. Feedback gains for correcting small perturbations to standing posture. IEEE Trans. Autom. Control 1991, 36, 322–332. [Google Scholar] [CrossRef]
- Lockhart, D.B.; Ting, L.H. Optimal sensorimotor transformations for balance. Nat. Neurosci. 2007, 10, 1329–1336. [Google Scholar] [CrossRef]
- Kilavik, B.E.; Zaepffel, M.; Brovelli, A.; Mackay, W.A.; Riehle, A. The ups and downs of beta oscillations in sensorimotor cortex. Exp. Neurol. 2013, 245, 15–26. [Google Scholar] [CrossRef] [Green Version]
- Baker, S.N.; Kilner, J.M.; Pinches, E.M.; Lemon, R.N. The role of synchrony and oscillations in the motor output. Exp. Brain Res. 1999, 128, 109–117. [Google Scholar] [CrossRef]
- Witham, C.L.; Riddle, C.N.; Baker, M.R.; Baker, S.N. Contributions of descending and ascending pathways to corticomuscular coherence in humans. J. Physiol. 2011, 589, 3789–3800. [Google Scholar] [CrossRef] [Green Version]
- Gaetz, W.; Cheyne, D. Localization of sensorimotor cortical rhythms induced by tactile stimulation using spatially filtered MEG. NeuroImage 2006, 30, 899–908. [Google Scholar] [CrossRef]
- Jurkiewicz, M.T.; Gaetz, W.C.; Bostan, A.C.; Cheyne, D. Post-movement beta rebound is generated in motor cortex: Evidence from neuromagnetic recordings. NeuroImage 2006, 32, 1281–1289. [Google Scholar] [CrossRef]
- Salmelin, R.; Hámáaláinen, M.; Kajola, M.; Hari, R. Functional Segregation of Movement-Related Rhythmic Activity in the Human Brain. NeuroImage 1995, 2, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Fioravanti, C.; Kajal, S.; Carboni, M.; Mazzetti, C.; Ziemann, U.; Braun, C. Inhibition in the somatosensory system: An integrative neuropharmacological and neuroimaging approach. NeuroImage 2019, 202, 116139. [Google Scholar] [CrossRef] [PubMed]
- Yamawaki, N.; Stanford, I.; Hall, S.; Woodhall, G. Pharmacologically induced and stimulus evoked rhythmic neuronal oscillatory activity in the primary motor cortex in vitro. Neuroscience 2008, 151, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Lalo, E.; Gilbertson, T.; Doyle, L.; Di Lazzaro, V.; Cioni, B.; Brown, P. Phasic increases in cortical beta activity are associated with alterations in sensory processing in the human. Exp. Brain Res. 2006, 177, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Murase, N.; Duque, J.; Mazzocchio, R.; Cohen, L.G. Influence of interhemispheric interactions on motor function in chronic stroke. Ann. Neurol. 2004, 55, 400–409. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghosn, N.J.; Palmer, J.A.; Borich, M.R.; Ting, L.H.; Payne, A.M. Cortical Beta Oscillatory Activity Evoked during Reactive Balance Recovery Scales with Perturbation Difficulty and Individual Balance Ability. Brain Sci. 2020, 10, 860. https://doi.org/10.3390/brainsci10110860
Ghosn NJ, Palmer JA, Borich MR, Ting LH, Payne AM. Cortical Beta Oscillatory Activity Evoked during Reactive Balance Recovery Scales with Perturbation Difficulty and Individual Balance Ability. Brain Sciences. 2020; 10(11):860. https://doi.org/10.3390/brainsci10110860
Chicago/Turabian StyleGhosn, Nina J., Jacqueline A. Palmer, Michael R. Borich, Lena H. Ting, and Aiden M. Payne. 2020. "Cortical Beta Oscillatory Activity Evoked during Reactive Balance Recovery Scales with Perturbation Difficulty and Individual Balance Ability" Brain Sciences 10, no. 11: 860. https://doi.org/10.3390/brainsci10110860
APA StyleGhosn, N. J., Palmer, J. A., Borich, M. R., Ting, L. H., & Payne, A. M. (2020). Cortical Beta Oscillatory Activity Evoked during Reactive Balance Recovery Scales with Perturbation Difficulty and Individual Balance Ability. Brain Sciences, 10(11), 860. https://doi.org/10.3390/brainsci10110860





