Auditory Brainstem Responses (ABR) of Rats during Experimentally Induced Tinnitus: Literature Review
Abstract
:1. Introduction
2. Materials and Methods
- Science Direct
- The Search Engine Tool for Scientific (Scopus)
- US National Library of Medicine National Institutes of Health (PubMed)
- Web of Science
- “auditory evoked potential” AND “tinnitus” AND “rat”
- “auditory brainstem response” AND “tinnitus” AND “rat”
- “ototoxicity” AND “tinnitus” AND “rat”
- Articles published between January 2000 and August 2020
- Research dedicated to an animal model of tinnitus induced by salicylate administration (When in addition to salicylate, other drugs were applied, only the data related to salicylate were acquired), blast or noise exposure, when the authors used the ABR to measure auditory abilities of animals
- Using rats
- Original research
- Literature review, editorials
- Full text not available
- Articles not published in English.
- Aim of the article
- Age, sex, and strain of rats
- Sample size
- Methods used to induce tinnitus (salicylate, noise, blast)
- Methods used to determine the presence of tinnitus
- Stimulus and acquisition characteristics of ABR
- The system used to measure ABR
- Signal intensity
- Rate of signal
- Polarity of signal
- The placement of the electrodes
- Filters
- ABR protocols
- ABR outcome.
3. Results
3.1. Study Selection
3.2. Strain, Gender, and Age
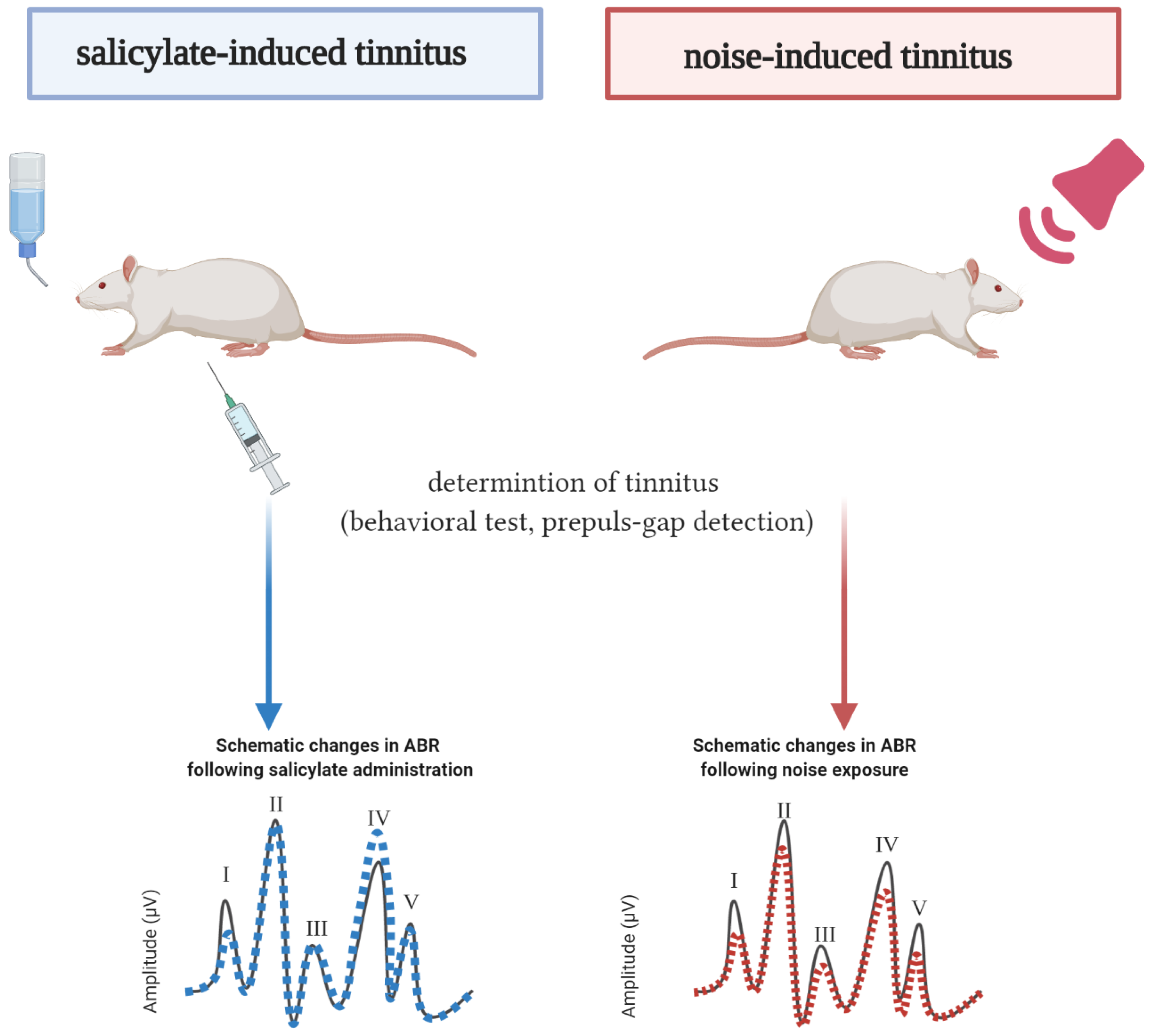
3.3. Methods Used for Tinnitus Induction
3.4. Methods Used to Determine the Presence of Tinnitus
3.5. Salicylate-Induced Tinnitus
3.6. Noise-Induced Tinnitus
The Noise Characteristics
3.7. Methods of ABR Measurement
3.7.1. ABR Recording Systems
3.7.2. The Stimulus Signal
3.7.3. Signal to Noise Ratio and Thresholds
3.7.4. Electrode Placement
3.7.5. Filters and Polarity
3.8. Protocols Used for ABR Recordings
3.8.1. Anesthesia
3.8.2. Additional Information
3.9. ABR Evaluation after Salicylate Treatment
3.9.1. Hearing Thresholds after Salicylate Treatment
3.9.2. Effects of Salicylate on ABR Amplitudes and Latencies
3.10. ABR Evaluation after Noise Exposure
3.10.1. Hearing Thresholds after Noise Exposure
3.10.2. Effects of Noise on ABR Amplitudes and Latencies
4. Discussion
5. Future Directions
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Dawes, P.; Fortnum, H.; Moore, D.R.; Emsley, R.; Norman, P.; Cruickshanks, K.; Davis, A.; Edmondson-Jones, M.; McCormack, A.; Lutman, M.; et al. Hearing in middle age: A population snapshot of 40- to 69-year olds in the United Kingdom. Ear Hear. 2014, 35, e44–e51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCormack, A.; Edmondson-Jones, M.; Fortnum, H.; Dawes, P.D.; Middleton, H.; Munro, K.J.; Moore, D.R. Investigating the association between tinnitus severity and symptoms of depression and anxiety, while controlling for neuroticism, in a large middle-aged UK population. Int. J. Audiol. 2015, 54, 599–604. [Google Scholar] [CrossRef] [PubMed]
- Makar, S.K.; Mukundan, G.; Gore, G. Treatment of Tinnitus: A Scoping Review. Int. Tinnitus J. 2017, 21, 144–156. [Google Scholar] [CrossRef] [PubMed]
- Henry, J.A.; Dennis, K.C.; Schechter, M.A. General review of tinnitus: Prevalence, mechanisms, effects, and management. J. Speech Lang. Hear. Res. 2005, 48, 1204–1235. [Google Scholar] [CrossRef]
- Nondahl, D.M.; Cruickshanks, K.J.; Dalton, D.S.; Klein, B.E.; Klein, R.; Schubert, C.R.; Tweed, T.S.; Wiley, T.L. The impact of tinnitus on quality of life in older adults. J. Am. Acad. Audiol. 2007, 18, 257–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Møller, A.R. Pathophysiology of tinnitus. Otolaryngol. Clin. N. Am. 2003, 36, 249–266. [Google Scholar] [CrossRef]
- Mazurek, B.; Olze, H.; Haupt, H.; Szczepek, A.J. The more the worse: The grade of noise-induced hearing loss associates with the severity of tinnitus. Int. J. Environ. Res. Public Health 2010, 7, 3071–3079. [Google Scholar] [CrossRef] [Green Version]
- Jastreboff, P.J. Tinnitus retraining therapy. In Progress in Brain Research; Langguth, B., Hajak, G., Kleinjung, T., Cacace, A., Møller, A.R., Eds.; Elsevier: Amsterdam, Netherlands, 2007; Volume 166, pp. 415–423. [Google Scholar]
- Eggermont, J.J. Can Animal Models Contribute to Understanding Tinnitus Heterogeneity in Humans? Front. Aging Neurosci. 2016, 8, 265. [Google Scholar] [CrossRef] [Green Version]
- Jastreboff, P.J.; Brennan, J.F.; Coleman, J.K.; Sasaki, C.T. Phantom auditory sensation in rats: An animal model for tinnitus. Behav. Neurosci. 1988, 102, 811–822. [Google Scholar] [CrossRef]
- Puel, J.L.; Guitton, M.J. Salicylate-induced tinnitus: Molecular mechanisms and modulation by anxiety. Prog. Brain Res. 2007, 166, 141–146. [Google Scholar] [CrossRef]
- Jastreboff, P.J.; Sasaki, C.T. An animal model of tinnitus: A decade of development. Am. J. Otol. 1994, 15, 19–27. [Google Scholar]
- Ralli, M.; Lobarinas, E.; Fetoni, A.R.; Stolzberg, D.; Paludetti, G.; Salvi, R. Comparison of salicylate- and quinine-induced tinnitus in rats: Development, time course, and evaluation of audiologic correlates. Otol. Neurotol. 2010, 31, 823–831. [Google Scholar] [CrossRef] [Green Version]
- Knipper, M.; Van Dijk, P.; Nunes, I.; Rüttiger, L.; Zimmermann, U. Advances in the neurobiology of hearing disorders: Recent developments regarding the basis of tinnitus and hyperacusis. Prog. Neurobiol. 2013, 111, 17–33. [Google Scholar] [CrossRef]
- Bing, D.; Lee, S.C.; Campanelli, D.; Xiong, H.; Matsumoto, M.; Panford-Walsh, R.; Wolpert, S.; Praetorius, M.; Zimmermann, U.; Chu, H.; et al. Cochlear NMDA receptors as a therapeutic target of noise-induced tinnitus. Cell. Physiol. Biochem. 2015, 35, 1905–1923. [Google Scholar] [CrossRef] [PubMed]
- Eggermont, J.J.; Roberts, L.E. The neuroscience of tinnitus. Trends Neurosci. 2004, 27, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Milloy, V.; Fournier, P.; Benoit, D.; Noreña, A.; Koravand, A. Auditory Brainstem Responses in Tinnitus: A Review of Who, How, and What? Front. Aging Neurosci. 2017, 9, 237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciorba, A.; Hatzopoulos, S.; Corazzi, V.; Cogliandolo, C.; Aimoni, C.; Bianchini, C.; Stomeo, F.; Pelucchi, S. Newborn hearing screening at the Neonatal Intensive Care Unit and Auditory Brainstem Maturation in preterm infants. Int. J. Pediatr. Otorhinolaryngol. 2019, 123, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Markand, O.N. Brainstem auditory evoked potentials. J. Clin. Neurophysiol. 1994, 11, 319–342. [Google Scholar] [CrossRef]
- Rupa, V.; Job, A.; George, M.; Rajshekhar, V. Cost-effective initial screening for vestibular schwannoma: Auditory brainstem response or magnetic resonance imaging? Otolaryngol. Head Neck Surg. 2003, 128, 823–828. [Google Scholar] [CrossRef]
- Edwards, M.S.; Gordon, D.G.; Levin, V.A. Evaluation of desmethylmisonidazole-induced neurotoxicity in the rat using brainstem auditory evoked potentials. Int. J. Radiat. Oncol. Biol. Phys. 1984, 10, 1377–1379. [Google Scholar] [CrossRef]
- Cai, R.; Montgomery, S.C.; Graves, K.A.; Caspary, D.M.; Cox, B.C. The FBN rat model of aging: Investigation of ABR waveforms and ribbon synapse changes. Neurobiol. Aging 2018, 62, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Alvarado, J.C.; Fuentes-Santamaría, V.; Jareño-Flores, T.; Blanco, J.L.; Juiz, J.M. Normal variations in the morphology of auditory brainstem response (ABR) waveforms: A study in Wistar rats. Neurosci. Res. 2012, 73, 302–311. [Google Scholar] [CrossRef]
- Blatchley, B.J.; Cooper, W.A.; Coleman, J.R. Development of auditory brainstem response to tone pip stimuli in the rat. Brain Res. 1987, 429, 75–84. [Google Scholar] [CrossRef]
- Szczepek, A.J.; Dietz, G.P.H.; Reich, U.; Hegend, O.; Olze, H.; Mazurek, B. Differences in Stress-Induced Modulation of the Auditory System Between Wistar and Lewis Rats. Front. Neurosci. 2018, 12. [Google Scholar] [CrossRef] [PubMed]
- Fabiani, M.; Sohmer, H.; Tait, C.; Gafni, M.; Kinarti, R. A functional measure of brain activity: Brain stem transmission time. Electroencephalogr. Clin. Neurophysiol. 1979, 47, 483–491. [Google Scholar] [CrossRef]
- Zhang, W.; Peng, Z.; Yu, S.K.; Song, Q.L.; Qu, T.F.; He, L.; Liu, K.; Gong, S.S. Loss of Cochlear Ribbon Synapse Is a Critical Contributor to Chronic Salicylate Sodium Treatment-Induced Tinnitus without Change Hearing Threshold. Neural Plast. 2020, 2020. [Google Scholar] [CrossRef] [PubMed]
- Castañeda, R.; Natarajan, S.; Jeong, S.Y.; Hong, B.N.; Kang, T.H. Electrophysiological changes in auditory evoked potentials in rats with salicylate-induced tinnitus. Brain Res. 2019, 1715, 235–244. [Google Scholar] [CrossRef]
- Liu, X.P.; Chen, L. Auditory brainstem response as a possible objective indicator for salicylate-induced tinnitus in rats. Brain Res. 2012, 1485, 88–94. [Google Scholar] [CrossRef]
- Albuquerque, A.A.S.; Rossato, M.; de Oliveira, J.A.A.; Hyppolito, M.A. Understanding the anatomy of ears from guinea pigs and rats and its use in basic otologic research. Braz. J. Otorhinolaryngol. 2009, 75, 43–49. [Google Scholar] [CrossRef] [Green Version]
- Heffner, H.E.; Heffner, R.S. Hearing ranges of laboratory animals. J. Am. Assoc. Lab. Anim. Sci. 2007, 46, 20–22. [Google Scholar]
- Holt, A.G.; Kühl, A.; Braun, R.D.; Altschuler, R. The rat as a model for studying noise injury and otoprotection. J. Acoust. Soc. Am. 2019, 146, 3681. [Google Scholar] [CrossRef] [PubMed]
- Willott, J.F. Factors affecting hearing in mice, rats, and other laboratory animals. J. Am. Assoc. Lab. Anim. Sci. 2007, 46, 23–27. [Google Scholar] [PubMed]
- Reijntjes, D.O.J.; Pyott, S.J. The afferent signaling complex: Regulation of type I spiral ganglion neuron responses in the auditory periphery. Hear. Res. 2016, 336, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guitton, M.J. Tinnitus-provoking salicylate treatment triggers social impairments in mice. J. Psychosom. Res. 2009, 67, 273–276. [Google Scholar] [CrossRef]
- Escabi, C.D.; Frye, M.D.; Trevino, M.; Lobarinas, E. The rat animal model for noise-induced hearing loss. J. Acoust. Soc. Am. 2019, 146, 3692. [Google Scholar] [CrossRef] [Green Version]
- Lobarinas, E.; Spankovich, C.; Le Prell, C.G. Evidence of “hidden hearing loss” following noise exposures that produce robust TTS and ABR wave-I amplitude reductions. Hear. Res. 2017, 349, 155–163. [Google Scholar] [CrossRef]
- von der Behrens, W. Animal models of subjective tinnitus. Neural Plast. 2014, 2014, 741452. [Google Scholar] [CrossRef] [Green Version]
- Turner, J.G.; Parrish, J.L.; Hughes, L.F.; Toth, L.A.; Caspary, D.M. Hearing in laboratory animals: Strain differences and nonauditory effects of noise. Comp. Med. 2005, 55, 12–23. [Google Scholar]
- Cederroth, C.R.; Dyhrfjeld-Johnsen, J.; Langguth, B. An update: Emerging drugs for tinnitus. Expert Opin. Emerg. Drugs 2018, 23, 251–260. [Google Scholar] [CrossRef]
- Mazurek, B.; Haupt, H.; Joachim, R.; Klapp, B.F.; Stöver, T.; Szczepek, A.J. Stress induces transient auditory hypersensitivity in rats. Hear. Res. 2010, 259, 55–63. [Google Scholar] [CrossRef]
- Mazurek, B.; Haupt, H.; Klapp, B.F.; Szczepek, A.J.; Olze, H. Exposure of Wistar rats to 24-h psycho-social stress alters gene expression in the inferior colliculus. Neurosci. Lett. 2012, 527, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The, P.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laundrie, E.; Sun, W. Acoustic trauma-induced auditory cortex enhancement and tinnitus. J. Otol. 2014, 9, 111–116. [Google Scholar] [CrossRef] [Green Version]
- Ropp, T.J.; Tiedemann, K.L.; Young, E.D.; May, B.J. Effects of unilateral acoustic trauma on tinnitus-related spontaneous activity in the inferior colliculus. J. Assoc. Res. Otolaryngol. 2014, 15, 1007–1022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahmood, G.; Mei, Z.; Hojjat, H.; Pace, E.; Kallakuri, S.; Zhang, J.S. Therapeutic effect of sildenafil on blast-induced tinnitus and auditory impairment. Neuroscience 2014, 269, 367–382. [Google Scholar] [CrossRef]
- Mao, J.C.; Pace, E.; Pierozynski, P.; Kou, Z.; Shen, Y.; VandeVord, P.; Haacke, E.M.; Zhang, X.; Zhang, J. Blast-induced tinnitus and hearing loss in rats: Behavioral and imaging assays. J. Neurotrauma 2012, 29, 430–444. [Google Scholar] [CrossRef] [Green Version]
- Ouyang, J.; Pace, E.; Lepczyk, L.; Kaufman, M.; Zhang, J.; Perrine, S.A.; Zhang, J. Blast-Induced Tinnitus and Elevated Central Auditory and Limbic Activity in Rats: A Manganese-Enhanced MRI and Behavioral Study. Sci. Rep. 2017, 7, 4852. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.D.; Kermany, M.H.; D’Elia, A.; Ralli, M.; Tanaka, C.; Bielefeld, E.C.; Ding, D.; Henderson, D.; Salvi, R. Too much of a good thing: Long-term treatment with salicylate strengthens outer hair cell function but impairs auditory neural activity. Hear. Res. 2010, 265, 63–69. [Google Scholar] [CrossRef] [Green Version]
- Duron, J.; Monconduit, L.; Avan, P. Auditory Brainstem Changes in Timing may Underlie Hyperacusis in a Salicylate-induced Acute Rat Model. Neuroscience 2020, 426, 129–140. [Google Scholar] [CrossRef]
- Jang, C.H.; Lee, S.; Park, I.Y.; Song, A.; Moon, C.; Cho, G.W. Memantine Attenuates Salicylate-induced Tinnitus Possibly by Reducing NR2B Expression in Auditory Cortex of Rat. Exp. Neurobiol. 2019, 28, 495–503. [Google Scholar] [CrossRef]
- Ralli, M.; Troiani, D.; Podda, M.V.; Paciello, F.; Eramo, S.L.; de Corso, E.; Salvi, R.; Paludetti, G.; Fetoni, A.R. The effect of the NMDA channel blocker memantine on salicylate-induced tinnitus in rats. Acta Otorhinolaryngol. Ital. 2014, 34, 198–204. [Google Scholar] [PubMed]
- Sawka, B.; Wei, S. The Effects of Salicylate on Auditory Evoked Potential Amplitwde from the Auditory Cortex and Auditory Brainstem. J. Otol. 2014, 9, 30–35. [Google Scholar] [CrossRef] [Green Version]
- Ahsan, S.F.; Luo, H.; Zhang, J.; Kim, E.; Xu, Y. An animal model of deep brain stimulation for treating tinnitus: A proof of concept study. Laryngoscope 2018, 128, 1213–1222. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.C.; Kim, H.; Kim, Y.S.; Jung, S.K.; Park, I.Y.; Choung, Y.H.; Jang, J.H. Objective Verification of Acute Tinnitus and Validation of Efficacy of Systemic Steroids in Rats. J. Korean Med. Sci. 2020, 35, e81. [Google Scholar] [CrossRef]
- van Zwieten, G.; Jahanshahi, A.; van Erp, M.L.; Temel, Y.; Stokroos, R.J.; Janssen, M.L.F.; Smit, J.V. Alleviation of Tinnitus With High-Frequency Stimulation of the Dorsal Cochlear Nucleus: A Rodent Study. Trends Hear. 2019, 23, 2331216519835080. [Google Scholar] [CrossRef] [Green Version]
- van Zwieten, G.; Janssen, M.L.F.; Smit, J.V.; Janssen, A.M.L.; Roet, M.; Jahanshahi, A.; Stokroos, R.J.; Temel, Y. Inhibition of Experimental Tinnitus With High Frequency Stimulation of the Rat Medial Geniculate Body. Neuromodulation 2019, 22, 416–424. [Google Scholar] [CrossRef] [Green Version]
- Fang, L.; Fu, Y.; Zhang, T.Y. Salicylate-Induced Hearing Loss Trigger Structural Synaptic Modifications in the Ventral Cochlear Nucleus of Rats via Medial Olivocochlear (MOC) Feedback Circuit. Neurochem. Res. 2016, 41, 1343–1353. [Google Scholar] [CrossRef]
- Zhang, W.; Peng, Z.; Yu, S.; Song, Q.L.; Qu, T.F.; Liu, K.; Gong, S.S. Exposure to sodium salicylate disrupts VGLUT3 expression in cochlear inner hair cells and contributes to tinnitus. Physiol. Res. 2020, 69, 181–190. [Google Scholar] [CrossRef]
- Liu, X.P.; Chen, L. Forward acoustic masking enhances the auditory brainstem response in a diotic, but not dichotic, paradigm in salicylate-induced tinnitus. Hear. Res. 2015, 323, 51–60. [Google Scholar] [CrossRef]
- Zheng, Y.; Hamilton, E.; Begum, S.; Smith, P.F.; Darlington, C.L. The effects of acoustic trauma that can cause tinnitus on spatial performance in rats. Neuroscience 2011, 186, 48–56. [Google Scholar] [CrossRef]
- Zheng, Y.; McNamara, E.; Stiles, L.; Darlington, C.L.; Smith, P.F. Evidence that Memantine Reduces Chronic Tinnitus Caused by Acoustic Trauma in Rats. Front. Neurol. 2012, 3, 127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, Y.; McPherson, K.; Smith, P.F. Effects of early and late treatment with L-baclofen on the development and maintenance of tinnitus caused by acoustic trauma in rats. Neuroscience 2014, 258, 410–421. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Reid, P.; Smith, P.F. Cannabinoid CB1 Receptor Agonists Do Not Decrease, but may Increase Acoustic Trauma-Induced Tinnitus in Rats. Front. Neurol. 2015, 6, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singer, W.; Zuccotti, A.; Jaumann, M.; Lee, S.C.; Panford-Walsh, R.; Xiong, H.; Zimmermann, U.; Franz, C.; Geisler, H.S.; Köpschall, I.; et al. Noise-induced inner hair cell ribbon loss disturbs central arc mobilization: A novel molecular paradigm for understanding tinnitus. Mol. Neurobiol. 2013, 47, 261–279. [Google Scholar] [CrossRef]
- Bauer, C.A.; Brozoski, T.J.; Holder, T.M.; Caspary, D.M. Effects of chronic salicylate on GABAergic activity in rat inferior colliculus. Hear. Res. 2000, 147, 175–182. [Google Scholar] [CrossRef]
- Brozoski, T.; Wisner, K.; Randall, M.; Caspary, D. Chronic Sound-induced Tinnitus and Auditory Attention in Animals. Neuroscience 2019, 407, 200–212. [Google Scholar] [CrossRef]
- Brozoski, T.J.; Wisner, K.W.; Sybert, L.T.; Bauer, C.A. Bilateral dorsal cochlear nucleus lesions prevent acoustic-trauma induced tinnitus in an animal model. J. Assoc. Res. Otolaryngol. 2012, 13, 55–66. [Google Scholar] [CrossRef] [Green Version]
- Pace, E.; Zhang, J. Noise-induced tinnitus using individualized gap detection analysis and its relationship with hyperacusis, anxiety, and spatial cognition. PLoS ONE 2013, 8, e75011. [Google Scholar] [CrossRef] [Green Version]
- Turner, J.G.; Larsen, D. Effects of noise exposure on development of tinnitus and hyperacusis: Prevalence rates 12 months after exposure in middle-aged rats. Hear. Res. 2016, 334, 30–36. [Google Scholar] [CrossRef]
- Wang, H.; Brozoski, T.J.; Turner, J.G.; Ling, L.; Parrish, J.L.; Hughes, L.F.; Caspary, D.M. Plasticity at glycinergic synapses in dorsal cochlear nucleus of rats with behavioral evidence of tinnitus. Neuroscience 2009, 164, 747–759. [Google Scholar] [CrossRef] [Green Version]
- Ohlemiller, K.K.; Jones, S.M.; Johnson, K.R. Application of Mouse Models to Research in Hearing and Balance. J. Assoc. Res. Otolaryngol. 2016, 17, 493–523. [Google Scholar] [CrossRef] [PubMed]
- Jamesdaniel, S.; Ding, D.; Kermany, M.H.; Jiang, H.; Salvi, R.; Coling, D. Analysis of cochlear protein profiles of Wistar, Sprague-Dawley, and Fischer 344 rats with normal hearing function. J. Proteome Res. 2009, 8, 3520–3528. [Google Scholar] [CrossRef]
- Zheng, Y.; Hamilton, E.; McNamara, E.; Smith, P.F.; Darlington, C.L. The effects of chronic tinnitus caused by acoustic trauma on social behaviour and anxiety in rats. Neuroscience 2011, 193, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J. Blast-induced tinnitus: Animal models. J. Acoust. Soc. Am. 2019, 146, 3811. [Google Scholar] [CrossRef]
- Kaltenbach, J.A.; Rachel, J.D.; Mathog, T.A.; Zhang, J.; Falzarano, P.R.; Lewandowski, M. Cisplatin-induced hyperactivity in the dorsal cochlear nucleus and its relation to outer hair cell loss: Relevance to tinnitus. J. Neurophysiol. 2002, 88, 699–714. [Google Scholar] [CrossRef] [PubMed]
- Jastreboff, P.J.; Brennan, J.F.; Sasaki, C.T. Quinine-induced tinnitus in rats. Arch. Otolaryngol. Head Neck Surg. 1991, 117, 1162–1166. [Google Scholar] [CrossRef] [PubMed]
- Santos-Sacchi, J.; Song, L.; Zheng, J.; Nuttall, A.L. Control of mammalian cochlear amplification by chloride anions. J. Neurosci. 2006, 26, 3992–3998. [Google Scholar] [CrossRef] [Green Version]
- Oliver, D.; He, D.Z.; Klöcker, N.; Ludwig, J.; Schulte, U.; Waldegger, S.; Ruppersberg, J.P.; Dallos, P.; Fakler, B. Intracellular anions as the voltage sensor of prestin, the outer hair cell motor protein. Science 2001, 292, 2340–2343. [Google Scholar] [CrossRef]
- Guitton, M.J.; Caston, J.; Ruel, J.; Johnson, R.M.; Pujol, R.; Puel, J.L. Salicylate induces tinnitus through activation of cochlear NMDA receptors. J. Neurosci. 2003, 23, 3944–3952. [Google Scholar] [CrossRef] [Green Version]
- Su, Y.Y.; Luo, B.; Wang, H.T.; Chen, L. Differential effects of sodium salicylate on current-evoked firing of pyramidal neurons and fast-spiking interneurons in slices of rat auditory cortex. Hear. Res. 2009, 253, 60–66. [Google Scholar] [CrossRef]
- Stolzberg, D.; Salvi, R.J.; Allman, B.L. Salicylate toxicity model of tinnitus. Front. Syst. Neurosci. 2012, 6, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clark, W.W.; Bohne, B.A.; Boettcher, F.A. Effect of periodic rest on hearing loss and cochlear damage following exposure to noise. J. Acoust. Soc. Am. 1987, 82, 1253–1264. [Google Scholar] [CrossRef] [PubMed]
- Fredelius, L.; Johansson, B.; Bagger-Sjöbäck, D.; Wersäll, J. Qualitative and quantitative changes in the guinea pig organ of Corti after pure tone acoustic overstimulation. Hear. Res. 1987, 30, 157–167. [Google Scholar] [CrossRef]
- Clark, W.W. Recent studies of temporary threshold shift (TTS) and permanent threshold shift (PTS) in animals. J. Acoust. Soc. Am. 1991, 90, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.W.; Ahn, J.H.; Kim, J.Y.; Lee, H.J.; Kang, H.H.; Lee, Y.K.; Kim, J.U.; Koo, S.W. The effect of isoflurane, halothane and pentobarbital on noise-induced hearing loss in mice. Anesth. Analg. 2007, 104, 1404–1408. [Google Scholar] [CrossRef]
- Rüttiger, L.; Singer, W.; Panford-Walsh, R.; Matsumoto, M.; Lee, S.C.; Zuccotti, A.; Zimmermann, U.; Jaumann, M.; Rohbock, K.; Xiong, H.; et al. The reduced cochlear output and the failure to adapt the central auditory response causes tinnitus in noise exposed rats. PLoS ONE 2013, 8, e57247. [Google Scholar] [CrossRef] [Green Version]
- Eggermont, J.J.; Spoor, A. Cochlear adaptation in guinea pigs. A quantitative description. Audiology 1973, 12, 193–220. [Google Scholar]
- Wang, Y.; Liberman, M.C. Restraint stress and protection from acoustic injury in mice. Hear. Res. 2002, 165, 96–102. [Google Scholar] [CrossRef]
- Gold, S.; Cahani, M.; Sohmer, H.; Horowitz, M.; Shahar, A. Effects of body temperature elevation on auditory nerve-brain-stem evoked responses and EEGs in rats. Electroencephalogr. Clin. Neurophysiol. 1985, 60, 146–153. [Google Scholar] [CrossRef]
- Wang, Y.; Urioste, R.T.; Wei, Y.; Wilder, D.M.; Arun, P.; Sajja, V.; Gist, I.D.; Fitzgerald, T.S.; Chang, W.; Kelley, M.W.; et al. Blast-induced hearing impairment in rats is associated with structural and molecular changes of the inner ear. Sci. Rep. 2020, 10, 10652. [Google Scholar] [CrossRef]
- Berger, J.I.; Coomber, B. Tinnitus-related changes in the inferior colliculus. Front. Neurol. 2015, 6, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Valenzuela, C.; Gárate-Pérez, M.F.; Sotomayor-Zárate, R.; Delano, P.H.; Dagnino-Subiabre, A. Reboxetine Improves Auditory Attention and Increases Norepinephrine Levels in the Auditory Cortex of Chronically Stressed Rats. Front. Neural Circuits 2016, 10, 108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shim, H.J.; An, Y.H.; Kim, D.H.; Yoon, J.E.; Yoon, J.H. Comparisons of auditory brainstem response and sound level tolerance in tinnitus ears and non-tinnitus ears in unilateral tinnitus patients with normal audiograms. PLoS ONE 2017, 12, e0189157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehraei, G.; Hickox, A.E.; Bharadwaj, H.M.; Goldberg, H.; Verhulst, S.; Liberman, M.C.; Shinn-Cunningham, B.G. Auditory Brainstem Response Latency in Noise as a Marker of Cochlear Synaptopathy. J. Neurosci. 2016, 36, 3755–3764. [Google Scholar] [CrossRef] [PubMed]
- Liberman, M.C.; Kujawa, S.G. Cochlear synaptopathy in acquired sensorineural hearing loss: Manifestations and mechanisms. Hear. Res. 2017, 349, 138–147. [Google Scholar] [CrossRef] [PubMed]
- ABR User Guide. 2020. Available online: https://www.tdt.com/files/manuals/ABRGuide.pdf (accessed on 20 October 2020).
- Gärtner, K.; Büttner, D.; Döhler, K.; Friedel, R.; Lindena, J.; Trautschold, I. Stress response of rats to handling and experimental procedures. Lab. Anim. 1980, 14, 267–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Backoff, P.M.; Caspary, D.M. Age-related changes in auditory brainstem responses in Fischer 344 rats: Effects of rate and intensity. Hear. Res. 1994, 73, 163–172. [Google Scholar] [CrossRef]
- Church, M.W.; Williams, H.L.; Holloway, J.A. Brain-stem auditory evoked potentials in the rat: Effects of gender, stimulus characteristics and ethanol sedation. Electroencephalogr. Clin. Neurophysiol. 1984, 59, 328–339. [Google Scholar] [CrossRef]
- Ruebhausen, M.R.; Brozoski, T.J.; Bauer, C.A. A comparison of the effects of isoflurane and ketamine anesthesia on auditory brainstem response (ABR) thresholds in rats. Hear. Res. 2012, 287, 25–29. [Google Scholar] [CrossRef]
- Sheppard, A.M.; Zhao, D.L.; Salvi, R. Isoflurane anesthesia suppresses distortion product otoacoustic emissions in rats. J. Otol. 2018, 13, 59–64. [Google Scholar] [CrossRef]
- Hatzopoulos, S.; Petruccelli, J.; Laurell, G.; Finesso, M.; Martini, A. Evaluation of anesthesia effects in a rat animal model using otoacoustic emission protocols. Hear. Res. 2002, 170, 12–21. [Google Scholar] [CrossRef]
- Maria, P.L.S.; Redmond, S.L.; Atlas, M.D.; Ghassemifar, R. Histology of the healing tympanic membrane following perforation in rats. Laryngoscope 2010, 120, 2061–2070. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G.T.; Britt, R.H. Effects of hypothermia on the cat brain-stem auditory evoked response. Electroencephalogr. Clin. Neurophysiol. 1984, 57, 143–155. [Google Scholar] [CrossRef]
- Jones, T.A.; Stockard, J.J.; Weidner, W.J. The effects of temperature and acute alcohol intoxication on brain stem auditory evoked potentials in the cat. Electroencephalogr. Clin. Neurophysiol. 1980, 49, 23–30. [Google Scholar] [CrossRef]
- Balogová, Z.; Popelář, J.; Chiumenti, F.; Chumak, T.; Burianová, J.S.; Rybalko, N.; Syka, J. Age-Related Differences in Hearing Function and Cochlear Morphology between Male and Female Fischer 344 Rats. Front. Aging Neurosci. 2017, 9, 428. [Google Scholar] [CrossRef] [Green Version]
- Marcondes, F.K.; Bianchi, F.J.; Tanno, A.P. Determination of the estrous cycle phases of rats: Some helpful considerations. Braz. J. Biol. 2002, 62, 609–614. [Google Scholar] [CrossRef] [Green Version]
- Church, M.W.; Williams, H.L.; Holloway, J.A. Postnatal development of the brainstem auditory evoked potential and far-field cochlear microphonic in non-sedated rat pups. Brain Res. 1984, 316, 23–31. [Google Scholar] [CrossRef]
- Meltser, I.; Canlon, B. Protecting the auditory system with glucocorticoids. Hear. Res. 2011, 281, 47–55. [Google Scholar] [CrossRef]
- Serra, A.; Maiolino, L.; Agnello, C.; Messina, A.; Caruso, S. Auditory brain stem response throughout the menstrual cycle. Ann. Otol. Rhinol. Laryngol. 2003, 112, 549–553. [Google Scholar] [CrossRef]
- Mann, N.; Sidhu, R.S.; Babbar, R. Brainstem auditory evoked responses in different phases of menstrual cycle. J. Clin. Diagn. Res. JCDR 2012, 6, 1640–1643. [Google Scholar] [CrossRef]
- Schaette, R.; McAlpine, D. Tinnitus with a normal audiogram: Physiological evidence for hidden hearing loss and computational model. J. Neurosci. 2011, 31, 13452–13457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kehrle, H.M.; Granjeiro, R.C.; Sampaio, A.L.; Bezerra, R.; Almeida, V.F.; Oliveira, C.A. Comparison of auditory brainstem response results in normal-hearing patients with and without tinnitus. Arch. Otolaryngol. Head Neck Surg. 2008, 134, 647–651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, J.W.; Herrmann, B.S.; Levine, R.A.; Melcher, J.R. Brainstem auditory evoked potentials suggest a role for the ventral cochlear nucleus in tinnitus. J. Assoc. Res. Otolaryngol. 2012, 13, 819–833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Attias, J.; Pratt, H.; Reshef, I.; Bresloff, I.; Horowitz, G.; Polyakov, A.; Shemesh, Z. Detailed analysis of auditory brainstem responses in patients with noise-induced tinnitus. Audiology 1996, 35, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Attias, J.; Urbach, D.; Gold, S.; Shemesh, Z. Auditory event related potentials in chronic tinnitus patients with noise induced hearing loss. Hear. Res. 1993, 71, 106–113. [Google Scholar] [CrossRef]



| Strain | Sprague–Dawley (Used in 17 Studies) | Wistar (Used in 13 Studies) | Long-Evans (Used in 4 Studies) | Fischer FBN; 344 (Used in 3 Studies) | |||||
|---|---|---|---|---|---|---|---|---|---|
| gender | Male (used in 16 studies) | Female (used in 1 study) | Male (used in 8 studies) | Female (used in 3 studies) | Female and male (used in 2 studies) | Male (used in 4 studies) | Female (no publications | Male (used in 2 studies) | Female (no publications) |
| age (range) | 1.5–4 months | 1.5–5 months | 1.5–5 months | 2–5 months | 2–3 months | - | 3–6 months old | ||
| Article | Sodium Salicylate Concentration | Frequency of Salicylate Application | Lenght of Salicylate Exposure | Method of Application | Tinnitus Measurement Method |
|---|---|---|---|---|---|
| Bauer et al., 2000 [66] | 8 mg/mL | 4 weeks | 4 weeks | orally (in drinking water) | No assessment |
| Chen et al., 2010 [49] | 200 mg/kg | Once a day | 5 days per week for 3 weeks | injection | No assessment |
| Zhang et al., 2020 [27,59] | 200 mg/kg | Once a day | 10 consecutive days | intraperitoneal injection | GPIAS |
| Fang et al., 2016 [58] | 300 mg/kg | Once a day | 8 consecutive days | intraperitoneal injection | GPIAS |
| Jang et al., 2019 [51] | 400 mg/kg | Once a day | 7 consecutive days | intraperitoneal injection | GPIAS |
| Ralli et al., 2010, 2014 [13,52] | 300 mg/kg | Once a day | 4 consecutive days | injection | GPIAS |
| Castañeda et al., 2019 [28] | 350 mg/kg | Once a day | 3 consecutive days | orally (by gavage) | an operant conditioned-suppression procedure |
| Lee et al., 2019 [51] | 350 mg/kg | single application | single application | intraperitoneal injection | No assessment |
| Liu and Chen, 2015 [60] | 300 mg/kg | single application | single application | intraperitoneal injection | GPIAS |
| Liu and Chen, 2012 [29] | 300 mg/kg | single application | single application | injection | No assessment |
| Sawka and Wei, 2014 [53] | 250 mg/kg | single application | single application | intraperitoneal injection | No assessment |
| Duron et al., 2020 [50] | 150 mg/kg | single application | single application | intraperitoneal injection | No assessment |
| Article | Laterality of Noise Application | Noise Intensity | Noise Duration | Anesthetic Used during Noise Exposure | Determination of Tinnitus in Rats/Sample Size | Timepoint of Tinnitus Determination |
|---|---|---|---|---|---|---|
| Kim et al., 2020 [55] | Bilateral | 16 kHz, 112 dB SPL | 4 h | Data not available | Data not available (GPIAS) | One day after the noise and 1 and 10 days after completing DEX administration |
| Brozoski et al., 2019 [67] | Unilateral (contralateral ear-plugged) | 16 kHz, 120 dB SPL | 1 h | Isoflurane (1.7%) | Not all (an operant conditioned-suppression procedure) | 3 and 9 months after noise |
| van Zwieten et al., 2019 [57] | Unilateral (contralateral ear-plugged) | 16 kHz, 115 dB SPL | 1.5 h | Ketamine (90 mg/kg) + Xylazine (10 mg/kg) | 11/11 (GPIAS) | 4–6 weeks after noise |
| van Zwieten et al., 2019 [56] | Unilateral (contralateral ear-plugged) | 16 kHz, 115 dB SPL | 1.5 h | Ketamine (90 mg/kg) + Xylazine (10 mg/kg) | (Data not available GPIAS) | 4–6 weeks after noise |
| Ahsan et al., 2018 [54] | Unilateral (contralateral ear-plugged) | 8–16 kHz, 115 dB | 2 h | Isoflurane (2–3%) | 4/6 (GPIAS) | After noise (no details) |
| Turner and Larsen, 2016 [70] | Unilateral (contralateral ear-not announced) | 16 kHz, 110, 116 or 122 dB SPL; 8 or 32 kHz or BBN, 110 dB SPL | 0.5 h, 1 h, or 2 h | Ketamine + Xylazine (doses—not announced) | Data not available (GPIAS) | On Day 1, 3, 7, 14, 21, 28 after noise exposure and monthly thereafter over until 1 year |
| Bing et al., 2015 [15] | Unilateral (contralateral ear-plugged) | 10 kHz, 120 dB | 2 h | Medetomidine hydrochloride (0.33 mg/kg) | Data not available (the motor task) | 3 and 10 days after noise |
| Zheng et al., 2015 [64] | Unilateral (contralateral ear-plugged) | 16 kHz, 115 dB | 1 h | Fentanyl (0.2 mg/kg) + Medetomidine hydrochloride (0.5 mg/kg) | 14/30 (a conditioned lick suppression task) | 1 month after noise |
| Zheng, McPherson and Smith, 2014 [63] | Unilateral (contralateral ear-plugged) | 16 kHz, 115 dB, | 1 h | Medetomidine hydrochloride (0.33 mg/kg) | Data not available (a conditioned lick suppression task) | 2 weeks and then at 10 and 17.5 weeks after noise |
| Laundrie and Sun, 2014 [44] | Unilateral (contralateral ear-plugged) | 12 kHz, 120 dB SPL | 1 h | Isoflurane (1–2%) | Data not available GPIAS) | 4 h after noise |
| Ropp et al., 2014 [45] | Unilateral (contralateral ear-plugged) | 16 kHz, 116 dB SPL | 2 h | Unanesthetized (rat was held in a slowly rotating hardware cloth cage) | Data not available (GPIAS) | At various delays after noise (2–3 times a week for 1–4 months) |
| Rüttiger et al., 2013 [87] | Binaural | 10 kHz, 120 dB SPL | 1 h or 1.5 h | Ketamine (75 mg/kg) + Xylazine hydrochloride (5 mg/kg) | 5/15 and 5/17. (the motor task) | Before and at 6 day (1 h) or 30 days (1.5 h) after noise |
| Pace and Zhang, 2013 [69] | Unilateral (contralateral ear-plugged) | 10 kHz, 118–120 dB peak SPL | Two hours, five weeks later, the 2nd exposure for 3 h | First: Isoflurane (5%); second: while rats awake | 12/18 (GPIAS) | One day after the 1st noise and two times a week until six weeks after the 2nd noise |
| Singer et al., 2013 [65] | Binaural | 10 kHz, 80,100, 110, or 120 dB SPL | 1–2 h | Ketamine hydrochloride (75 mg/kg) + Xylazine hydrohloride (5 mg/kg) | Only rats subjected to 120 dB demonstrated tinnitus (the motor task) | 6–14 days after noise exposure |
| Brozoski et al., 2012 [68] | Unilateral (contralateral ear-plugged) | 16 kHz, 116 dB | 1 h | Data not available | Data not available (an operant conditioned-suppression) | Immediately after noise |
| Zheng et al., 2012 [62] | Unilateral (contralateral ear-plugged) | 16 kHz, 110 dB | 1 h | Ketamine hydrochloride (75 mg/kg) + Medetomidine hydrochloride (0.3 mg/kg) | 5/8 (a conditioned lick suppression task) | After noise (no details) |
| Zheng et al., 2012 [62] | Unilateral (contralateral ear-plugged) | 16 kHz, 110 dB | 1 h | Ketamine hydrochloride (75 mg/kg) + Medetomidine hydrochloride (0.3 mg/kg) | Data not available (a conditioned lick suppression task) | Two weeks after noise |
| Zheng et al., 2011 [61] | Unilateral (contralateral ear-plugged) | 16 kHz, 110 dB | 1 h | Ketamine hydrochloride (75 mg/kg) + Medetomidine hydrochloride (0.3 mg/kg) | Data not available (a conditioned lick suppression task) | 2 weeks and 10 months after noise |
| Wang et al., 2009 [88] | Unilateral (contralateral ear-plugged) | 17 kHz, 116 dB SPL | 1 h | Ketamine hydrochloride (50 mg/kg) + Xylazine (9 mg/kg) | 10/14 (GPIAS) | 20 days after noise every 2 weeks up to 16 weeks |
| Ouyang 2017 [48] | Unilateral (contralateral ear-plugged) | 194 dB SPL | Single blast exposure | Isoflurane (4%) or Ketamine (100 mg/kg) + Xylazine (10 mg/kg) | 8/13 (GPIAS) | After blast (2 times per week) |
| Mahmood et al., 2014 [46] | Unilateral (contralateral ear-plugged) | Data not available | 3 consecutive blast exposure | Isoflurane (3%) | Data not available (GPIAS) | 1 h after the last blast and for 8 weeks afterward |
| Mao et al., 2012 [47] | Bilateral | 194 dB SPL | Single blast exposure (10 ms) | Ketamine (100 mg/kg) + Xylazine (10 mg/kg) | Data not available (GPIAS) | 1, 14, 28, and 90 days after blast |
| Article | SAL Dose | Rats | Wave I | Wave II | Wave III | Wave IV | Wave V | Intervals | Threshold |
|---|---|---|---|---|---|---|---|---|---|
| Zhang et al., 2020 [27,59] | 200 mg/kg/day per 10 days | Male, Wis | ↓ __ | Not tested | Not tested | Not tested | Not tested | __ | __ (vs. control group) |
| Fang et al., 2015 [58] | 300 mg/kg per 8 days | male, Wis | Not tested | Not tested | ↓ | Not tested | Not tested | Not tested | Not tested |
| Duron et al., 2020 [50] | 150 mg/kg/day | male, SD | ↓ → | __ | __ | ↑ ← | Not tested | III–IV: ← | ↑ at 6–16 kHz |
| Castañeda et al., 2019 [28] | 350 mg/kg per 3 days | female, SD | ↓ | ↑ | __ | ↑ | __ | I–IV: ← II–IV: → | ↑ at 4–32 kHz |
| Sawka and Wei, 2014 [53] | 250 mg/kg/day | male, SD | Not tested | ↓ | Not tested | Not tested | ↓ | Not tested | Not tested |
| Chen et al., 2010 [49] | 200/mg/kg/day for three weeks (5 days per week) | male, SD | Not tested | Not tested | ↓ | Not tested | Not tested | Not tested | Not tested |
| Article | Noise Details | Rats | Wave I | Wave II | Wave III | Wave IV | Wave V | Intervals | Threshold |
|---|---|---|---|---|---|---|---|---|---|
| Bing et al., 2015 [15] * | 10 kHz, 120 dB, 2 h, unilateral | female, Wis | ↓ | Not tested | Not tested | ↓ | Not tested | Not tested | ↑ at 8–50 kHz |
| Singer et al., 2013 [65] | 10 kHz, 120 dB SPL for 1–2 h, binaural | female, Wis | ↓ | ↓ | ↓ | ↓ | ↓ | Not tested | ↑ at 2–4 kHz |
| Rüttiger et al., 2013 [65] | 10 kHz, 120 dB SPL, 1 or 1.5 h, binaural | female, Wis | ↓ | ↓ | ↓ | ↓ | ↓ | Not tested | ↑ at 1–45 kHz |
| Ouyang et al., 2017 [48] | blast exposure (194 dB SPL), unilateral | male, SD | ↓ | Not tested | Not tested | Not tested | Not tested | Not tested | _ |
| Factor | Influence on ABR | Suggestions Based on ABR User Guide [97] | Additional Recommendations |
|---|---|---|---|
| Experimental area | Cables, noise generators might generate electrical noise | A sound attenuating chamber with a built-in Faraday cage | Before starting experiments, conduct a saline test (to determine the noise floor) |
| Speaker placement | Affect the stimulus level | The speaker should be on the same plane as the tested ear and set at an angle from the sides of the enclosure | Place the speaker in a distance of 10 cm away from the animal |
| Electrode placement | Incorrect recording | Vertex (active); reference (ipsilateral ear); ground (contralateral ear or hind hip or base of tail) | Write the lot number of electrodes in the protocol |
| Electrode impedance | <4 kΩ, lower artifacts suppression, low quality of ABR recording | 1 k–3 kΩ | |
| Repetition rate | If the repetition rate increases, latency increases, too [88,98] With increased stimulus rates (click, 5/s to 50/s), aged Fischer 344 rats demonstrated an increase in latencies wave IV and V and overall amplitude reduction (I–V) [99] A similar result was observed in young Sprague–Dawley rats in response to click and pure tones [100] Increasing the repetition rate shortens the recovery time, but it also reduces ABR measurement times and shortens the time of anesthesia [87] | 21/s | The rate 21/s minimizes the effects of noise from the 50/60 Hz cycle of mains power (95) |
| Hearing range tested | Standard Testing Range: >4 kHz to 14/32 kHz | ||
| Type of stimulus | Data not available | Click: 100 μs, pure tone: 5 ms (2-1-2) | - |
| Number of averages | Impact on signal/noise ratio. Rats with hearing loss require more averages than rats with normal hearing | 512 averages | 512 ensures a balance between the signal quality and minimalization of the time to complete testing |
| Polarity | Data not available | Alternating | - |
| Anesthesia | Rats following isoflurane (1.5–2%; 4%) administration have higher hearing thresholds in comparison to ketamine + xylazine (50 mg/kg + 9 mg/kg) [101,102] In addition, isoflurane significantly impairs DPOAEs, whereas a normal dose of ketamine + xylazine (50 mg/kg + 10 mg/kg) in Sprague–Dawley rats does not [102,103] | A mixture of ketamine + xylazine (a weight-dependent mg/kg dosage) Ketamine can be used to keep the subject anesthetized longer than 45 min | Monitor rats under anesthesia |
| Tympanic membrane evaluation | Properly functioning ossicular chain conduction is a prerequisite for ABR recording | Evaluate tympanic membrane using otoscope before the start of the experiment | See picture in [104] |
| Body temperature of rats | Temperature decreasing by 0.5 °C or more degrees may significantly alter ABR latencies and amplitudes [105,106] | Heating pads should be used to maintain body temperature (37 °C) or control the temperature in the experimental room (25 °C) | Monitor the body temperature with a rectal probe throughout the recording |
| Gender | There were no significant gender-dependent differences in amplitudes or latencies between the ages of 14 and 70 days in Sprague–Dawley rats [100] However, adult female rats had shorter latencies (I–IV) than male rats [100,107] | - | In female rats >5 weeks old, the estrous cycle should be controlled [108] |
| Age | Immature auditory response of rat pups [109] Age-related effects on hearing | - | Write the age and the body weight in the protocol |
| Strain | The hearing range of laboratory subjects varies across different strains (95) | ||
| Day-night cycle | The sensitivity to noise varies at different daytimes. Two weeks after noise trauma (during the night 9 PM) ABR thresholds were elevated, whereas in mice exposed to noise at 9 AM (6–12 kHz, 100 dB SPL for 1 h) [110] | - | Note the time of experiments |
| Handling rats | Handling is a well-known source of stress-induced variation in animal studies [98] | - | |
| Housing rats | Disruption in factors below evokes stress reactions in rats, which mediates hearing abilities in rats. Factors: Maintaining a stable temperature, humidity, and light–dark cycle in the facility, free access to water and chow | - | Maintaining a stable temperature and humidity in the facility; Standard chow and water ad libitum; acclimatization one week before running the experiment; providing an enriched environment |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domarecka, E.; Olze, H.; Szczepek, A.J. Auditory Brainstem Responses (ABR) of Rats during Experimentally Induced Tinnitus: Literature Review. Brain Sci. 2020, 10, 901. https://doi.org/10.3390/brainsci10120901
Domarecka E, Olze H, Szczepek AJ. Auditory Brainstem Responses (ABR) of Rats during Experimentally Induced Tinnitus: Literature Review. Brain Sciences. 2020; 10(12):901. https://doi.org/10.3390/brainsci10120901
Chicago/Turabian StyleDomarecka, Ewa, Heidi Olze, and Agnieszka J. Szczepek. 2020. "Auditory Brainstem Responses (ABR) of Rats during Experimentally Induced Tinnitus: Literature Review" Brain Sciences 10, no. 12: 901. https://doi.org/10.3390/brainsci10120901
APA StyleDomarecka, E., Olze, H., & Szczepek, A. J. (2020). Auditory Brainstem Responses (ABR) of Rats during Experimentally Induced Tinnitus: Literature Review. Brain Sciences, 10(12), 901. https://doi.org/10.3390/brainsci10120901






