Haptic Perception in Extreme Obesity: qEEG Study Focused on Predictive Coding and Body Schema
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
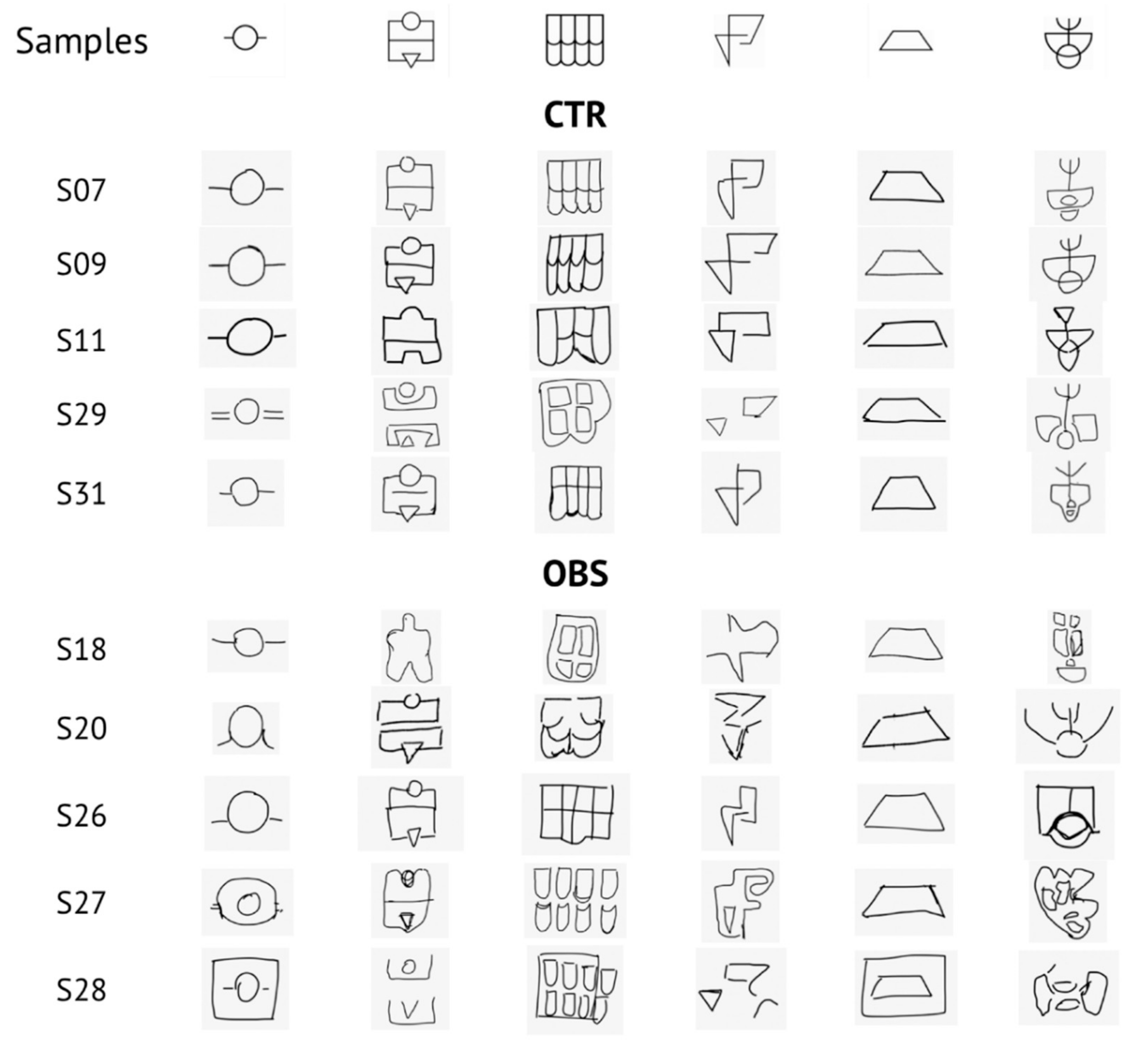
2.2. Haptic Task
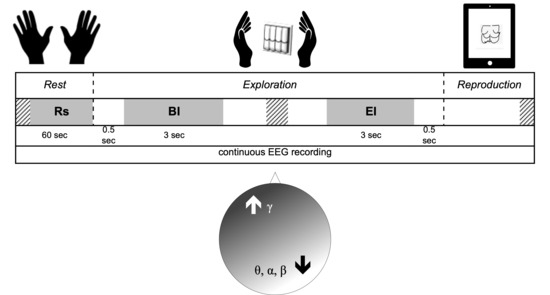
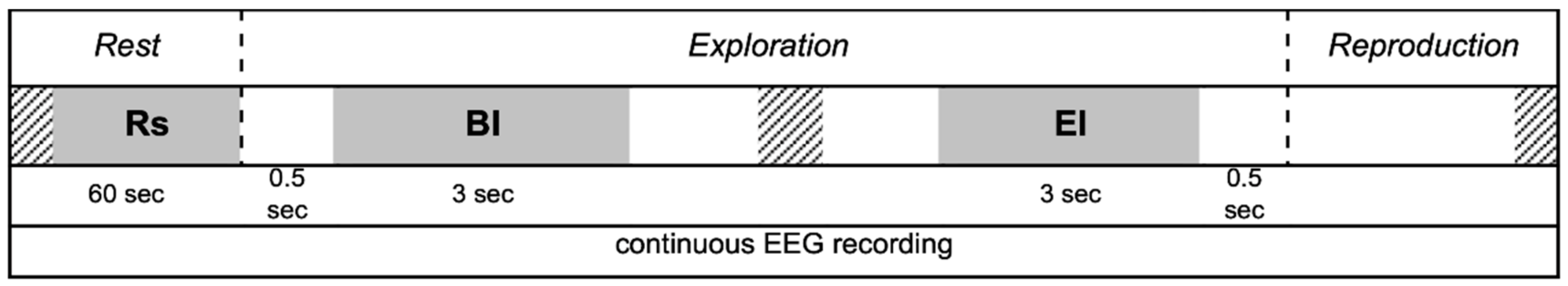
2.3. EEG Recordings
- Rs
- Rest (baseline): 60 s with eyes closed before starting exploration.
- BI
- Begin Interval: 3 s of continuous recording extracted after 0.5 s from the beginning of the exploration.
- EI
- End Interval: 3 s of continuous recording ending up 0.5 s before the end of exploration.
2.4. Statistical Analysis
3. Results
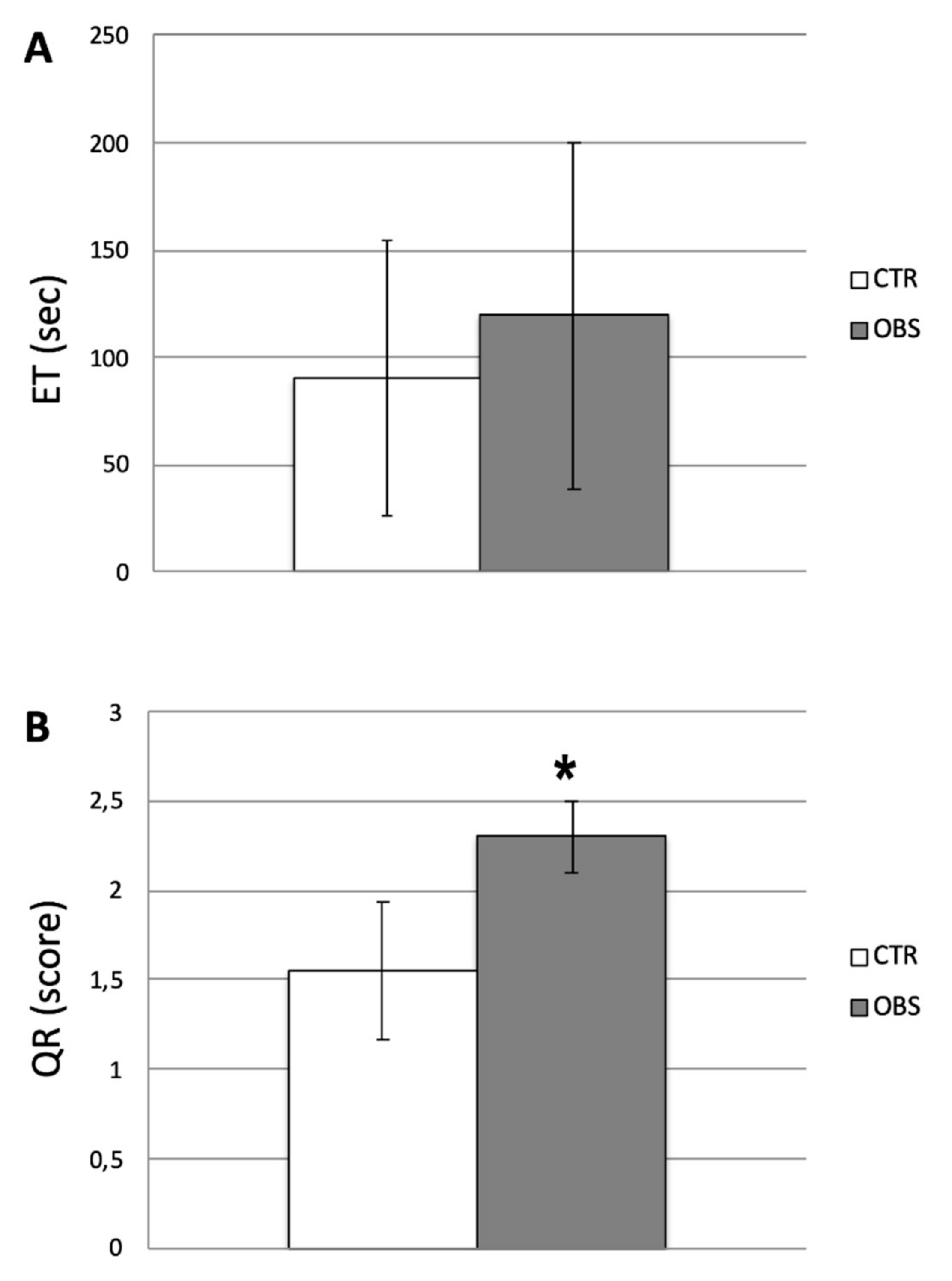
3.1. Descriptive and Behavioral Data
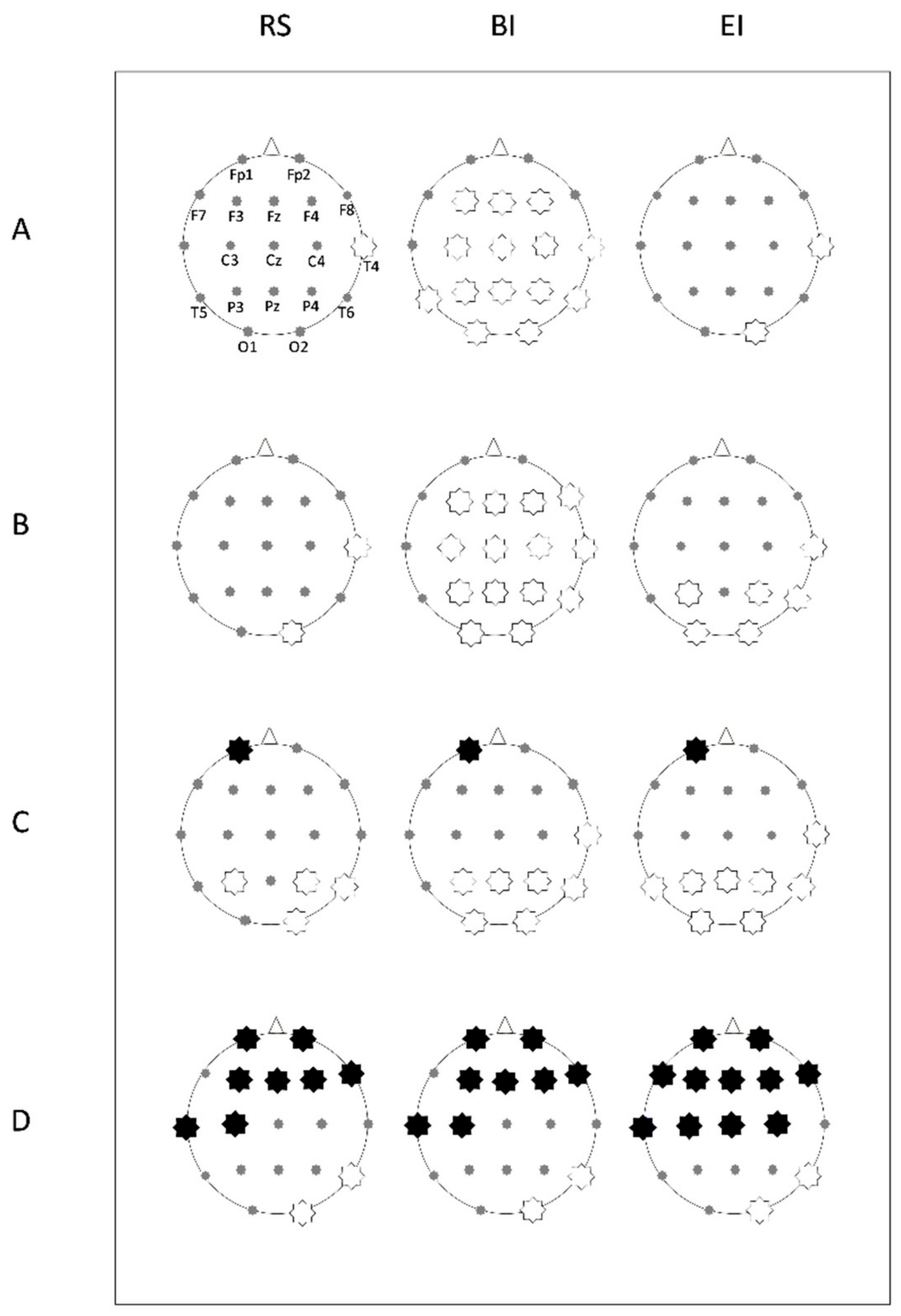
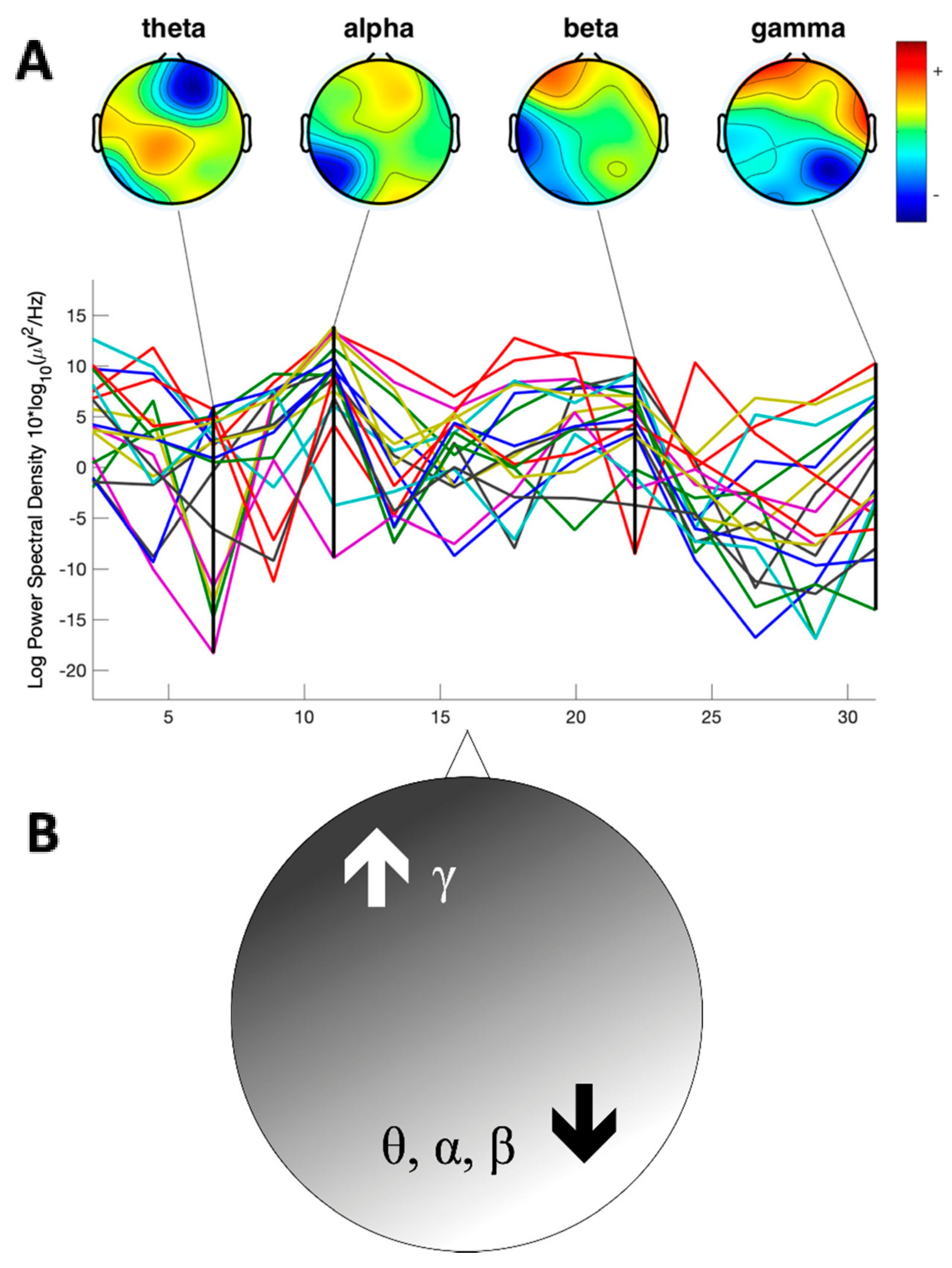
3.2. EEG Analysis
3.2.1. Theta Power
3.2.2. Alpha Power
3.2.3. Beta Power
3.2.4. Gamma Power
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Grunwald, M. Human Haptic Perception: Basics and Applications; Springer Science & Business Media: New York, NY, USA, 2008; ISBN 9783764376123. [Google Scholar]
- Lederman, S.J.; Klatzky, R.L. Haptic perception: A tutorial. Atten. Percept. Psychophys. 2009, 71, 1439–1459. [Google Scholar] [CrossRef]
- Kappers, A.M.L.; Bergmann Tiest, W.M. Haptic perception. Wiley Interdiscip. Rev. Cogn. Sci. 2013, 4, 357–374. [Google Scholar] [CrossRef] [PubMed]
- Costantini, M.; Urgesi, C.; Galati, G.; Romani, G.L.; Aglioti, S.M. Haptic perception and body representation in lateral and medial occipito-temporal cortices. Neuropsychol. 2011, 49, 821–829. [Google Scholar] [CrossRef] [PubMed]
- Engel, A.; Senkowski, D.; Schneider, T. Multisensory Integration through Neural Coherence. In The Neural Bases of Multisensory Processes; Murray, M.M., Wallace, M.T., Eds.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2012; ISBN 9781439812174. [Google Scholar]
- Sathian, K. Analysis of haptic information in the cerebral cortex. J. Neurophysiol. 2016, 116, 1795–1806. [Google Scholar] [CrossRef]
- Tamè, L.; Azañón, E.; Longo, M.R. A Conceptual Model of Tactile Processing across Body Features of Size, Shape, Side, and Spatial Location. Front. Psychol. 2019, 10, 291. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.P.N.; Ballard, D.H. Predictive coding in the visual cortex: A functional interpretation of some extra-classical receptive-field effects. Nat. Neurosci. 1999, 2, 79–87. [Google Scholar] [CrossRef]
- Tramper, J.J.; Flanders, M. Predictive mechanisms in the control of contour following. Exp. Brain Res. 2013, 227, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Friston, K.; Chu, C.; Mourão-Miranda, J.; Hulme, O.J.; Rees, G.; Penny, W.; Ashburner, J.; Penny, W. Bayesian decoding of brain images. NeuroImage 2008, 39, 181–205. [Google Scholar] [CrossRef]
- Ognibene, D.; Giglia, G. Use of hierarchical Bayesian framework in MTS studies to model different causes and novel possible forms of acquired MTS. Cogn. Neurosci. 2015, 6, 144–145. [Google Scholar] [CrossRef]
- Bruno, N.; Bertamini, M. Haptic perception after a change in hand size. Neuropsychol. 2010, 48, 1853–1856. [Google Scholar] [CrossRef] [PubMed]
- Grunwald, M.; Weiss, T.; Assmann, B.; Ettrich, C. Stable Asymmetric Interhemispheric Theta Power in Patients With Anorexia Nervosa During Haptic Perception Even After Weight Gain: A Longitudinal Study. J. Clin. Exp. Neuropsychol. 2004, 26, 608–620. [Google Scholar] [CrossRef] [PubMed]
- Grunwald, M.; Ettrich, C.; Assmann, B.; Dähne, A.; Krause, W.; Busse, F.; Gertz, H.-J. Deficits in haptic perception and right parietal theta power changes in patients with anorexia nervosa before and after weight gain. Int. J. Eat. Disord. 2001, 29, 417–428. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-J. Neurophysiological and Computational Principles of Cortical Rhythms in Cognition. Physiol. Rev. 2010, 90, 1195–1268. [Google Scholar] [CrossRef] [PubMed]
- Kurz, M.J.; Wiesman, A.I.; Coolidge, N.M.; Wilson, T.W. Haptic exploration attenuates and alters somatosensory cortical oscillations. J. Physiol. 2018, 596, 5051–5061. [Google Scholar] [CrossRef]
- Neuper, C.; Wörtz, M.; Pfurtscheller, G. ERD/ERS patterns reflecting sensorimotor activation and deactivation. Prog. Brain Res. 2006, 159, 211–222. [Google Scholar] [CrossRef]
- Bastos, A.M.; Litvak, V.; Moran, R.J.; Bosman, C.A.; Fries, P.; Friston, K.J. A DCM study of spectral asymmetries in feedforward and feedback connections between visual areas V1 and V4 in the monkey. NeuroImage 2015, 108, 460–475. [Google Scholar] [CrossRef]
- Babiloni, C.; Marzano, N.; Lizio, R.; Valenzano, A.; Triggiani, A.I.; Petito, A.; Bellomo, A.; Lecce, B.; Mundi, C.; Soricelli, A.; et al. Resting state cortical electroencephalographic rhythms in subjects with normal and abnormal body weight. NeuroImage 2011, 58, 698–707. [Google Scholar] [CrossRef]
- Fagundo, A.B.; de la Torre, R.; Jiménez-Murcia, S.; Agüera, Z.; Granero, R.; Tárrega, S.; Botella, C.; Baños, R.; Fernández-Real, J.M.; Rodríguez, R.; et al. Executive Functions Profile in Extreme Eating/Weight Conditions: From Anorexia Nervosa to Obesity. PLoS ONE 2012, 7, e43382. [Google Scholar] [CrossRef]
- Sardesai, V. Obesity and Eating Disorders. Introd. Clin. Nutr. 2003, 8 (Suppl. 1), 151–155. [Google Scholar] [CrossRef]
- Hume, D.J.; Howells, F.M.; Rauch, H.L.; Kroff, J.; Lambert, E.V. Electrophysiological indices of visual food cue-reactivity. Differences in obese, overweight and normal weight women. Appetite 2015, 85, 126–137. [Google Scholar] [CrossRef]
- Von Stein, A.; Sarnthein, J. Different frequencies for different scales of cortical integration: From local gamma to long range alpha/theta synchronization. Int. J. Psychophysiol. 2000, 38, 301–313. [Google Scholar] [CrossRef]
- Freeman, W.; Quiroga, R.Q. Imaging Brain Function with EEG: Advanced Temporal and Spatial Analysis of Electroencephalographic Signals; Springer Science & Business Media: New York, NY, USA, 2012; ISBN 9781461449843. [Google Scholar]
- Oldfield, R. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 1971, 9, 97–113. [Google Scholar] [CrossRef]
- Raven, J. The Raven’s Progressive Matrices: Change and Stability over Culture and Time. Cogn. Psychol. 2000, 41, 1–48. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013; ISBN 9780890425558. [Google Scholar]
- Grunwald, M.; Muniyandi, M.; Kim, H.; Kim, J.; Krause, F.; Mueller, S.; Srinivasan, M.A. Human haptic perception is interrupted by explorative stops of milliseconds. Front. Psychol. 2014, 5, 292. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schubert, T.W.; D’Ausilio, A.; Canto, R. Using Arduino microcontroller boards to measure response latencies. Behav. Res. Methods 2013, 45, 1332–1346. [Google Scholar] [CrossRef]
- Klem, G.H.; O Lüders, H.; Jasper, H.H.; Elger, C. The ten-twenty electrode system of the International Federation. The International Federation of Clinical Neurophysiology. Electroencephalogr. Clin. Neurophysiol. Suppl. 1999, 52, 3–6. [Google Scholar]
- Delorme, A.; Makeig, S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef]
- Mognon, A.; Jovicich, J.; Bruzzone, L.; Buiatti, M. ADJUST: An automatic EEG artifact detector based on the joint use of spatial and temporal features. Psychophysiology 2011, 48, 229–240. [Google Scholar] [CrossRef]
- Onton, J.; Westerfield, M.; Townsend, J.; Makeig, S. Imaging human EEG dynamics using independent component analysis. Neurosci. Biobehav. Rev. 2006, 30, 808–822. [Google Scholar] [CrossRef]
- Gerlach, G.; Herpertz, S.; Loeber, S. Personality traits and obesity: A systematic review. Obes. Rev. 2014, 16, 32–63. [Google Scholar] [CrossRef]
- La Grutta, S.; di Blasi, M.; la Barbera, D.; Alabastro, V.; Alfano, P.; Guttilla, G.; Matranga, D.; Epifanio, M.; Roccella, M.; Lo Baido, R. Meccanismi di difesa in un gruppo di persone con obesità. Minerva Psichiatr. 2013, 54, 239–246. [Google Scholar]
- Sominsky, L.; Spencer, S.J. Eating behavior and stress: A pathway to obesity. Front. Psychol. 2014, 5, 434. [Google Scholar] [CrossRef] [PubMed]
- Gaysina, D.; Hotopf, M.; Richards, M.; Colman, I.; Kuh, D.; Hardy, R. Symptoms of depression and anxiety, and change in body mass index from adolescence to adulthood: Results from a British birth cohort. Psychol. Med. 2010, 41, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.M.; Stojek, M.K.; MacKillop, J. Interrelationships among impulsive personality traits, food addiction, and Body Mass Index. Appetite 2014, 73, 45–50. [Google Scholar] [CrossRef]
- Nummenmaa, L.; Hirvonen, J.; Hannukainen, J.C.; Immonen, H.; Lindroos, M.M.; Salminen, P.; Nuutila, P. Dorsal Striatum and Its Limbic Connectivity Mediate Abnormal Anticipatory Reward Processing in Obesity. PLoS ONE 2012, 7, e31089. [Google Scholar] [CrossRef]
- Babiloni, C.; del Percio, C.; Valenzano, A.; Marzano, N.; de Rosas, M.; Petito, A.; Bellomo, A.; Rossi, G.; Lecce, B.; Mundi, C.; et al. Frontal attentional responses to food size are abnormal in obese subjects: An electroencephalographic study. Clin. Neurophysiol. 2009, 120, 1441–1448. [Google Scholar] [CrossRef]
- Navas-Olive, A.; Valero, M.; Jurado-Parras, T.; de Salas-Quiroga, A.; Averkin, R.G.; Gambino, G.; Cid, E.; de la Prida, L.M. Multimodal determinants of phase-locked dynamics across deep-superficial hippocampal sublayers during theta oscillations. Nat. Commun. 2020, 11, 1–14. [Google Scholar] [CrossRef]
- Gambino, G.; Allegra, M.; Sardo, P.; Attanzio, A.; Tesoriere, L.; Livrea, M.A.; Ferraro, G.; Carletti, F. Brain Distribution and Modulation of Neuronal Excitability by Indicaxanthin From Opuntia Ficus Indica Administered at Nutritionally-Relevant Amounts. Front. Aging Neurosci. 2018, 10, 133. [Google Scholar] [CrossRef]
- Carletti, F.; Sardo, P.; Gambino, G.; Liu, X.-A.; Ferraro, G.; Rizzo, V. Hippocampal Hyperexcitability is Modulated by Microtubule-Active Agent: Evidence from In Vivo and In Vitro Epilepsy Models in the Rat. Front. Cell. Neurosci. 2016, 10, 29. [Google Scholar] [CrossRef]
- Benito-León, J.; Mitchell, A.J.; Hernández-Gallego, J.; Bermejo-Pareja, F. Obesity and impaired cognitive functioning in the elderly: A population-based cross-sectional study (NEDICES). Eur. J. Neurol. 2013, 20, 899-e77. [Google Scholar] [CrossRef]
- Masouleh, S.K.; Arelin, K.; Horstmann, A.; Lampe, L.; Kipping, J.A.; Luck, T.; Riedel-Heller, S.G.; Schroeter, M.L.; Stumvoll, M.; Villringer, A.; et al. Higher body mass index in older adults is associated with lower gray matter volume: Implications for memory performance. Neurobiol. Aging 2016, 40, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Biessels, G.J.; Deary, I.J.; Ryan, C.M. Cognition and diabetes: A lifespan perspective. Lancet Neurol. 2008, 7, 184–190. [Google Scholar] [CrossRef]
- Shefer, G.; Marcus, Y.; Stern, N. Is obesity a brain disease? Neurosci. Biobehav. Rev. 2013, 37, 2489–2503. [Google Scholar] [CrossRef] [PubMed]
- Hrabosky, J.I.; Cash, T.F.; Veale, D.; Neziroglu, F.; Soll, E.A.; Garner, D.M.; Strachan-Kinser, M.; Bakke, B.; Clauss, L.J.; Phillips, K.A. Multidimensional body image comparisons among patients with eating disorders, body dysmorphic disorder, and clinical controls: A multisite study. Body Image 2009, 6, 155–163. [Google Scholar] [CrossRef]
- Urgesi, C.; Fornasari, L.; de Faccio, S.; Perini, L.; Mattiussi, E.; Ciano, R.; Rucci, P.; Fabbro, F.; Brambilla, P. Body schema and self-representation in patients with bulimia nervosa. Int. J. Eat. Disord. 2011, 44, 238–248. [Google Scholar] [CrossRef]
- Dijkerman, H.C.; de Haan, E.H.F. Somatosensory processes subserving perception and action. Behav. Brain Sci. 2007, 30, 189–201. [Google Scholar] [CrossRef]
- Ionta, S.; Gassert, R.; Brookes, M.J. Multi-Sensory and Sensorimotor Foundation of Bodily Self-Consciousness—An Interdisciplinary Approach. Front. Psychol. 2011, 2, 383. [Google Scholar] [CrossRef]
- Giglia, G.; Pia, L.; Folegatti, A.; Puma, A.; Fierro, B.; Cosentino, G.; Berti, A.; Brighina, F. Far Space Remapping by Tool Use: A rTMS Study Over the Right Posterior Parietal Cortex. Brain Stimul. 2015, 8, 795–800. [Google Scholar] [CrossRef]
- Case, L.K.; Wilson, R.C.; Ramachandran, V.S. Diminished size–weight illusion in anorexia nervosa: Evidence for visuo-proprioceptive integration deficit. Exp. Brain Res. 2011, 217, 79–87. [Google Scholar] [CrossRef]
- Eshkevari, E.; Rieger, E.; Longo, M.R.; Haggard, P.; Treasure, J. Increased plasticity of the bodily self in eating disorders. Psychol. Med. 2012, 42, 819–828. [Google Scholar] [CrossRef]
- Lozano-Serra, E.; Andrés-Perpiña, S.; Lázaro-García, L.; Castro-Fornieles, J. Adolescent Anorexia Nervosa: Cognitive performance after weight recovery. J. Psychosom. Res. 2014, 76, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Waldstein, S.R.; Katzel, L., I. Interactive relations of central versus total obesity and blood pressure to cognitive function. Int. J. Obes. 2005, 30, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Cruikshank, L.C.; Singhal, A.; Hueppelsheuser, M.; Caplan, J.B. Theta oscillations reflect a putative neural mechanism for human sensorimotor integration. J. Neurophysiol. 2012, 107, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Van Driel, J.; Knapen, T.; van Es, D.M.; Cohen, M.X. Interregional alpha-band synchrony supports temporal cross-modal integration. NeuroImage 2014, 101, 404–415. [Google Scholar] [CrossRef] [PubMed]
- Van Pelt, S.; Heil, L.; Kwisthout, J.; Ondobaka, S.; van Rooij, I.; Bekkering, H. Beta- and gamma-band activity reflect predictive coding in the processing of causal events. Soc. Cogn. Affect. Neurosci. 2016, 11, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Koster-Hale, J.; Saxe, R.R.; Dungan, J.; Young, L.L. Decoding moral judgments from neural representations of intentions. Proc. Natl. Acad. Sci. USA 2013, 110, 5648–5653. [Google Scholar] [CrossRef]
| Inclusion Criteria (Male, Right-Handed (LQ > 40; Age 18–60)) | |
|---|---|
| CTR | OBS |
| BMI: 18-25 kg/m2 | BMI > 40 kg/m2 |
| healthy | History of obesity for at least 7 years |
| No comorbidities | |
| Scheduled for bariatric surgery at the General Gastroenterologic Surgery- University Hospital “Paolo Giaccone”. | |
| CTR | OBS | |
|---|---|---|
| N | 10 | 10 |
| Age | 43.3 ± 5.7 | 44.4 ± 9.8 |
| BMI * | 23.3 ± 3.2 | 47.6 ± 7.8 |
| Education | 13.4 ± 4.3 | 12.2 ± 3.3 |
| SPM | 46.1 ± 7.4 | 46.7 ± 6.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gambino, G.; Giglia, G.; Schiera, G.; Di Majo, D.; Epifanio, M.S.; La Grutta, S.; Lo Baido, R.; Ferraro, G.; Sardo, P. Haptic Perception in Extreme Obesity: qEEG Study Focused on Predictive Coding and Body Schema. Brain Sci. 2020, 10, 908. https://doi.org/10.3390/brainsci10120908
Gambino G, Giglia G, Schiera G, Di Majo D, Epifanio MS, La Grutta S, Lo Baido R, Ferraro G, Sardo P. Haptic Perception in Extreme Obesity: qEEG Study Focused on Predictive Coding and Body Schema. Brain Sciences. 2020; 10(12):908. https://doi.org/10.3390/brainsci10120908
Chicago/Turabian StyleGambino, Giuditta, Giuseppe Giglia, Girolamo Schiera, Danila Di Majo, Maria Stella Epifanio, Sabina La Grutta, Rosa Lo Baido, Giuseppe Ferraro, and Pierangelo Sardo. 2020. "Haptic Perception in Extreme Obesity: qEEG Study Focused on Predictive Coding and Body Schema" Brain Sciences 10, no. 12: 908. https://doi.org/10.3390/brainsci10120908
APA StyleGambino, G., Giglia, G., Schiera, G., Di Majo, D., Epifanio, M. S., La Grutta, S., Lo Baido, R., Ferraro, G., & Sardo, P. (2020). Haptic Perception in Extreme Obesity: qEEG Study Focused on Predictive Coding and Body Schema. Brain Sciences, 10(12), 908. https://doi.org/10.3390/brainsci10120908