Does Aerobic and Resistance Exercise Influence Episodic Memory through Unique Mechanisms?
Abstract
:1. Introduction
2. Acute Exercise Modality on Memory
3. Chronic Exercise Modality on Memory
4. Combined Exercise Modality on Memory
5. Exercise Modality Mechanisms on Memory
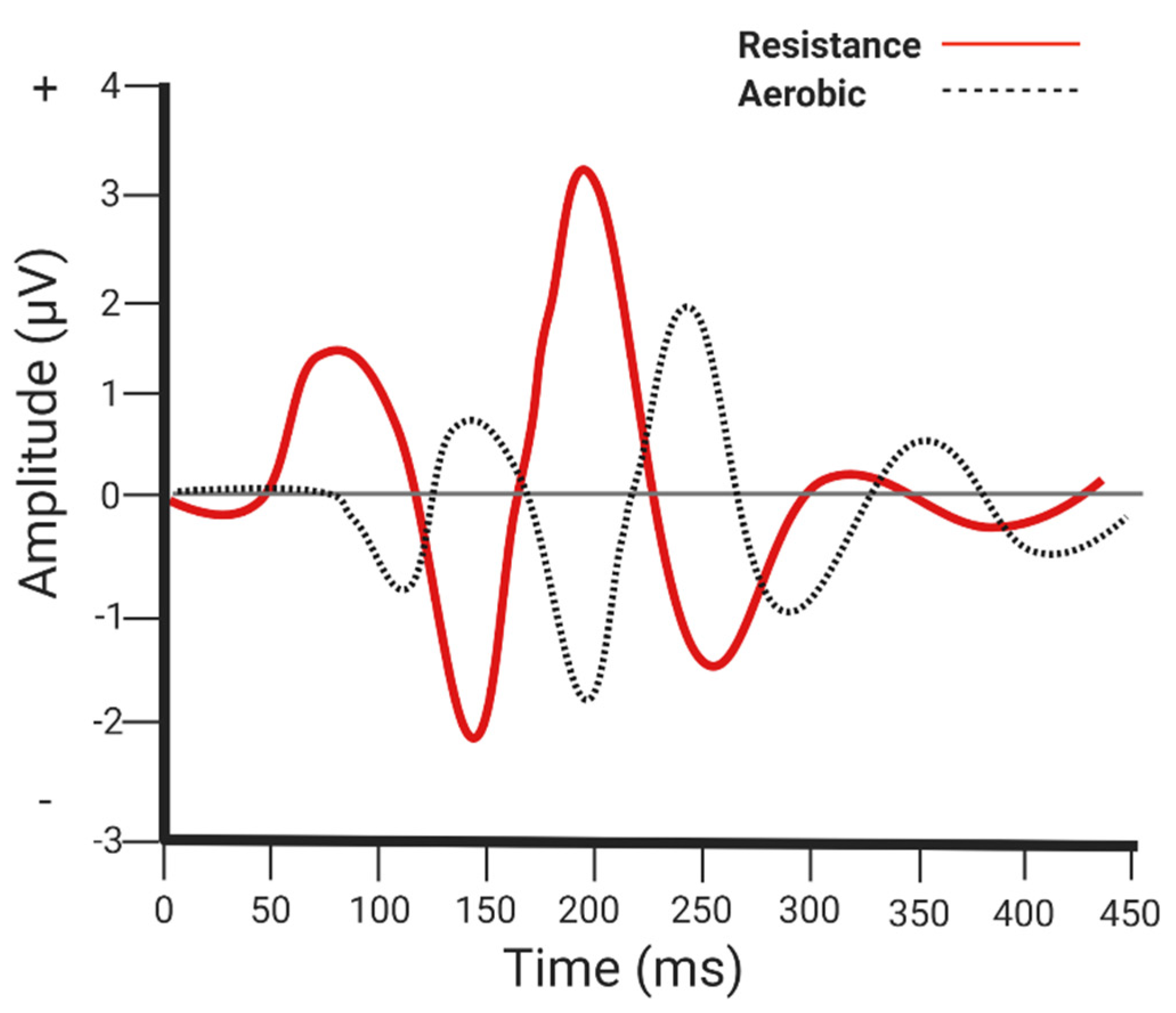
5.1. Neuroelectrical Parameters
5.2. Long-Term Potentiation and Related Parameters
5.3. Other Potential Candidate Mechanisms
6. Conclusions
Funding
Conflicts of Interest
References
- ACSM. ACSM’s Guidelines for Exercise Testing and Prescription, 10th ed.; ACSM: Indianapolis, IN, USA, 2017. [Google Scholar]
- Baechle, T.R.; Earle, R.W. Essentials of Strength Trianing and Conditioning, 3rd ed.; Human Kinetics: Champaign, IL, USA, 2008. [Google Scholar]
- Dankel, S.J.; Loenneke, J.P.; Loprinzi, P.D. Determining the Importance of Meeting Muscle-Strengthening Activity Guidelines: Is the Behavior or the Outcome of the Behavior (Strength) a More Important Determinant of All-Cause Mortality? Mayo Clin. Proc. 2016, 91, 166–174. [Google Scholar] [CrossRef]
- Stamatakis, E.; Lee, I.M.; Bennie, J.; Freeston, J.; Hamer, M.; O’Donovan, G.; Ding, D.; Bauman, A.; Mavros, Y. Does Strength-Promoting Exercise Confer Unique Health Benefits? A Pooled Analysis of Data on 11 Population Cohorts with All-Cause, Cancer, and Cardiovascular Mortality Endpoints. Am. J. Epidemiol. 2018, 187, 1102–1112. [Google Scholar] [CrossRef] [Green Version]
- Buckner, S.L.; Loenneke, J.P.; Loprinzi, P.D. Single and combined associations of accelerometer-assessed physical activity and muscle-strengthening activities on plasma homocysteine in a national sample. Clin. Physiol. Funct. Imaging 2017, 37, 669–674. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.K.; Pan, C.Y.; Chen, F.T.; Tsai, C.L.; Huang, C.C. Effect of resistance-exercise training on cognitive function in healthy older adults: A review. J. Aging Phys. Act. 2012, 20, 497–517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Northey, J.M.; Cherbuin, N.; Pumpa, K.L.; Smee, D.J.; Rattray, B. Exercise interventions for cognitive function in adults older than 50: A systematic review with meta-analysis. Br. J. Sports Med. 2017. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.K.; Labban, J.D.; Gapin, J.I.; Etnier, J.L. The effects of acute exercise on cognitive performance: A meta-analysis. Brain Res. 2012, 1453, 87–101. [Google Scholar] [CrossRef] [Green Version]
- Roig, M.; Nordbrandt, S.; Geertsen, S.S.; Nielsen, J.B. The effects of cardiovascular exercise on human memory: A review with meta-analysis. Neurosci. Biobehav. Rev. 2013, 37, 1645–1666. [Google Scholar] [CrossRef]
- Loprinzi, P.D.; Frith, E.; Edwards, M.K.; Sng, E.; Ashpole, N. The Effects of Exercise on Memory Function among Young to Middle-Aged Adults: Systematic Review and Recommendations for Future Research. Am. J. Health Promot. AJHP 2018, 32, 691–704. [Google Scholar] [CrossRef]
- Barha, C.K.; Davis, J.C.; Falck, R.S.; Nagamatsu, L.S.; Liu-Ambrose, T. Sex differences in exercise efficacy to improve cognition: A systematic review and meta-analysis of randomized controlled trials in older humans. Front. Neuroendocrinol. 2017, 46, 71–85. [Google Scholar] [CrossRef]
- Colcombe, S.; Kramer, A.F. Fitness effects on the cognitive function of older adults: A meta-analytic study. Psychol. Sci. 2003, 14, 125–130. [Google Scholar] [CrossRef]
- Loprinzi, P.D.; Frith, E.; Edwards, M.K. Resistance exercise and episodic memory function: A systematic review. Clin. Physiol. Funct. Imaging 2018. [Google Scholar] [CrossRef] [PubMed]
- Roig, M.; Thomas, R.; Mang, C.S.; Snow, N.J.; Ostadan, F.; Boyd, L.A.; Lundbye-Jensen, J. Time-Dependent Effects of Cardiovascular Exercise on Memory. Exerc. Sport Sci. Rev. 2016, 44, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Loprinzi, P.D.; Edwards, M.K.; Frith, E. Potential avenues for exercise to activate episodic memory-related pathways: A narrative review. Eur. J. Neurosci. 2017, 46, 2067–2077. [Google Scholar] [CrossRef] [PubMed]
- Guadagni, V.; Drogos, L.L.; Tyndall, A.V.; Davenport, M.H.; Anderson, T.J.; Eskes, G.A.; Longman, R.S.; Hill, M.D.; Hogan, D.B.; Poulin, M.J. Aerobic exercise improves cognition and cerebrovascular regulation in older adults. Neurology 2020, 94, e2245–e2257. [Google Scholar] [CrossRef] [PubMed]
- Erickson, K.I.; Hillman, C.; Stillman, C.M.; Ballard, R.M.; Bloodgood, B.; Conroy, D.E.; Macko, R.; Marquez, D.X.; Petruzzello, S.J.; Powell, K.E.; et al. Physical Activity, Cognition, and Brain Outcomes: A Review of the 2018 Physical Activity Guidelines. Med. Sci. Sports Exerc. 2019, 51, 1242–1251. [Google Scholar] [CrossRef]
- Landrigan, J.F.; Bell, T.; Crowe, M.; Clay, O.J.; Mirman, D. Lifting cognition: A meta-analysis of effects of resistance exercise on cognition. Psychol. Res. 2020, 84, 1167–1183. [Google Scholar] [CrossRef] [Green Version]
- Clark, C.M.; Guadagni, V.; Mazerolle, E.L.; Hill, M.; Hogan, D.B.; Pike, G.B.; Poulin, M.J. Effect of aerobic exercise on white matter microstructure in the aging brain. Behav. Brain Res. 2019, 373, 112042. [Google Scholar] [CrossRef]
- Tulving, E. Elements of Episodic Memory; Oxford University Press: Oxford, UK, 1983. [Google Scholar]
- Labban, J.D.; Etnier, J.L. Effects of acute exercise on long-term memory. Res. Q. Exerc. Sport 2011, 82, 712–721. [Google Scholar] [CrossRef] [Green Version]
- Etnier, J.L.; Wideman, L.; Labban, J.D.; Piepmeier, A.T.; Pendleton, D.M.; Dvorak, K.K.; Becofsky, K. The Effects of Acute Exercise on Memory and Brain-Derived Neurotrophic Factor (BDNF). J. Sport Exerc. Psychol. 2016, 38, 331–340. [Google Scholar] [CrossRef] [Green Version]
- Labban, J.D.; Etnier, J.L. The Effect of Acute Exercise on Encoding and Consolidation of Long-Term Memory. J. Sport Exerc. Psychol. 2018, 40, 336–342. [Google Scholar] [CrossRef]
- Frith, E.; Sng, E.; Loprinzi, P.D. Randomized controlled trial evaluating the temporal effects of high-intensity exercise on learning, short-term and long-term memory, and prospective memory. Eur. J. Neurosci. 2017, 46, 2557–2564. [Google Scholar] [CrossRef] [PubMed]
- Haynes, J.T.T.; Frith, E.; Sng, E.; Loprinzi, P.D. Experimental Effects of Acute Exercise on Episodic Memory Function: Considerations for the Timing of Exercise. Psychol. Rep. 2019, 122, 1744–1754. [Google Scholar] [CrossRef] [PubMed]
- Sng, E.; Frith, E.; Loprinzi, P.D. Temporal Effects of Acute Walking Exercise on Learning and Memory Function. Am. J. Health Promot. AJHP 2018, 32, 1518–1525. [Google Scholar] [CrossRef] [PubMed]
- Loprinzi, P.D.; Green, D.; Wages, S.; Cheke, L.G.; Jones, T. Experimental Effects of Acute High-Intensity Resistance Exercise on Episodic Memory Function: Consideration for Post-Exercise Recovery Period. J. Lifestyle Med. 2020, 10, 7–20. [Google Scholar] [CrossRef]
- Iuliano, E.; Fiorilli, G.; Aquino, G.; Di Costanzo, A.; Calcagno, G.; di Cagno, A. Twelve-Week Exercise Influences Memory Complaint but Not Memory Performance in Older Adults: A Randomized Controlled Study. J. Aging Phys. Act. 2017. [Google Scholar] [CrossRef]
- Best, J.R.; Chiu, B.K.; Liang Hsu, C.; Nagamatsu, L.S.; Liu-Ambrose, T. Long-Term Effects of Resistance Exercise Training on Cognition and Brain Volume in Older Women: Results from a Randomized Controlled Trial. J. Int. Neuropsychol. Soc. JINS 2015, 21, 745–756. [Google Scholar] [CrossRef]
- Jonasson, L.S.; Nyberg, L.; Kramer, A.F.; Lundquist, A.; Riklund, K.; Boraxbekk, C.J. Aerobic Exercise Intervention, Cognitive Performance, and Brain Structure: Results from the Physical Influences on Brain in Aging (PHIBRA) Study. Front. Aging Neurosci. 2016, 8, 336. [Google Scholar] [CrossRef]
- Komulainen, P.; Kivipelto, M.; Lakka, T.A.; Savonen, K.; Hassinen, M.; Kiviniemi, V.; Hanninen, T.; Rauramaa, R. Exercise, fitness and cognition—A randomised controlled trial in older individuals: The DR’s EXTRA study. Eur. Geriatr. Med. 2010, 1, 266–272. [Google Scholar] [CrossRef]
- Aquino, G.; Iuliano, E.; di Cagno, A.; Vardaro, A.; Fiorilli, G.; Moffa, S.; Di Costanzo, A.; De Simone, G.; Calcagno, G. Effects of combined training vs aerobic training on cognitive functions in COPD: A randomized controlled trial. Int. J. Chron. Obs. Pulmon. Dis. 2016, 11, 711–718. [Google Scholar] [CrossRef] [Green Version]
- Bossers, W.J.; van der Woude, L.H.; Boersma, F.; Hortobagyi, T.; Scherder, E.J.; van Heuvelen, M.J. A 9-Week Aerobic and Strength Training Program Improves Cognitive and Motor Function in Patients with Dementia: A Randomized, Controlled Trial. Am. J. Geriatr. Psychiatry Off. J. Am. Assoc. Geriatr. Psychiatry 2015, 23, 1106–1116. [Google Scholar] [CrossRef]
- Lin, T.W.; Chen, S.J.; Huang, T.Y.; Chang, C.Y.; Chuang, J.I.; Wu, F.S.; Kuo, Y.M.; Jen, C.J. Different types of exercise induce differential effects on neuronal adaptations and memory performance. Neurobiol. Learn. Mem. 2012, 97, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Umpierre, D.; Stein, R. Hemodynamic and vascular effects of resistance training: Implications for cardiovascular disease. Arq. Bras. Cardiol. 2007, 89, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Akazawa, N.; Choi, Y.; Miyaki, A.; Sugawara, J.; Ajisaka, R.; Maeda, S. Aerobic exercise training increases cerebral blood flow in postmenopausal women. Artery Res. 2012, 6, 124–129. [Google Scholar] [CrossRef]
- Van Pelt, D.W.; Guth, L.M.; Horowitz, J.F. Aerobic exercise elevates markers of angiogenesis and macrophage IL-6 gene expression in the subcutaneous adipose tissue of overweight-to-obese adults. J. Appl. Physiol. (1985) 2017, 123, 1150–1159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Praag, H.; Shubert, T.; Zhao, C.; Gage, F.H. Exercise enhances learning and hippocampal neurogenesis in aged mice. J. Neurosci. Off. J. Soc. Neurosci. 2005, 25, 8680–8685. [Google Scholar] [CrossRef] [PubMed]
- Novaes Gomes, F.G.; Fernandes, J.; Vannucci Campos, D.; Cassilhas, R.C.; Viana, G.M.; D’Almeida, V.; de Moraes Rego, M.K.; Buainain, P.I.; Cavalheiro, E.A.; Arida, R.M. The beneficial effects of strength exercise on hippocampal cell proliferation and apoptotic signaling is impaired by anabolic androgenic steroids. Psychoneuroendocrinology 2014, 50, 106–117. [Google Scholar] [CrossRef]
- Yau, S.Y.; Li, A.; So, K.F. Involvement of Adult Hippocampal Neurogenesis in Learning and Forgetting. Neural Plast. 2015, 2015, 717958. [Google Scholar] [CrossRef] [Green Version]
- Heo, S.; Prakash, R.S.; Voss, M.W.; Erickson, K.I.; Ouyang, C.; Sutton, B.P.; Kramer, A.F. Resting hippocampal blood flow, spatial memory and aging. Brain Res. 2010, 1315, 119–127. [Google Scholar] [CrossRef] [Green Version]
- Ozkaya, G.Y.; Aydin, H.; Toraman, F.N.; Kizilay, F.; Ozdemir, O.; Cetinkaya, V. Effect of strength and endurance training on cognition in older people. J. Sports Sci. Med. 2005, 4, 300–313. [Google Scholar]
- Fu, S.; Caggiano, D.M.; Greenwood, P.M.; Parasuraman, R. Event-related potentials reveal dissociable mechanisms for orienting and focusing visuospatial attention. Brain Res. Cogn. Brain Res. 2005, 23, 341–353. [Google Scholar] [CrossRef] [Green Version]
- Cheung, C.H.M.; McLoughlin, G.; Brandeis, D.; Banaschewski, T.; Asherson, P.; Kuntsi, J. Neurophysiological Correlates of Attentional Fluctuation in Attention-Deficit/Hyperactivity Disorder. Brain Topogr. 2017, 30, 320–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poo, M.M.; Pignatelli, M.; Ryan, T.J.; Tonegawa, S.; Bonhoeffer, T.; Martin, K.C.; Rudenko, A.; Tsai, L.H.; Tsien, R.W.; Fishell, G.; et al. What is memory? The present state of the engram. BMC Biol. 2016, 14, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugar, J.; Moser, M.B. Episodic memory: Neuronal codes for what, where, and when. Hippocampus 2019, 29, 1190–1205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takeda, M. Brain mechanisms of visual long-term memory retrieval in primates. Neurosci. Res. 2019, 142, 7–15. [Google Scholar] [CrossRef]
- Lu, Y.; Christian, K.; Lu, B. BDNF: A key regulator for protein synthesis-dependent LTP and long-term memory? Neurobiol. Learn. Mem. 2008, 89, 312–323. [Google Scholar] [CrossRef] [Green Version]
- Kida, S. A Functional Role for CREB as a Positive Regulator of Memory Formation and LTP. Exp. Neurobiol. 2012, 21, 136–140. [Google Scholar] [CrossRef]
- Deak, F.; Sonntag, W.E. Aging, synaptic dysfunction, and insulin-like growth factor (IGF)-1. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2012, 67, 611–625. [Google Scholar] [CrossRef] [Green Version]
- Lisman, J.; Yasuda, R.; Raghavachari, S. Mechanisms of CaMKII action in long-term potentiation. Nat. Rev. Neurosci. 2012, 13, 169–182. [Google Scholar] [CrossRef] [Green Version]
- Ehrlich, I.; Malinow, R. Postsynaptic density 95 controls AMPA receptor incorporation during long-term potentiation and experience-driven synaptic plasticity. J. Neurosci. Off. J. Soc. Neurosci. 2004, 24, 916–927. [Google Scholar] [CrossRef] [Green Version]
- Muller, D.; Buchs, P.A.; Stoppini, L.; Boddeke, H. Long-term potentiation, protein kinase C, and glutamate receptors. Mol. Neurobiol. 1991, 5, 277–288. [Google Scholar] [CrossRef]
- Luscher, C.; Malenka, R.C. NMDA receptor-dependent long-term potentiation and long-term depression (LTP/LTD). Cold Spring Harb. Perspect. Biol. 2012, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minichiello, L. TrkB signalling pathways in LTP and learning. Nat. Rev. Neurosci. 2009, 10, 850–860. [Google Scholar] [CrossRef] [PubMed]
- Jankowsky, J.L.; Derrick, B.E.; Patterson, P.H. Cytokine responses to LTP induction in the rat hippocampus: A comparison of in vitro and in vivo techniques. Learn. Mem. 2000, 7, 400–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jankowsky, J.L.; Patterson, P.H. Cytokine and growth factor involvement in long-term potentiation. Mol. Cell. Neurosci. 1999, 14, 273–286. [Google Scholar] [CrossRef]
- Li, A.J.; Katafuchi, T.; Oda, S.; Hori, T.; Oomura, Y. Interleukin-6 inhibits long-term potentiation in rat hippocampal slices. Brain Res. 1997, 748, 30–38. [Google Scholar] [CrossRef]
- Kim, K.B. Effect of different training mode on Interleukin-6 (IL-6) and C-reactive protein (CRP) in type 2 diabetes mellitus (T2DM) patients. J. Exerc. Nutr. Biochem. 2014, 18, 371–378. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, Z.; Shang, C.; Yan, F.; Shi, Y.; Zhang, J.; Qu, B.; Han, H.; Wang, Y.; Li, D.; et al. IGF1-Dependent Synaptic Plasticity of Mitral Cells in Olfactory Memory during Social Learning. Neuron 2017, 95, 106–122.e105. [Google Scholar] [CrossRef] [Green Version]
- Kopec, A.M.; Carew, T.J. Growth factor signaling and memory formation: Temporal and spatial integration of a molecular network. Learn. Mem. 2013, 20, 531–539. [Google Scholar] [CrossRef] [Green Version]
- Llorens-Martin, M.; Torres-Aleman, I.; Trejo, J.L. Mechanisms mediating brain plasticity: IGF1 and adult hippocampal neurogenesis. Neurosci. Rev. J. Bringing Neurobiol. Neurol. Psychiatry 2009, 15, 134–148. [Google Scholar] [CrossRef]
- Le Greves, M.; Steensland, P.; Le Greves, P.; Nyberg, F. Growth hormone induces age-dependent alteration in the expression of hippocampal growth hormone receptor and N-methyl-D-aspartate receptor subunits gene transcripts in male rats. Proc. Natl. Acad. Sci. USA 2002, 99, 7119–7123. [Google Scholar] [CrossRef] [Green Version]
- Zheng, W.H.; Quirion, R. Insulin-like growth factor-1 (IGF-1) induces the activation/phosphorylation of Akt kinase and cAMP response element-binding protein (CREB) by activating different signaling pathways in PC12 cells. BMC Neurosci. 2006, 7, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arazi, H.; Khanmohammadi, A.; Asadi, A.; Haff, G.G. The effect of resistance training set configuration on strength, power, and hormonal adaptation in female volleyball players. Appl. Physiol. Nutr. Metab. 2017, 43, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.-L.; Wang, C.-H.; Pan, C.-Y.; Chen, F.-C. The effects of long-term resistance exercise on the relationship between neurocognitive performance and GH, IGF-1, and homocysteine levels in the elderly. Front. Behav. Neurosci. 2015, 9, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strickland, J.C.; Smith, M.A. Animal models of resistance exercise and their application to neuroscience research. J. Neurosci. Methods 2016, 273, 191–200. [Google Scholar] [CrossRef] [Green Version]
- Ari, Z.; Kutlu, N.; Uyanik, B.S.; Taneli, F.; Buyukyazi, G.; Tavli, T. Serum testosterone, growth hormone, and insulin-like growth factor-1 levels, mental reaction time, and maximal aerobic exercise in sedentary and long-term physically trained elderly males. Int. J. Neurosci. 2004, 114, 623–637. [Google Scholar] [CrossRef]
- Gordon, S.E.; Kraemer, W.J.; Looney, D.P.; Flanagan, S.D.; Comstock, B.A.; Hymer, W.C. The influence of age and exercise modality on growth hormone bioactivity in women. Growth Horm. IGF Res. 2014, 24, 95–103. [Google Scholar] [CrossRef]
- Arikawa, A.Y.; Kurzer, M.S.; Thomas, W.; Schmitz, K.H. No effect of exercise on insulin-like growth factor-I, insulin, and glucose in young women participating in a 16-week randomized controlled trial. Cancer Epidemiol. Biomark. Prev. 2010, 19, 2987–2990. [Google Scholar] [CrossRef] [Green Version]
- Borst, S.E.; De Hoyos, D.V.; Garzarella, L.; Vincent, K.; Pollock, B.H.; Lowenthal, D.T.; Pollock, M.L. Effects of resistance training on insulin-like growth factor-I and IGF binding proteins. Med. Sci. Sports Exerc. 2001, 33, 648–653. [Google Scholar] [CrossRef] [Green Version]
- Cassilhas, R.C.; Antunes, H.K.; Tufik, S.; de Mello, M.T. Mood, anxiety, and serum IGF-1 in elderly men given 24 weeks of high resistance exercise. Percept. Mot. Ski. 2010, 110, 265–276. [Google Scholar] [CrossRef] [Green Version]
- Correia, P.R.; Pansani, A.; Machado, F.; Andrade, M.; Silva, A.C.; Scorza, F.A.; Cavalheiro, E.A.; Arida, R.M. Acute strength exercise and the involvement of small or large muscle mass on plasma brain-derived neurotrophic factor levels. Clinics 2010, 65, 1123–1126. [Google Scholar] [CrossRef] [Green Version]
- Goekint, M.; De Pauw, K.; Roelands, B.; Njemini, R.; Bautmans, I.; Mets, T.; Meeusen, R. Strength training does not influence serum brain-derived neurotrophic factor. Eur. J. Appl. Physiol. 2010, 110, 285–293. [Google Scholar] [CrossRef] [PubMed]
- McTiernan, A.; Sorensen, B.; Yasui, Y.; Tworoger, S.S.; Ulrich, C.M.; Irwin, M.L.; Rudolph, R.E.; Stanczyk, F.Z.; Schwartz, R.S.; Potter, J.D. No effect of exercise on insulin-like growth factor 1 and insulin-like growth factor binding protein 3 in postmenopausal women: A 12-month randomized clinical trial. Cancer Epidemiol. Biomark. Prev. 2005, 14, 1020–1021. [Google Scholar] [CrossRef] [Green Version]
- Vale, R.G.; de Oliveira, R.D.; Pernambuco, C.S.; de Meneses, Y.P.; Novaes Jda, S.; de Andrade Ade, F. Effects of muscle strength and aerobic training on basal serum levels of IGF-1 and cortisol in elderly women. Arch. Gerontol. Geriatr. 2009, 49, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Dinoff, A.; Herrmann, N.; Swardfager, W.; Liu, C.S.; Sherman, C.; Chan, S.; Lanctot, K.L. The Effect of Exercise Training on Resting Concentrations of Peripheral Brain-Derived Neurotrophic Factor (BDNF): A Meta-Analysis. PLoS ONE 2016, 11, e0163037. [Google Scholar] [CrossRef] [PubMed]
- Knaepen, K.; Goekint, M.; Heyman, E.M.; Meeusen, R. Neuroplasticity-exercise-induced response of peripheral brain-derived neurotrophic factor: A systematic review of experimental studies in human subjects. Sports Med. 2010, 40, 765–801. [Google Scholar] [CrossRef] [PubMed]
- Church, D.D.; Hoffman, J.R.; Mangine, G.T.; Jajtner, A.R.; Townsend, J.R.; Beyer, K.S.; Wang, R.; La Monica, M.B.; Fukuda, D.H.; Stout, J.R. Comparison of high-intensity vs. high-volume resistance training on the BDNF response to exercise. J. Appl. Physiol. (1985) 2016, 121, 123–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Meireles, L.C.F.; Galvao, F., Jr.; Walker, D.M.; Cechinel, L.R.; de Souza Grefenhagen, A.I.; Andrade, G.; Palazzo, R.P.; Lovatel, G.A.; Basso, C.G.; Nestler, E.J.; et al. Exercise Modalities Improve Aversive Memory and Survival Rate in Aged Rats: Role of Hippocampal Epigenetic Modifications. Mol. Neurobiol. 2019, 56, 8408–8419. [Google Scholar] [CrossRef]
- Yarrow, J.F.; White, L.J.; McCoy, S.C.; Borst, S.E. Training augments resistance exercise induced elevation of circulating brain derived neurotrophic factor (BDNF). Neurosci. Lett. 2010, 479, 161–165. [Google Scholar] [CrossRef]
- Frystyk, J. Exercise and the growth hormone-insulin-like growth factor axis. Med. Sci. Sports Exerc. 2010, 42, 58–66. [Google Scholar] [CrossRef]
- Carro, E.; Nunez, A.; Busiguina, S.; Torres-Aleman, I. Circulating insulin-like growth factor I mediates effects of exercise on the brain. J. Neurosci. 2000, 20, 2926–2933. [Google Scholar] [CrossRef]
- Cassilhas, R.C.; Lee, K.S.; Fernandes, J.; Oliveira, M.G.; Tufik, S.; Meeusen, R.; de Mello, M.T. Spatial memory is improved by aerobic and resistance exercise through divergent molecular mechanisms. Neuroscience 2012, 202, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Cassilhas, R.C.; Tufik, S.; de Mello, M.T. Physical exercise, neuroplasticity, spatial learning and memory. Cell. Mol. Life Sci. 2016, 73, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.C.; Okamoto, M.; Liu, Y.F.; Inoue, K.; Matsui, T.; Nogami, H.; Soya, H. Voluntary resistance running with short distance enhances spatial memory related to hippocampal BDNF signaling. J. Appl. Physiol. 2012, 113, 1260–1266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vilela, T.C.; Muller, A.P.; Damiani, A.P.; Macan, T.P.; da Silva, S.; Canteiro, P.B.; de Sena Casagrande, A.; Pedroso, G.D.S.; Nesi, R.T.; de Andrade, V.M.; et al. Strength and Aerobic Exercises Improve Spatial Memory in Aging Rats through Stimulating Distinct Neuroplasticity Mechanisms. Mol. Neurobiol. 2017, 54, 7928–7937. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Kang, Y.T.; Yin, B.; Sun, L.J.; Fan, X.S. Effects of weight-bearing ladder and aerobic treadmill exercise on learning and memory ability of diabetic rats and its mechanism. Zhongguo Ying Yong Sheng Li Xue Za Zhi 2017, 33, 436–440. [Google Scholar] [CrossRef] [PubMed]
- Voss, M.W.; Vivar, C.; Kramer, A.F.; van Praag, H. Bridging animal and human models of exercise-induced brain plasticity. Trends Cogn. Sci. 2013, 17, 525–544. [Google Scholar] [CrossRef] [Green Version]
- Zentall, T.R. Animal Cognition: The Bridge Between Animal Learning and Human Cognition. Psychol. Sci. 1999, 10, 206–208. [Google Scholar] [CrossRef]
- Sejnowski, T.J.; Paulsen, O. Network oscillations: Emerging computational principles. J. Neurosci. Off. J. Soc. Neurosci. 2006, 26, 1673–1676. [Google Scholar] [CrossRef] [Green Version]
- Capocchi, G.; Zampolini, M.; Larson, J. Theta burst stimulation is optimal for induction of LTP at both apical and basal dendritic synapses on hippocampal CA1 neurons. Brain Res. 1992, 591, 332–336. [Google Scholar] [CrossRef]
- Buzsaki, G. Theta oscillations in the hippocampus. Neuron 2002, 33, 325–340. [Google Scholar] [CrossRef] [Green Version]
- McFarland, W.L.; Teitelbaum, H.; Hedges, E.K. Relationship between hippocampal theta activity and running speed in the rat. J. Comp. Physiol. Psychol. 1975, 88, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Vanderwolf, C.H. Hippocampal electrical activity and voluntary movement in the rat. Electroencephalogr. Clin. Neurophysiol. 1969, 26, 407–418. [Google Scholar] [CrossRef]
- Feter, N.; Spanevello, R.M.; Soares, M.S.P.; Spohr, L.; Pedra, N.S.; Bona, N.P.; Freitas, M.P.; Gonzales, N.G.; Ito, L.; Stefanello, F.M.; et al. How does physical activity and different models of exercise training affect oxidative parameters and memory? Physiol. Behav. 2018. [Google Scholar] [CrossRef] [PubMed]
- Handayaningsih, A.E.; Iguchi, G.; Fukuoka, H.; Nishizawa, H.; Takahashi, M.; Yamamoto, M.; Herningtyas, E.H.; Okimura, Y.; Kaji, H.; Chihara, K.; et al. Reactive oxygen species play an essential role in IGF-I signaling and IGF-I-induced myocyte hypertrophy in C2C12 myocytes. Endocrinology 2011, 152, 912–921. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Protein | Role in Influencing LTP | Does Blocking This Parameter Influence Memory? | References |
|---|---|---|---|
| BDNF | Facilitates function and structural changes at the synapse. Induces the transformation of E-LTP to L-LTP by, for example, activating PI3K/AKT (protein transcription) and ERK (regulates dendritic and spine morphology) pathways and phosphorylation of CREB. | Yes | [48] |
| CREB | L-LTP via transcription of regulatory proteins. | Yes | [49] |
| IGF-1 | Phosphorylation of voltage-gated calcium channels (increasing calcium influx and neurotransmitter release) and activates PI3K-AKT pathway. | Yes | [50] |
| β-CaMKII | Phosphorylation of AMPA receptors and exocytosis of AMPA receptors. | Yes | [51] |
| PSD-95 | Receptor (e.g., AMPA-R incorporation) and synapse stabilization. | Yes | [52] |
| PKC | LTP induction mechanisms: increased release of pre-synaptic neurotransmitters; closure of dendritic chloride conductances. | Yes | [53] |
| Receptor | |||
| NMDA | Calcium influx, redistribution of AMPA receptors, downstream activation of proteins to maintain L-LTP. | Yes | [54] |
| TrkB | Activation of MAPK, PI3K, and PLCγ pathways. MAPK and PI3K pathways ultimately have effects on neuronal survival and protein transcription; PLCγ activation increases release of intracellular calcium. | Yes | [55] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loprinzi, P.D.; Moore, D.; Loenneke, J.P. Does Aerobic and Resistance Exercise Influence Episodic Memory through Unique Mechanisms? Brain Sci. 2020, 10, 913. https://doi.org/10.3390/brainsci10120913
Loprinzi PD, Moore D, Loenneke JP. Does Aerobic and Resistance Exercise Influence Episodic Memory through Unique Mechanisms? Brain Sciences. 2020; 10(12):913. https://doi.org/10.3390/brainsci10120913
Chicago/Turabian StyleLoprinzi, Paul D., Damien Moore, and Jeremy P. Loenneke. 2020. "Does Aerobic and Resistance Exercise Influence Episodic Memory through Unique Mechanisms?" Brain Sciences 10, no. 12: 913. https://doi.org/10.3390/brainsci10120913
APA StyleLoprinzi, P. D., Moore, D., & Loenneke, J. P. (2020). Does Aerobic and Resistance Exercise Influence Episodic Memory through Unique Mechanisms? Brain Sciences, 10(12), 913. https://doi.org/10.3390/brainsci10120913





