Genome-Wide Scan for Five Brain Oscillatory Phenotypes Identifies a New QTL Associated with Theta EEG Band
Abstract
1. Introduction
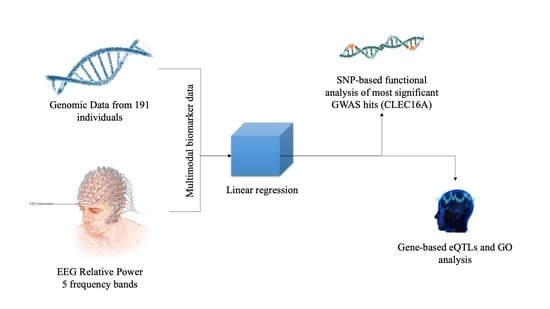
2. Materials and Methods
2.1. Subjects
2.2. EEG Recording and Processing
2.3. Genotyping and Genome-Wide Association Analysis
2.4. Common Variant QTL and eQTL Enrichment Analysis
2.5. Statistical Power Computation
3. Results
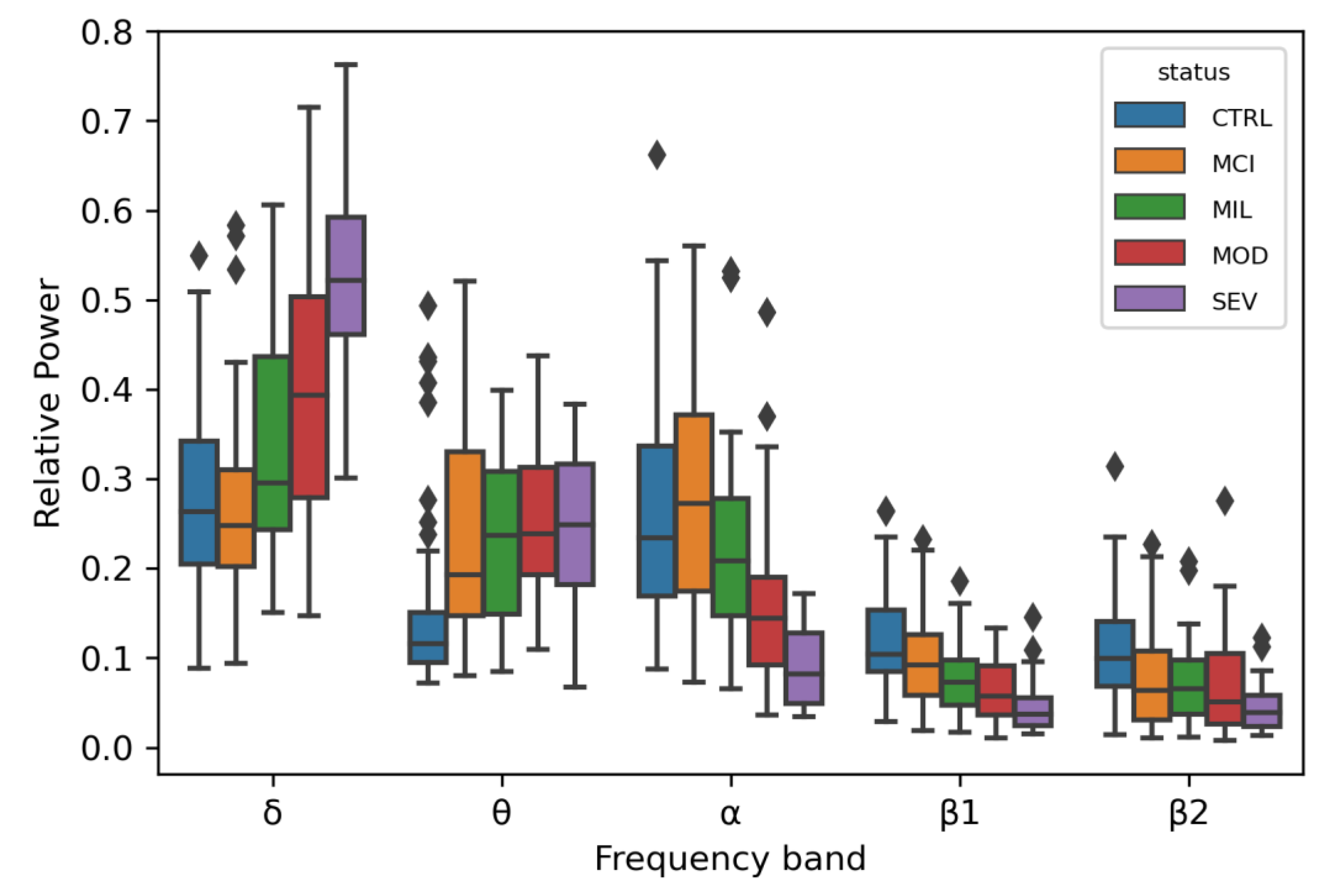
3.1. Linear Regression for 5 Oscillatory Phenotypes Identified One New Association
3.2. SNP-Based Functional Analysis of Most Significant GWAS Hits
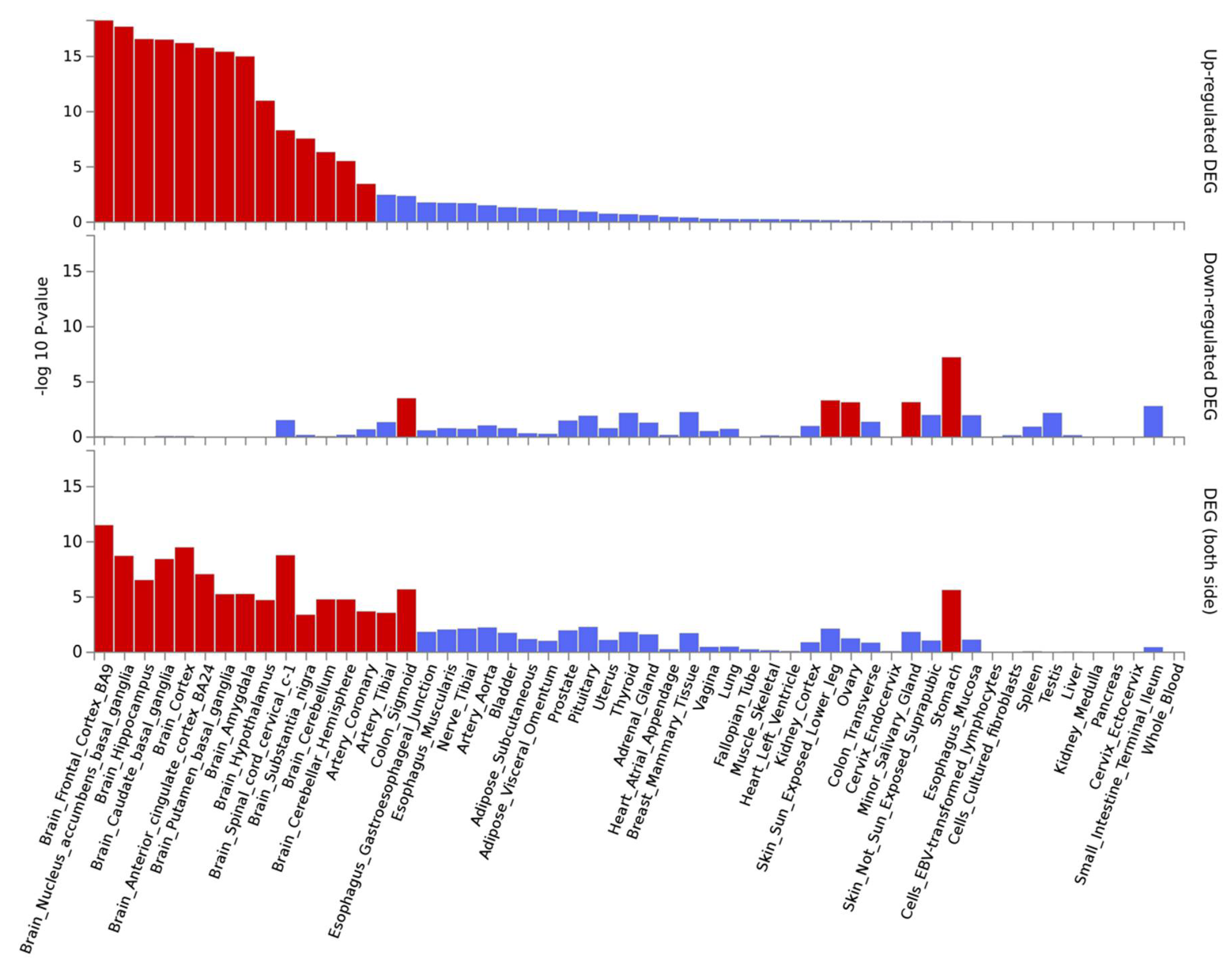
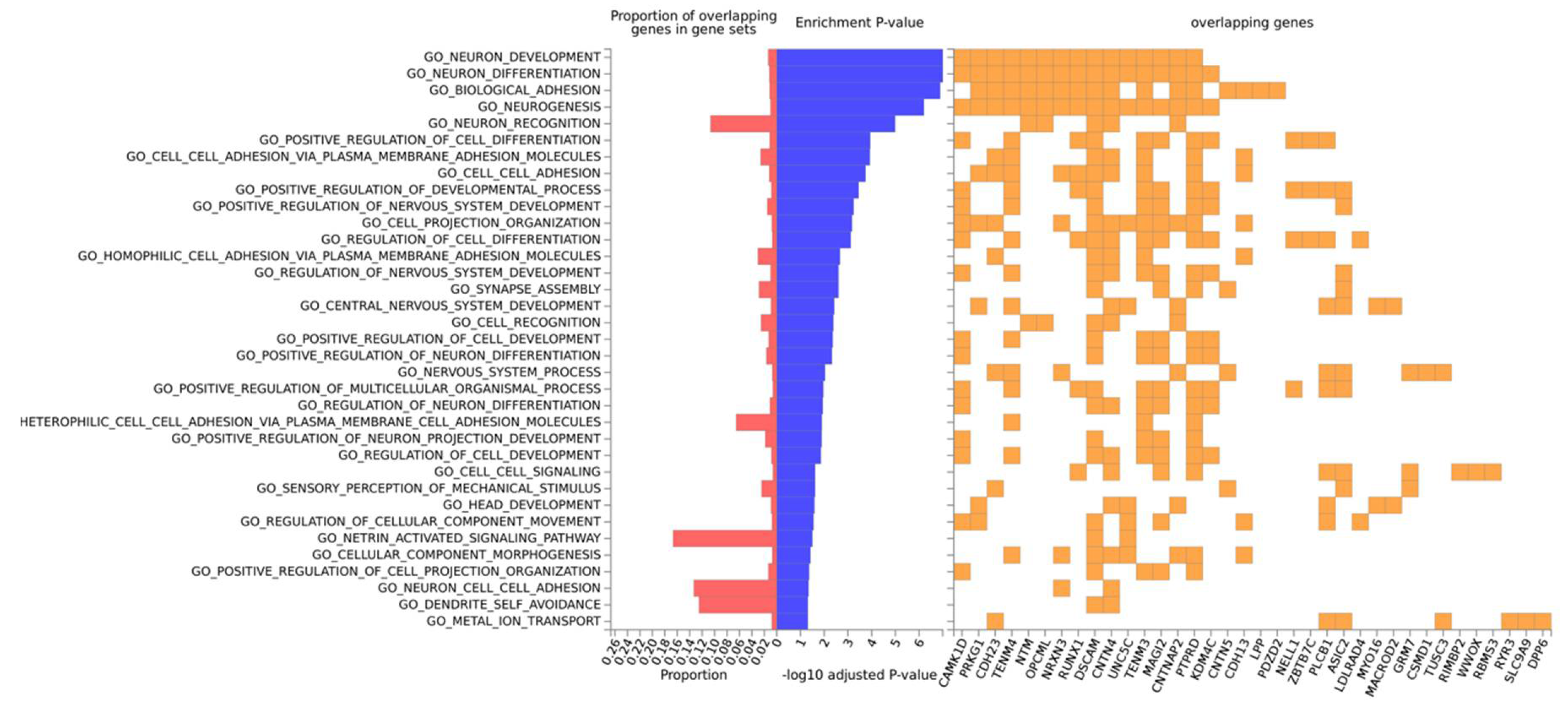
3.3. Gene-Based Expression and GO Analysis
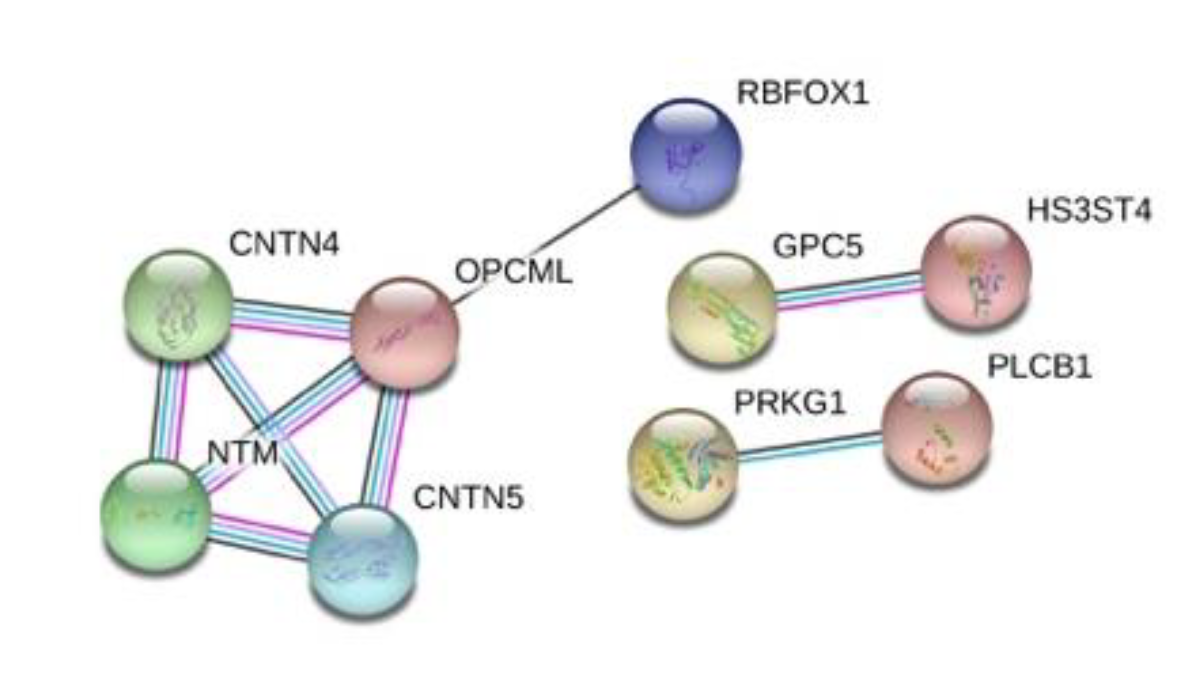
3.4. Protein–Protein Interactions
3.5. Links to Brain Phenotypes and Neuropathologies
3.6. Statistical Power and Effect Size
4. Discussion
4.1. Linear Regression for 5 Oscillatory Phenotypes Identified One New θ QTL
4.2. SNP and Gene-Based Functional Analysis of Most Significant GWAS Hits and Their Link to Neurological Traits and Pathologies
4.3. Limitations and Future Research Lines
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Da Silva, F.L. EEG: Origin and Measurement. In EEG-fMRI; Mulert, C., Lemieux, L., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 19–38. ISBN 978-3-540-87918-3. [Google Scholar]
- Nunez, P.L.; Srinivasan, R.; Fields, R.D. EEG functional connectivity, axon delays and white matter disease. Clin. Neurophysiol. 2015, 126, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Voytek, B.; Knight, R.T. Dynamic Network Communication as a Unifying Neural Basis for Cognition, Development, Aging, and Disease. Biol. Psychiatry 2015, 77, 1089–1097. [Google Scholar] [CrossRef] [PubMed]
- Babiloni, C.; Blinowska, K.; Bonanni, L.; Cichocki, A.; De Haan, W.; Del Percio, C.; Dubois, B.; Escudero, J.; Fernández, A.; Frisoni, G.; et al. What electrophysiology tells us about Alzheimer’s disease: A window into the synchronization and connectivity of brain neurons. Neurobiol. Aging 2020, 85, 58–73. [Google Scholar] [CrossRef] [PubMed]
- Cremer, R.; Zeef, E.J. What Kind of Noise Increases With Age? J. Gerontol. 1987, 42, 515–518. [Google Scholar] [CrossRef]
- Holtzheimer, P.E.; Mayberg, H.S. Stuck in a rut: Rethinking depression and its treatment. Trends Neurosci. 2011, 34, 1–9. [Google Scholar] [CrossRef]
- Jelic, V.; Johansson, S.-E.; Almkvist, O.; Shigeta, M.; Julin, P.; Nordberg, A.; Winblad, B.; Wahlund, L.-O. Quantitative electroencephalography in mild cognitive impairment: Longitudinal changes and possible prediction of Alzheimer’s disease. Neurobiol. Aging 2000, 21, 533–540. [Google Scholar] [CrossRef]
- Jeong, J. EEG dynamics in patients with Alzheimer’s disease. Clin. Neurophysiol. 2004, 115, 1490–1505. [Google Scholar] [CrossRef]
- Weinger, P.M.; Zemon, V.; Soorya, L.; Gordon, J. Low-contrast response deficits and increased neural noise in children with autism spectrum disorder. Neuropsychologia 2014, 63, 10–18. [Google Scholar] [CrossRef]
- Winterer, G.; Weinberger, D.R. Genes, dopamine and cortical signal-to-noise ratio in schizophrenia. Trends Neurosci. 2004, 27, 683–690. [Google Scholar] [CrossRef]
- Tang, Y.; Chorlian, D.B.; Rangaswamy, M.; Porjesz, B.; Bauer, L.; Kuperman, S.; O’Connor, S.; Rohrbaugh, J.; Schuckit, M.; Stimus, A.; et al. Genetic influences on bipolar EEG power spectra. Int. J. Psychophysiol. 2007, 65, 2–9. [Google Scholar] [CrossRef]
- Zietsch, B.P.; Hansen, J.L.; Hansell, N.K.; Geffen, G.M.; Martin, N.G.; Wright, M.J. Common and specific genetic influences on EEG power bands delta, theta, alpha, and beta. Biol. Psychol. 2007, 75, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Smit, D.J.A.; Posthuma, D.; Boomsma, D.I.; Geus, E.J.C. Heritability of background EEG across the power spectrum. Psychophysiology 2005, 42, 691–697. [Google Scholar] [CrossRef] [PubMed]
- De Geus, E.J. From genotype to EEG endophenotype: A route for post-genomic understanding of complex psychiatric disease? Genome Med. 2010, 2, 63. [Google Scholar] [CrossRef] [PubMed]
- Loo, S.K.; Lenartowicz, A.; Makeig, S. Research Review: Use of EEG biomarkers in child psychiatry research-current state and future directions. J. Child Psychol. Psychiatry 2016, 57, 4–17. [Google Scholar] [CrossRef]
- De Geus, E.J.C.; Boomsma, D.I. A Genetic Neuroscience Approach to Human Cognition. Eur. Psychol. 2001, 6, 241–253. [Google Scholar] [CrossRef]
- Gottesman, I.I.; Gould, T.D. The Endophenotype Concept in Psychiatry: Etymology and Strategic Intentions. Am. J. Psychiatry 2003, 160, 636–645. [Google Scholar] [CrossRef]
- Psychiatric GWAS Consortium Coordinating Committee. Genomewide Association Studies: History, Rationale, and Prospects for Psychiatric Disorders. Am. J. Psychiatry 2009, 166, 540–556. [Google Scholar] [CrossRef]
- Lamparter, D.; Marbach, D.; Rueedi, R.; Kutalik, Z.; Bergmann, S. Fast and Rigorous Computation of Gene and Pathway Scores from SNP-Based Summary Statistics. PLoS Comput. Biol. 2016, 12, e1004714. [Google Scholar] [CrossRef]
- De Leeuw, C.A.; Mooij, J.M.; Heskes, T.; Posthuma, D. MAGMA: Generalized Gene-Set Analysis of GWAS Data. PLoS Comput. Biol. 2015, 11, e1004219. [Google Scholar] [CrossRef]
- Watanabe, K.; Taskesen, E.; van Bochoven, A.; Posthuma, D. Functional mapping and annotation of genetic associations with FUMA. Nat. Commun. 2017, 8, 1826. [Google Scholar] [CrossRef]
- Smit, D.J.A.; Wright, M.J.; Meyers, J.L.; Martin, N.G.; Ho, Y.Y.W.; Malone, S.M.; Zhang, J.; Burwell, S.J.; Chorlian, D.B.; de Geus, E.J.C.; et al. Genome-wide association analysis links multiple psychiatric liability genes to oscillatory brain activity. Hum. Brain Mapp. 2018, 39, 4183–4195. [Google Scholar] [CrossRef] [PubMed]
- Jack, C.R.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J.; et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018, 14, 535–562. [Google Scholar] [CrossRef] [PubMed]
- Núñez, P.; Poza, J.; Gómez, C.; Rodríguez-González, V.; Hillebrand, A.; Tola-Arribas, M.A.; Cano, M.; Hornero, R. Characterizing the fluctuations of dynamic resting-state electrophysiological functional connectivity: Reduced neuronal coupling variability in mild cognitive impairment and dementia due to Alzheimer’s disease. J. Neural Eng. 2019, 16, 056030. [Google Scholar] [CrossRef]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.R.; Bender, D.; Maller, J.; Sklar, P.; de Bakker, P.I.W.; Daly, M.J.; et al. PLINK: A Tool Set for Whole-Genome Association and Population-Based Linkage Analyses. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef] [PubMed]
- Price, A.L.; Patterson, N.J.; Plenge, R.M.; Weinblatt, M.E.; Shadick, N.A.; Reich, D. Principal components analysis corrects for stratification in genome-wide association studies. Nat. Genet. 2006, 38, 904–909. [Google Scholar] [CrossRef] [PubMed]
- Gómez, C.; Pérez-Macías, J.M.; Poza, J.; Fernández, A.; Hornero, R. Spectral changes in spontaneous MEG activity across the lifespan. J. Neural Eng. 2013, 10, 066006. [Google Scholar] [CrossRef]
- Patterson, N.; Price, A.L.; Reich, D. Population Structure and Eigenanalysis. PLoS Genet. 2006, 2, e190. [Google Scholar] [CrossRef]
- Schmitt, A.D.; Hu, M.; Jung, I.; Xu, Z.; Qiu, Y.; Tan, C.L.; Li, Y.; Lin, S.; Lin, Y.; Barr, C.L.; et al. A Compendium of Chromatin Contact Maps Reveals Spatially Active Regions in the Human Genome. Cell Rep. 2016, 17, 2042–2059. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein–protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef]
- Welter, D.; MacArthur, J.; Morales, J.; Burdett, T.; Hall, P.; Junkins, H.; Klemm, A.; Flicek, P.; Manolio, T.; Hindorff, L.; et al. The NHGRI GWAS Catalog, a curated resource of SNP-trait associations. Nucleic Acids Res. 2014, 42, D1001–D1006. [Google Scholar] [CrossRef]
- Visscher, P.M.; Wray, N.R.; Zhang, Q.; Sklar, P.; McCarthy, M.I.; Brown, M.A.; Yang, J. 10 Years of GWAS Discovery: Biology, Function, and Translation. Am. J. Hum. Genet. 2017, 101, 5–22. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, W. Über Vererbungsgesetze beim Menschen. Z. Indukt. Abstamm.-Vererb. 1908, 1, 440–460. [Google Scholar] [CrossRef][Green Version]
- Hardy, G.H. Mendelian proportions in a mixed population. Science 1908, 28, 49–50. [Google Scholar] [CrossRef]
- Shim, H.; Chasman, D.I.; Smith, J.D.; Mora, S.; Ridker, P.M.; Nickerson, D.A.; Krauss, R.M.; Stephens, M. A Multivariate Genome-Wide Association Analysis of 10 LDL Subfractions, and Their Response to Statin Treatment, in 1868 Caucasians. PLoS ONE 2015, 10, e0120758. [Google Scholar] [CrossRef] [PubMed]
- Hamm, V.; Héraud, C.; Cassel, J.-C.; Mathis, C.; Goutagny, R. Precocious Alterations of Brain Oscillatory Activity in Alzheimer’s Disease: A Window of Opportunity for Early Diagnosis and Treatment. Front. Cell. Neurosci. 2015, 9. [Google Scholar] [CrossRef] [PubMed]
- Fisher, S.E.; Scharff, C. FOXP2 as a molecular window into speech and language. Trends Genet. 2009, 25, 166–177. [Google Scholar] [CrossRef] [PubMed]
- Konopka, G.; Bomar, J.M.; Winden, K.; Coppola, G.; Jonsson, Z.O.; Gao, F.; Peng, S.; Preuss, T.M.; Wohlschlegel, J.A.; Geschwind, D.H. Human-specific transcriptional regulation of CNS development genes by FOXP2. Nature 2009, 462, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Teramitsu, I. Parallel FoxP1 and FoxP2 Expression in Songbird and Human Brain Predicts Functional Interaction. J. Neurosci. 2004, 24, 3152–3163. [Google Scholar] [CrossRef] [PubMed]
- Andrews, S.J.; Das, D.; Cherbuin, N.; Anstey, K.J.; Easteal, S. Association of genetic risk factors with cognitive decline: The PATH through life project. Neurobiol. Aging 2016, 41, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Huovinen, J.; Helisalmi, S.; Paananen, J.; Laiterä, T.; Kojoukhova, M.; Sutela, A.; Vanninen, R.; Laitinen, M.; Rauramaa, T.; Koivisto, A.M.; et al. Alzheimer’s Disease-Related Polymorphisms in Shunt-Responsive Idiopathic Normal Pressure Hydrocephalus. J. Alzheimers Dis. 2017, 60, 1077–1085. [Google Scholar] [CrossRef]
- Jung, I.; Schmitt, A.; Diao, Y.; Lee, A.J.; Liu, T.; Yang, D.; Tan, C.; Eom, J.; Chan, M.; Chee, S.; et al. A compendium of promoter-centered long-range chromatin interactions in the human genome. Nat. Genet. 2019, 51, 1442–1449. [Google Scholar] [CrossRef] [PubMed]
- Lindholm, P.; Voutilainen, M.H.; Laurén, J.; Peränen, J.; Leppänen, V.-M.; Andressoo, J.-O.; Lindahl, M.; Janhunen, S.; Kalkkinen, N.; Timmusk, T.; et al. Novel neurotrophic factor CDNF protects and rescues midbrain dopamine neurons in vivo. Nature 2007, 448, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Ait-Si-Ali, S.; Guasconi, V.; Fritsch, L.; Yahi, H.; Sekhri, R.; Naguibneva, I.; Robin, P.; Cabon, F.; Polesskaya, A.; Harel-Bellan, A. A Suv39h-dependent mechanism for silencing S-phase genes in differentiating but not in cycling cells. EMBO J. 2004, 23, 605–615. [Google Scholar] [CrossRef] [PubMed]
- Duong, H.A.; Weitz, C.J. Temporal orchestration of repressive chromatin modifiers by circadian clock Period complexes. Nat. Struct. Mol. Biol. 2014, 21, 126–132. [Google Scholar] [CrossRef]
- Kato, A.S.; Gill, M.B.; Ho, M.T.; Yu, H.; Tu, Y.; Siuda, E.R.; Wang, H.; Qian, Y.-W.; Nisenbaum, E.S.; Tomita, S.; et al. Hippocampal AMPA Receptor Gating Controlled by Both TARP and Cornichon Proteins. Neuron 2010, 68, 1082–1096. [Google Scholar] [CrossRef]
- Guipponi, M.; Chentouf, A.; Webling, K.E.B.; Freimann, K.; Crespel, A.; Nobile, C.; Lemke, J.R.; Hansen, J.; Dorn, T.; Lesca, G.; et al. Galanin pathogenic mutations in temporal lobe epilepsy. Hum. Mol. Genet. 2015, 24, 3082–3091. [Google Scholar] [CrossRef]
- Messanvi, F.; Perkins, A.; du Hoffmann, J.; Chudasama, Y. Fronto-temporal galanin modulates impulse control. Psychopharmacology 2019. [Google Scholar] [CrossRef]
- Osterhout, J.A.; Stafford, B.K.; Nguyen, P.L.; Yoshihara, Y.; Huberman, A.D. Contactin-4 Mediates Axon-Target Specificity and Functional Development of the Accessory Optic System. Neuron 2015, 86, 985–999. [Google Scholar] [CrossRef]
- Kleijer, K.T.E.; van Nieuwenhuize, D.; Spierenburg, H.A.; Gregorio-Jordan, S.; Kas, M.J.H.; Burbach, J.P.H. Structural abnormalities in the primary somatosensory cortex and a normal behavioral profile in Contactin-5 deficient mice. Cell Adhes. Migr. 2018, 12, 5–18. [Google Scholar] [CrossRef]
- Gil, O.D.; Zanazzi, G.; Struyk, A.F.; Salzer, J.L. Neurotrimin Mediates Bifunctional Effects on Neurite Outgrowth via Homophilic and Heterophilic Interactions. J. Neurosci. 1998, 18, 9312–9325. [Google Scholar] [CrossRef]
- Krizsan-Agbas, D.; Pedchenko, T.; Smith, P.G. Neurotrimin is an estrogen-regulated determinant of peripheral sympathetic innervation. J. Neurosci. Res. 2008, 86, 3086–3095. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-A.; Damianov, A.; Lin, C.-H.; Fontes, M.; Parikshak, N.N.; Anderson, E.S.; Geschwind, D.H.; Black, D.L.; Martin, K.C. Cytoplasmic Rbfox1 Regulates the Expression of Synaptic and Autism-Related Genes. Neuron 2016, 89, 113–128. [Google Scholar] [CrossRef] [PubMed]
- European Alzheimer’s Disease Initiative (EADI); Genetic and Environmental Risk in Alzheimer’s Disease (GERAD); Alzheimer’s Disease Genetic Consortium (ADGC); Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE); Lambert, J.-C.; Ibrahim-Verbaas, C.A.; Harold, D.; Naj, A.C.; Sims, R.; Bellenguez, C.; et al. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Nat. Genet. 2013, 45, 1452–1458. [Google Scholar] [CrossRef] [PubMed]
- Jansen, I.E.; Savage, J.E.; Watanabe, K.; Bryois, J.; Williams, D.M.; Steinberg, S.; Sealock, J.; Karlsson, I.K.; Hägg, S.; Athanasiu, L.; et al. Genome-wide meta-analysis identifies new loci and functional pathways influencing Alzheimer’s disease risk. Nat. Genet. 2019, 51, 404–413. [Google Scholar] [CrossRef]
- Li, Z.; Chen, J.; Yu, H.; He, L.; Xu, Y.; Zhang, D.; Yi, Q.; Li, C.; Li, X.; Shen, J.; et al. Genome-wide association analysis identifies 30 new susceptibility loci for schizophrenia. Nat. Genet. 2017, 49, 1576–1583. [Google Scholar] [CrossRef]
- Yan, X.; Nykänen, N.-P.; Brunello, C.A.; Haapasalo, A.; Hiltunen, M.; Uronen, R.-L.; Huttunen, H.J. FRMD4A–cytohesin signaling modulates the cellular release of tau. J. Cell Sci. 2016, 129, 2003–2015. [Google Scholar] [CrossRef]
- Malone, S.M.; Burwell, S.J.; Vaidyanathan, U.; Miller, M.B.; Mcgue, M.; Iacono, W.G. Heritability and molecular-genetic basis of resting EEG activity: A genome-wide association study: Genome-wide association study of resting EEG. Psychophysiology 2014, 51, 1225–1245. [Google Scholar] [CrossRef]
- Van Luijn, M.M.; Kreft, K.L.; Jongsma, M.L.; Mes, S.W.; Wierenga-Wolf, A.F.; van Meurs, M.; Melief, M.-J.; van der Kant, R.; Janssen, L.; Janssen, H.; et al. Multiple sclerosis-associated CLEC16A controls HLA class II expression via late endosome biogenesis. Brain 2015, 138, 1531–1547. [Google Scholar] [CrossRef]
- Radford, R.A.; Morsch, M.; Rayner, S.L.; Cole, N.J.; Pountney, D.L.; Chung, R.S. The established and emerging roles of astrocytes and microglia in amyotrophic lateral sclerosis and frontotemporal dementia. Front. Cell. Neurosci. 2015, 9. [Google Scholar] [CrossRef]
- Gong, N.; Li, Y.; Cai, G.-Q.; Niu, R.-F.; Fang, Q.; Wu, K.; Chen, Z.; Lin, L.-N.; Xu, L.; Fei, J.; et al. GABA Transporter-1 Activity Modulates Hippocampal Theta Oscillation and Theta Burst Stimulation-Induced Long-Term Potentiation. J. Neurosci. 2009, 29, 15836–15845. [Google Scholar] [CrossRef]
- Van Welie, I.; Smith, I.T.; Watt, A.J. The metamorphosis of the developing cerebellar microcircuit. Curr. Opin. Neurobiol. 2011, 21, 245–253. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Du, X.; Rowland, L.M.; Summerfelt, A.; Choa, F.-S.; Wittenberg, G.F.; Wisner, K.; Wijtenburg, A.; Chiappelli, J.; Kochunov, P.; Hong, L.E. Cerebellar-Stimulation Evoked Prefrontal Electrical Synchrony Is Modulated by GABA. Cerebellum 2018, 17, 550–563. [Google Scholar] [CrossRef] [PubMed]
- Casula, E.P.; Pellicciari, M.C.; Ponzo, V.; Stampanoni Bassi, M.; Veniero, D.; Caltagirone, C.; Koch, G. Cerebellar theta burst stimulation modulates the neural activity of interconnected parietal and motor areas. Sci. Rep. 2016, 6, 36191. [Google Scholar] [CrossRef]
- Redmann, V.; Lamb, C.A.; Hwang, S.; Orchard, R.C.; Kim, S.; Razi, M.; Milam, A.; Park, S.; Yokoyama, C.C.; Kambal, A.; et al. Clec16a is Critical for Autolysosome Function and Purkinje Cell Survival. Sci. Rep. 2016, 6, 23326. [Google Scholar] [CrossRef] [PubMed]
- Bruce, H.A.; Margolis, R.L. FOXP2: Novel exons, splice variants, and CAG repeat length stability. Hum. Genet. 2002, 111, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Ikenouchi, J.; Umeda, M. FRMD4A regulates epithelial polarity by connecting Arf6 activation with the PAR complex. Proc. Natl. Acad. Sci. USA 2010, 107, 748–753. [Google Scholar] [CrossRef] [PubMed]
- Alarcón, M.; Abrahams, B.S.; Stone, J.L.; Duvall, J.A.; Perederiy, J.V.; Bomar, J.M.; Sebat, J.; Wigler, M.; Martin, C.L.; Ledbetter, D.H.; et al. Linkage, Association, and Gene-Expression Analyses Identify CNTNAP2 as an Autism-Susceptibility Gene. Am. J. Hum. Genet. 2008, 82, 150–159. [Google Scholar] [CrossRef]
- Friedman, J.I.; Vrijenhoek, T.; Markx, S.; Janssen, I.M.; van der Vliet, W.A.; Faas, B.H.W.; Knoers, N.V.; Cahn, W.; Kahn, R.S.; Edelmann, L.; et al. CNTNAP2 gene dosage variation is associated with schizophrenia and epilepsy. Mol. Psychiatry 2008, 13, 261–266. [Google Scholar] [CrossRef]
- Rodenas-Cuadrado, P.M.; Mengede, J.; Baas, L.; Devanna, P.; Schmid, T.A.; Yartsev, M.; Firzlaff, U.; Vernes, S.C. Mapping the distribution of language related genes FoxP1, FoxP2, and CntnaP2 in the brains of vocal learning bat species. J. Comp. Neurol. 2018, 526, 1235–1266. [Google Scholar] [CrossRef]
- Whitehouse, A.J.O.; Bishop, D.V.M.; Ang, Q.W.; Pennell, C.E.; Fisher, S.E. CNTNAP2 variants affect early language development in the general population. Genes Brain Behav. 2011, 10, 451–456. [Google Scholar] [CrossRef]
- Peñagarikano, O.; Abrahams, B.S.; Herman, E.I.; Winden, K.D.; Gdalyahu, A.; Dong, H.; Sonnenblick, L.I.; Gruver, R.; Almajano, J.; Bragin, A.; et al. Absence of CNTNAP2 Leads to Epilepsy, Neuronal Migration Abnormalities, and Core Autism-Related Deficits. Cell 2011, 147, 235–246. [Google Scholar] [CrossRef]
- Fine, D.; Flusser, H.; Markus, B.; Shorer, Z.; Gradstein, L.; Khateeb, S.; Langer, Y.; Narkis, G.; Birk, R.; Galil, A.; et al. A syndrome of congenital microcephaly, intellectual disability and dysmorphism with a homozygous mutation in FRMD4A. Eur. J. Hum. Genet. 2015, 23, 1729–1734. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Du, R.; Zhou, J.; Lorenzano, S.; Liu, W.; Charoenvimolphan, N.; Qian, B.; Xu, J.; Wang, J.; Zhang, X.; Wang, X.; et al. Integrative Mouse and Human Studies Implicate ANGPT1 and ZBTB7C as Susceptibility Genes to Ischemic Injury. Stroke 2015, 46, 3514–3522. [Google Scholar] [CrossRef] [PubMed]
- Haliloglu, G.; Jobard, F.; Oguz, K.; Anlar, B.; Akalan, N.; Coskun, T.; Sass, J.; Fischer, J.; Topcu, M. L-2-Hydroxyglutaric Aciduria and Brain Tumors in Children with Mutations in the L2HGDH Gene: Neuroimaging Findings. Neuropediatrics 2008, 39, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Sun, R.; Jiang, B.; Gao, J.; Deng, W.; Liu, P.; He, R.; Cui, J.; Ji, M.; Yi, W.; et al. L2hgdh Deficiency Accumulates l-2-Hydroxyglutarate with Progressive Leukoencephalopathy and Neurodegeneration. Mol. Cell. Biol. 2017, 37, e00492-16. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Lazar, M.A. Clocks, Metabolism, and the Epigenome. Mol. Cell 2012, 47, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Cacioppo, S.; Bangee, M.; Balogh, S.; Cardenas-Iniguez, C.; Qualter, P.; Cacioppo, J.T. Loneliness and implicit attention to social threat: A high-performance electrical neuroimaging study. Cogn. Neurosci. 2016, 7, 138–159. [Google Scholar] [CrossRef]
- Ceballos, N.A.; Bauer, L.O.; Houston, R.J. Recent EEG and ERP Findings in Substance Abusers. Clin. EEG Neurosci. 2009, 40, 122–128. [Google Scholar] [CrossRef]
- Clarke, A.R.; Barry, R.J.; Indraratna, A.; Dupuy, F.E.; McCarthy, R.; Selikowitz, M. EEG activity in children with Asperger’s Syndrome. Clin. Neurophysiol. 2016, 127, 442–451. [Google Scholar] [CrossRef]
- Dvey-Aharon, Z.; Fogelson, N.; Peled, A.; Intrator, N. Schizophrenia Detection and Classification by Advanced Analysis of EEG Recordings Using a Single Electrode Approach. PLoS ONE 2015, 10, e0123033. [Google Scholar] [CrossRef]
- Jamal, W.; Das, S.; Maharatna, K.; Pan, I.; Kuyucu, D. Brain connectivity analysis from EEG signals using stable phase-synchronized states during face perception tasks. Phys. A Stat. Mech. Appl. 2015, 434, 273–295. [Google Scholar] [CrossRef]
- Putilov, A.A.; Donskaya, O.G.; Verevkin, E.G.; Putilov, D.A. Associations of waking EEG structure with chronotype and trototype of 130 sleep deprived individuals. Biol. Rhythm Res. 2010, 41, 113–136. [Google Scholar] [CrossRef]
- Håvik, B.; Le Hellard, S.; Rietschel, M.; Lybæk, H.; Djurovic, S.; Mattheisen, M.; Mühleisen, T.W.; Degenhardt, F.; Priebe, L.; Maier, W.; et al. The Complement Control-Related Genes CSMD1 and CSMD2 Associate to Schizophrenia. Biol. Psychiatry 2011, 70, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Steen, V.M.; Nepal, C.; Ersland, K.M.; Holdhus, R.; Nævdal, M.; Ratvik, S.M.; Skrede, S.; Håvik, B. Neuropsychological Deficits in Mice Depleted of the Schizophrenia Susceptibility Gene CSMD1. PLoS ONE 2013, 8, e79501. [Google Scholar] [CrossRef] [PubMed]
- Bauß, K.; Knapp, B.; Jores, P.; Roepman, R.; Kremer, H.; v. Wijk, E.; Märker, T.; Wolfrum, U. Phosphorylation of the Usher syndrome 1G protein SANS controls Magi2-mediated endocytosis. Hum. Mol. Genet. 2014, 23, 3923–3942. [Google Scholar] [CrossRef]
- Koide, T.; Banno, M.; Aleksic, B.; Yamashita, S.; Kikuchi, T.; Kohmura, K.; Adachi, Y.; Kawano, N.; Kushima, I.; Nakamura, Y.; et al. Common Variants in MAGI2 Gene Are Associated with Increased Risk for Cognitive Impairment in Schizophrenic Patients. PLoS ONE 2012, 7, e36836. [Google Scholar] [CrossRef]
- Musiek, E.S. Circadian clock disruption in neurodegenerative diseases: Cause and effect? Front. Pharmacol. 2015, 6. [Google Scholar] [CrossRef] [PubMed]

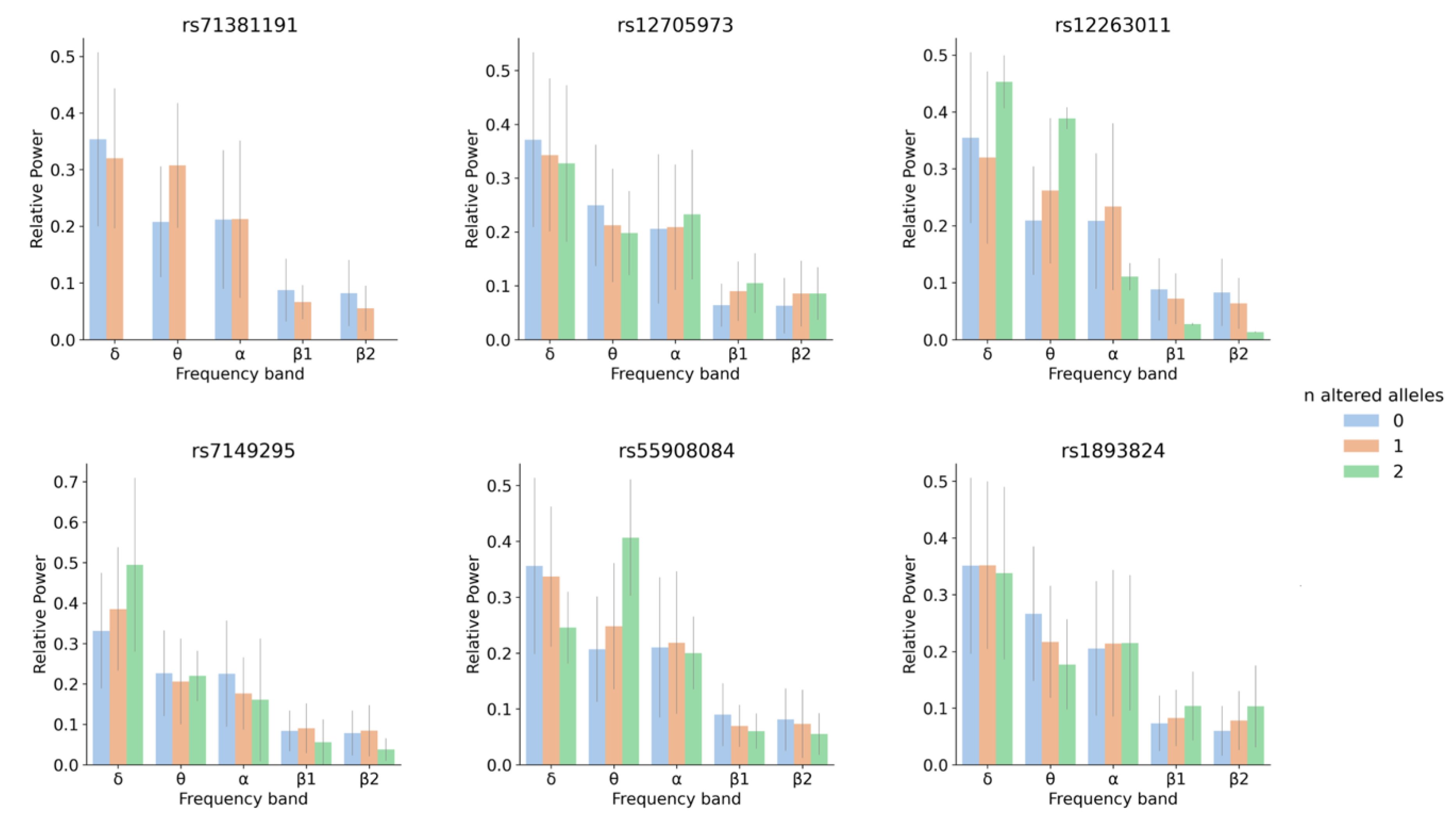
| rsID | p_δ | p_θ | p_α | p_β1 | p_β2 | b1 |
|---|---|---|---|---|---|---|
| rs7516534 | 2.76 × 10−6 | −0.08 | ||||
| rs12720066 | 1.39 × 10−6 | 0.10 | ||||
| rs12705973 | 2.65 × 10−6 | 0.02 | ||||
| rs10108126 | 1.97 × 10−6 | 0.05 | ||||
| rs10104429 | 1.55 × 10−7 | 0.05 | ||||
| rs7125249 | 3.70 × 10−6 | 0.03 | ||||
| rs6692346 | 2.32 × 10−6 | 0.04 | ||||
| rs4658030 | 2.04 × 10−6 | −0.03 | ||||
| rs77599684 | 9.17 × 10−7 | 0.05 | ||||
| rs6106856 | 2.21 × 10−6 | 0.03 | ||||
| rs7149295 | 4.88 × 10−6 | 0.09 | ||||
| rs12263011 | 4.98 × 10−6 | 0.07 | ||||
| rs71381191 | 2.64 × 10−8 1 | 0.11 | ||||
| rs12443654 | 4.19 × 10−6 | 0.07 | ||||
| rs9930193 | 4.57 × 10−6 | 0.07 | ||||
| rs55908084 | 2.65 × 10−6 | 0.06 | ||||
| rs9960516 2 | 3.09 × 10−6 | 0.05 | ||||
| rs72919581 2 | 1.82 × 10−6 | 0.06 | ||||
| rs1893824 | 1.66 × 10−6 | −0.05 |
| Locus | p | Sig_SNPs | Gene | Type | eqtlMapts | ciMapts |
|---|---|---|---|---|---|---|
| 1:31318133:C:T | 2.76 × 10−6 | rs7516534 | LAPTM5 | p_coding | Adult_Cortex | |
| RN7SKP91 | misc RNA | |||||
| RP1-65J11.5 | antisense | |||||
| 1:192427329:C:G | 2.04 × 10−6 | rs4658030 | ||||
| 7:87169702:A:C | 1.39 × 10−6 | rs12720066 | ||||
| 7:114313199:A:G | 2.65 × 10−6 | rs12705973 | FOXP2 | p_coding | Brain_Caudate_basal_ganglia | Fetal_Cortex: Neural_Progen_Cell |
| AC073626.2 | antisense | Fetal_Cortex: Neural_Progen_Cell | ||||
| MIR3666 | miRNA | |||||
| 8:74257947:A:G | 1.55 × 10−7 | rs10104429 | ||||
| rs10108126 | ||||||
| 10:13865505:A:C | 4.98 × 10−6 | rs12263011 | PRPF18 | p_coding | Fetal_Cortex | |
| FRMD4A | p_coding | |||||
| CDNF | p_coding | Adult_Cortex: Fetal_Cortex | ||||
| HSPA14 | p_coding | Adult_Cortex: Fetal_Cortex | ||||
| RP11-398C13.6 | lincRNA | Fetal_Cortex | ||||
| SUV39H2 | p_coding | Fetal_Cortex | ||||
| 11:45062339:G:T | 3.70 × 10−6 | rs7125249 | ||||
| 14:51283148:C:T | 4.88 × 10−6 | rs7149295 | L2HGDH | p_coding | Fetal_Cortex | |
| ATP5S | p_coding | Fetal_Cortex | ||||
| NIN | p_coding | Adult_Cortex: Hippocampus | ||||
| RP11-286O18.1 | antisense | |||||
| PYGL | p_coding | Adult_Cortex | ||||
| 16:11156812:A:G | 2.64 × 10−8 | rs71381191 | CLEC16A | p_coding | ||
| RPL7P46 | pseudogene | |||||
| RP11-66H6.3 | antisense | |||||
| 16:19375297:C:T | 4.19 × 10−6 | rs12443654 | RPS15A | p_coding | Brain_Spinal_cord_cervical_c-1 | |
| rs9930193 | CTA-363E6.2 | lincRNA | ||||
| 17:65034162:C:G | 2.65 × 10−6 | rs55908084 | CACNG4 | p_coding | ||
| AC005544.1 | p_coding | |||||
| RP11-74H8.1 | antisense | |||||
| 18:21849024:A:G | 9.17 × 10−7 | rs77599684 | OSBPL1A | p_coding | ||
| RN7SL247P | misc_RNA | |||||
| 18:45885064:C:T | 1.82 × 10−6 | rs72919581 | ZBTB7C | p_coding | ||
| rs9960516 | ||||||
| 18:74959125:G:T | 1.66 × 10−6 | rs1893824 | RP11-17M16.2 | antisense | Brain_Hypothalamus | |
| GALR1 | p_coding | Brain_Anterior_cingulate_cortex_BA24 | ||||
| 20:24313473:A:G | 2.21 × 10−6 | rs6106856 |
| Gene | nSNPs | p-Value |
|---|---|---|
| CSMD1 | 35 | 3.01 × 10−16 |
| CDH13 | 27 | 4.85 × 10−14 |
| PTPRD | 21 | 7.89 × 10−14 |
| SORCS2 | 21 | 1.23 × 10−13 |
| RBFOX1 | 20 | 2.05 × 10−13 |
| MACROD2 | 20 | 1.39 × 10−12 |
| ZBTB7C | 12 | 2.29 × 10−12 |
| RBFOX3 | 18 | 6.64 × 10−12 |
| RUNX1 | 14 | 1.17 × 10−11 |
| WWOX | 23 | 2.39 × 10−11 |
| FRMD4A | 14 | 9.68 × 10−11 |
| PLCB1 | 13 | 1.08 × 10−10 |
| LRP1B | 12 | 1.55 × 10−10 |
| OPCML | 11 | 2.74 × 10−10 |
| CAMK1D | 13 | 3.29 × 10−10 |
| FHIT | 13 | 3.97 × 10−10 |
| PDZD2 | 13 | 4.84 × 10−10 |
| CNTN4 | 14 | 5.03 × 10−10 |
| CDH23 | 8 | 7.60 × 10−10 |
| LPP | 10 | 8.21 × 10−10 |
| MYO16 | 11 | 8.79 × 10−10 |
| CNTNAP2 | 12 | 8.92 × 10−10 |
| KDM4C | 12 | 1.06 × 10−9 |
| NTM | 11 | 1.09 × 10−9 |
| ASIC2 | 9 | 1.13 × 10−9 |
| RYR3 | 12 | 1.22 × 10−9 |
| RPA3-AS1 | 13 | 1.39 × 10−9 |
| OFCC1 | 10 | 1.41 × 10−9 |
| DSCAM | 12 | 1.46 × 10−9 |
| TMEM132C | 12 | 1.53 × 10−9 |
| MTUS2 | 11 | 1.98 × 10−9 |
| SLC9A9 | 10 | 2.12 × 10−9 |
| LDLRAD4 | 11 | 3.99 × 10−9 |
| FSTL5 | 11 | 4.06 × 10−9 |
| RBMS3 | 10 | 4.79 × 10−9 |
| TENM4 | 13 | 7.66 × 10−9 |
| NELL1 | 10 | 1.06 × 10−8 |
| TENM3 | 8 | 1.06 × 10−8 |
| PRKG1 | 11 | 1.06 × 10−8 |
| GRM7 | 8 | 1.19 × 10−8 |
| HS3ST4 | 8 | 1.46 × 10−8 |
| CNTN5 | 10 | 1.46 × 10−8 |
| TUSC3 | 14 | 1.60 × 10−8 |
| NRXN3 | 7 | 1.86 × 10−8 |
| DPP6 | 11 | 1.92 × 10−8 |
| UNC5C | 9 | 2.06 × 10−8 |
| MAGI2 | 10 | 2.08 × 10−8 |
| ADARB2 | 8 | 2.27 × 10−8 |
| RIMBP2 | 7 | 2.53 × 10−8 |
| GPC5 | 7 | 2.59 × 10−8 |
| GeneSet | N | n | p-Value | Adjusted p-Value | Genes |
|---|---|---|---|---|---|
| Chronotype | 556 | 13 | 7.05 × 10−13 | 6.39 × 10−10 | CNTN5, GPC5, MYO16, NRXN3, RBFOX1, ASIC2, MACROD2, CNTN4, GRM7, FSTL5, MAGI2, CSMD1, PTPRD |
| Intracranial aneurysm | 72 | 6 | 8.85 × 10−10 | 3.97 × 10−7 | RBFOX1, DSCAM, RBMS3, FHIT, PDZD2, PTPRD |
| Response to amphetamines | 33 | 5 | 1.09 × 10−9 | 3.97 × 10−7 | NELL1, WWOX, CDH13, FHIT, TENM3 |
| Schizophrenia | 827 | 12 | 1.43 × 10−9 | 4.31 × 10−7 | PRKG1, NELL1, NRXN3, RYR3, CDH13, CNTN4, RBMS3, FHIT, TENM3, MAGI2, CSMD1, KDM4C |
| Amyotrophic lateral sclerosis (sporadic) | 164 | 7 | 3.58 × 10−9 | 8.76 × 10−7 | CNTN5, OPCML, RYR3, ASIC2, MACROD2, DSCAM, CSMD1 |
| Night sleep phenotypes | 538 | 10 | 3.86 × 10−9 | 8.76 × 10−7 | TENM4, CDH13, LRP1B, CNTN4, GRM7, RBMS3, SLC9A9, OFCC1, MAGI2, CSMD1 |
| Cognitive ability, years of educational attainment or schizophrenia (pleiotropy) | 197 | 6 | 3.72 × 10−7 | 4.22 × 10−5 | CAMK1D, NTM, GPC5, CDH13, LRP1B, CNTN4 |
| Loneliness (multivariate analysis) | 29 | 3 | 9.65 × 10−6 | 7.00 × 10−4 | PRKG1, CNTN5, PTPRD |
| Asperger disorder | 6 | 2 | 2.97 × 10−5 | 1.54 × 10−3 | NTM, FHIT |
| Middle childhood and early adolescence aggressive behavior | 6 | 2 | 2.97 × 10−5 | 1.54 × 10−3 | OPCML, CNTN4 |
| Daytime sleep phenotypes | 259 | 5 | 3.38 × 10−5 | 1.66 × 10−3 | WWOX, CDH13, PLCB1, CNTN4, GRM7 |
| Brain connectivity | 7 | 2 | 4.15 × 10−5 | 1.88 × 10−3 | MACROD2, CNTN4 |
| Short-term memory (digit-span task) | 7 | 2 | 4.15 × 10−5 | 1.88 × 10−3 | CDH13, PTPRD |
| Dimensional psychopathology (Negative) | 9 | 2 | 7.10 × 10−5 | 2.53 × 10−3 | CDH13, MACROD2 |
| Hippocampal sclerosis | 9 | 2 | 7.10 × 10−5 | 2.53 × 10−3 | NELL1, SORCS2 |
| Bipolar disorder (body mass index interaction) | 10 | 2 | 8.86 × 10−5 | 2.87 × 10−3 | CDH23, WWOX |
| Aggressiveness in attention deficit hyperactivity disorder | 11 | 2 | 1.08 × 10−4 | 3.33 × 10−3 | NTM, CSMD1 |
| Alzheimer’s disease (64°) | 70 | 3 | 1.39 × 10−4 | 3.93 × 10−3 | FRMD4A, MYO16, CNTNAP2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rebelo, M.Â.; Gómez, C.; Gomes, I.; Poza, J.; Martins, S.; Maturana-Candelas, A.; Ruiz-Gómez, S.J.; Durães, L.; Sousa, P.; Figueruelo, M.; et al. Genome-Wide Scan for Five Brain Oscillatory Phenotypes Identifies a New QTL Associated with Theta EEG Band. Brain Sci. 2020, 10, 870. https://doi.org/10.3390/brainsci10110870
Rebelo MÂ, Gómez C, Gomes I, Poza J, Martins S, Maturana-Candelas A, Ruiz-Gómez SJ, Durães L, Sousa P, Figueruelo M, et al. Genome-Wide Scan for Five Brain Oscillatory Phenotypes Identifies a New QTL Associated with Theta EEG Band. Brain Sciences. 2020; 10(11):870. https://doi.org/10.3390/brainsci10110870
Chicago/Turabian StyleRebelo, Miguel Ângelo, Carlos Gómez, Iva Gomes, Jesús Poza, Sandra Martins, Aarón Maturana-Candelas, Saúl J. Ruiz-Gómez, Luis Durães, Patrícia Sousa, Manuel Figueruelo, and et al. 2020. "Genome-Wide Scan for Five Brain Oscillatory Phenotypes Identifies a New QTL Associated with Theta EEG Band" Brain Sciences 10, no. 11: 870. https://doi.org/10.3390/brainsci10110870
APA StyleRebelo, M. Â., Gómez, C., Gomes, I., Poza, J., Martins, S., Maturana-Candelas, A., Ruiz-Gómez, S. J., Durães, L., Sousa, P., Figueruelo, M., Rodríguez, M., Pita, C., Arenas, M., Álvarez, L., Hornero, R., Pinto, N., & Lopes, A. M. (2020). Genome-Wide Scan for Five Brain Oscillatory Phenotypes Identifies a New QTL Associated with Theta EEG Band. Brain Sciences, 10(11), 870. https://doi.org/10.3390/brainsci10110870