Does Aerobic and Resistance Exercise Influence Episodic Memory through Unique Mechanisms?
Abstract
1. Introduction
2. Acute Exercise Modality on Memory
3. Chronic Exercise Modality on Memory
4. Combined Exercise Modality on Memory
5. Exercise Modality Mechanisms on Memory
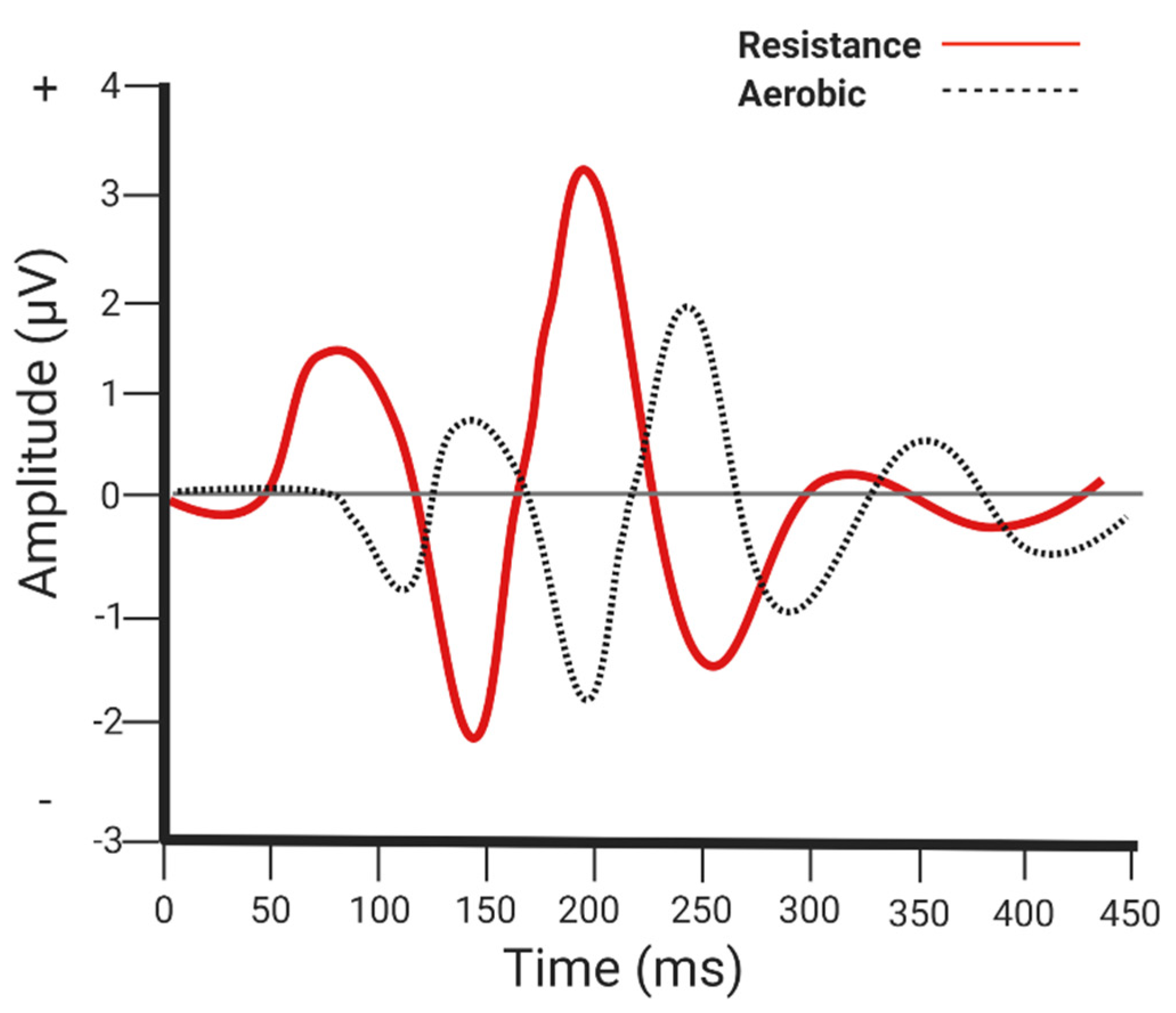
5.1. Neuroelectrical Parameters
5.2. Long-Term Potentiation and Related Parameters
5.3. Other Potential Candidate Mechanisms
6. Conclusions
Funding
Conflicts of Interest
References
- ACSM. ACSM’s Guidelines for Exercise Testing and Prescription, 10th ed.; ACSM: Indianapolis, IN, USA, 2017. [Google Scholar]
- Baechle, T.R.; Earle, R.W. Essentials of Strength Trianing and Conditioning, 3rd ed.; Human Kinetics: Champaign, IL, USA, 2008. [Google Scholar]
- Dankel, S.J.; Loenneke, J.P.; Loprinzi, P.D. Determining the Importance of Meeting Muscle-Strengthening Activity Guidelines: Is the Behavior or the Outcome of the Behavior (Strength) a More Important Determinant of All-Cause Mortality? Mayo Clin. Proc. 2016, 91, 166–174. [Google Scholar] [CrossRef]
- Stamatakis, E.; Lee, I.M.; Bennie, J.; Freeston, J.; Hamer, M.; O’Donovan, G.; Ding, D.; Bauman, A.; Mavros, Y. Does Strength-Promoting Exercise Confer Unique Health Benefits? A Pooled Analysis of Data on 11 Population Cohorts with All-Cause, Cancer, and Cardiovascular Mortality Endpoints. Am. J. Epidemiol. 2018, 187, 1102–1112. [Google Scholar] [CrossRef]
- Buckner, S.L.; Loenneke, J.P.; Loprinzi, P.D. Single and combined associations of accelerometer-assessed physical activity and muscle-strengthening activities on plasma homocysteine in a national sample. Clin. Physiol. Funct. Imaging 2017, 37, 669–674. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.K.; Pan, C.Y.; Chen, F.T.; Tsai, C.L.; Huang, C.C. Effect of resistance-exercise training on cognitive function in healthy older adults: A review. J. Aging Phys. Act. 2012, 20, 497–517. [Google Scholar] [CrossRef] [PubMed]
- Northey, J.M.; Cherbuin, N.; Pumpa, K.L.; Smee, D.J.; Rattray, B. Exercise interventions for cognitive function in adults older than 50: A systematic review with meta-analysis. Br. J. Sports Med. 2017. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.K.; Labban, J.D.; Gapin, J.I.; Etnier, J.L. The effects of acute exercise on cognitive performance: A meta-analysis. Brain Res. 2012, 1453, 87–101. [Google Scholar] [CrossRef]
- Roig, M.; Nordbrandt, S.; Geertsen, S.S.; Nielsen, J.B. The effects of cardiovascular exercise on human memory: A review with meta-analysis. Neurosci. Biobehav. Rev. 2013, 37, 1645–1666. [Google Scholar] [CrossRef]
- Loprinzi, P.D.; Frith, E.; Edwards, M.K.; Sng, E.; Ashpole, N. The Effects of Exercise on Memory Function among Young to Middle-Aged Adults: Systematic Review and Recommendations for Future Research. Am. J. Health Promot. AJHP 2018, 32, 691–704. [Google Scholar] [CrossRef]
- Barha, C.K.; Davis, J.C.; Falck, R.S.; Nagamatsu, L.S.; Liu-Ambrose, T. Sex differences in exercise efficacy to improve cognition: A systematic review and meta-analysis of randomized controlled trials in older humans. Front. Neuroendocrinol. 2017, 46, 71–85. [Google Scholar] [CrossRef]
- Colcombe, S.; Kramer, A.F. Fitness effects on the cognitive function of older adults: A meta-analytic study. Psychol. Sci. 2003, 14, 125–130. [Google Scholar] [CrossRef]
- Loprinzi, P.D.; Frith, E.; Edwards, M.K. Resistance exercise and episodic memory function: A systematic review. Clin. Physiol. Funct. Imaging 2018. [Google Scholar] [CrossRef] [PubMed]
- Roig, M.; Thomas, R.; Mang, C.S.; Snow, N.J.; Ostadan, F.; Boyd, L.A.; Lundbye-Jensen, J. Time-Dependent Effects of Cardiovascular Exercise on Memory. Exerc. Sport Sci. Rev. 2016, 44, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Loprinzi, P.D.; Edwards, M.K.; Frith, E. Potential avenues for exercise to activate episodic memory-related pathways: A narrative review. Eur. J. Neurosci. 2017, 46, 2067–2077. [Google Scholar] [CrossRef] [PubMed]
- Guadagni, V.; Drogos, L.L.; Tyndall, A.V.; Davenport, M.H.; Anderson, T.J.; Eskes, G.A.; Longman, R.S.; Hill, M.D.; Hogan, D.B.; Poulin, M.J. Aerobic exercise improves cognition and cerebrovascular regulation in older adults. Neurology 2020, 94, e2245–e2257. [Google Scholar] [CrossRef] [PubMed]
- Erickson, K.I.; Hillman, C.; Stillman, C.M.; Ballard, R.M.; Bloodgood, B.; Conroy, D.E.; Macko, R.; Marquez, D.X.; Petruzzello, S.J.; Powell, K.E.; et al. Physical Activity, Cognition, and Brain Outcomes: A Review of the 2018 Physical Activity Guidelines. Med. Sci. Sports Exerc. 2019, 51, 1242–1251. [Google Scholar] [CrossRef]
- Landrigan, J.F.; Bell, T.; Crowe, M.; Clay, O.J.; Mirman, D. Lifting cognition: A meta-analysis of effects of resistance exercise on cognition. Psychol. Res. 2020, 84, 1167–1183. [Google Scholar] [CrossRef]
- Clark, C.M.; Guadagni, V.; Mazerolle, E.L.; Hill, M.; Hogan, D.B.; Pike, G.B.; Poulin, M.J. Effect of aerobic exercise on white matter microstructure in the aging brain. Behav. Brain Res. 2019, 373, 112042. [Google Scholar] [CrossRef]
- Tulving, E. Elements of Episodic Memory; Oxford University Press: Oxford, UK, 1983. [Google Scholar]
- Labban, J.D.; Etnier, J.L. Effects of acute exercise on long-term memory. Res. Q. Exerc. Sport 2011, 82, 712–721. [Google Scholar] [CrossRef]
- Etnier, J.L.; Wideman, L.; Labban, J.D.; Piepmeier, A.T.; Pendleton, D.M.; Dvorak, K.K.; Becofsky, K. The Effects of Acute Exercise on Memory and Brain-Derived Neurotrophic Factor (BDNF). J. Sport Exerc. Psychol. 2016, 38, 331–340. [Google Scholar] [CrossRef]
- Labban, J.D.; Etnier, J.L. The Effect of Acute Exercise on Encoding and Consolidation of Long-Term Memory. J. Sport Exerc. Psychol. 2018, 40, 336–342. [Google Scholar] [CrossRef]
- Frith, E.; Sng, E.; Loprinzi, P.D. Randomized controlled trial evaluating the temporal effects of high-intensity exercise on learning, short-term and long-term memory, and prospective memory. Eur. J. Neurosci. 2017, 46, 2557–2564. [Google Scholar] [CrossRef] [PubMed]
- Haynes, J.T.T.; Frith, E.; Sng, E.; Loprinzi, P.D. Experimental Effects of Acute Exercise on Episodic Memory Function: Considerations for the Timing of Exercise. Psychol. Rep. 2019, 122, 1744–1754. [Google Scholar] [CrossRef] [PubMed]
- Sng, E.; Frith, E.; Loprinzi, P.D. Temporal Effects of Acute Walking Exercise on Learning and Memory Function. Am. J. Health Promot. AJHP 2018, 32, 1518–1525. [Google Scholar] [CrossRef] [PubMed]
- Loprinzi, P.D.; Green, D.; Wages, S.; Cheke, L.G.; Jones, T. Experimental Effects of Acute High-Intensity Resistance Exercise on Episodic Memory Function: Consideration for Post-Exercise Recovery Period. J. Lifestyle Med. 2020, 10, 7–20. [Google Scholar] [CrossRef]
- Iuliano, E.; Fiorilli, G.; Aquino, G.; Di Costanzo, A.; Calcagno, G.; di Cagno, A. Twelve-Week Exercise Influences Memory Complaint but Not Memory Performance in Older Adults: A Randomized Controlled Study. J. Aging Phys. Act. 2017. [Google Scholar] [CrossRef]
- Best, J.R.; Chiu, B.K.; Liang Hsu, C.; Nagamatsu, L.S.; Liu-Ambrose, T. Long-Term Effects of Resistance Exercise Training on Cognition and Brain Volume in Older Women: Results from a Randomized Controlled Trial. J. Int. Neuropsychol. Soc. JINS 2015, 21, 745–756. [Google Scholar] [CrossRef]
- Jonasson, L.S.; Nyberg, L.; Kramer, A.F.; Lundquist, A.; Riklund, K.; Boraxbekk, C.J. Aerobic Exercise Intervention, Cognitive Performance, and Brain Structure: Results from the Physical Influences on Brain in Aging (PHIBRA) Study. Front. Aging Neurosci. 2016, 8, 336. [Google Scholar] [CrossRef]
- Komulainen, P.; Kivipelto, M.; Lakka, T.A.; Savonen, K.; Hassinen, M.; Kiviniemi, V.; Hanninen, T.; Rauramaa, R. Exercise, fitness and cognition—A randomised controlled trial in older individuals: The DR’s EXTRA study. Eur. Geriatr. Med. 2010, 1, 266–272. [Google Scholar] [CrossRef]
- Aquino, G.; Iuliano, E.; di Cagno, A.; Vardaro, A.; Fiorilli, G.; Moffa, S.; Di Costanzo, A.; De Simone, G.; Calcagno, G. Effects of combined training vs aerobic training on cognitive functions in COPD: A randomized controlled trial. Int. J. Chron. Obs. Pulmon. Dis. 2016, 11, 711–718. [Google Scholar] [CrossRef]
- Bossers, W.J.; van der Woude, L.H.; Boersma, F.; Hortobagyi, T.; Scherder, E.J.; van Heuvelen, M.J. A 9-Week Aerobic and Strength Training Program Improves Cognitive and Motor Function in Patients with Dementia: A Randomized, Controlled Trial. Am. J. Geriatr. Psychiatry Off. J. Am. Assoc. Geriatr. Psychiatry 2015, 23, 1106–1116. [Google Scholar] [CrossRef]
- Lin, T.W.; Chen, S.J.; Huang, T.Y.; Chang, C.Y.; Chuang, J.I.; Wu, F.S.; Kuo, Y.M.; Jen, C.J. Different types of exercise induce differential effects on neuronal adaptations and memory performance. Neurobiol. Learn. Mem. 2012, 97, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Umpierre, D.; Stein, R. Hemodynamic and vascular effects of resistance training: Implications for cardiovascular disease. Arq. Bras. Cardiol. 2007, 89, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Akazawa, N.; Choi, Y.; Miyaki, A.; Sugawara, J.; Ajisaka, R.; Maeda, S. Aerobic exercise training increases cerebral blood flow in postmenopausal women. Artery Res. 2012, 6, 124–129. [Google Scholar] [CrossRef]
- Van Pelt, D.W.; Guth, L.M.; Horowitz, J.F. Aerobic exercise elevates markers of angiogenesis and macrophage IL-6 gene expression in the subcutaneous adipose tissue of overweight-to-obese adults. J. Appl. Physiol. (1985) 2017, 123, 1150–1159. [Google Scholar] [CrossRef] [PubMed]
- Van Praag, H.; Shubert, T.; Zhao, C.; Gage, F.H. Exercise enhances learning and hippocampal neurogenesis in aged mice. J. Neurosci. Off. J. Soc. Neurosci. 2005, 25, 8680–8685. [Google Scholar] [CrossRef] [PubMed]
- Novaes Gomes, F.G.; Fernandes, J.; Vannucci Campos, D.; Cassilhas, R.C.; Viana, G.M.; D’Almeida, V.; de Moraes Rego, M.K.; Buainain, P.I.; Cavalheiro, E.A.; Arida, R.M. The beneficial effects of strength exercise on hippocampal cell proliferation and apoptotic signaling is impaired by anabolic androgenic steroids. Psychoneuroendocrinology 2014, 50, 106–117. [Google Scholar] [CrossRef]
- Yau, S.Y.; Li, A.; So, K.F. Involvement of Adult Hippocampal Neurogenesis in Learning and Forgetting. Neural Plast. 2015, 2015, 717958. [Google Scholar] [CrossRef]
- Heo, S.; Prakash, R.S.; Voss, M.W.; Erickson, K.I.; Ouyang, C.; Sutton, B.P.; Kramer, A.F. Resting hippocampal blood flow, spatial memory and aging. Brain Res. 2010, 1315, 119–127. [Google Scholar] [CrossRef]
- Ozkaya, G.Y.; Aydin, H.; Toraman, F.N.; Kizilay, F.; Ozdemir, O.; Cetinkaya, V. Effect of strength and endurance training on cognition in older people. J. Sports Sci. Med. 2005, 4, 300–313. [Google Scholar]
- Fu, S.; Caggiano, D.M.; Greenwood, P.M.; Parasuraman, R. Event-related potentials reveal dissociable mechanisms for orienting and focusing visuospatial attention. Brain Res. Cogn. Brain Res. 2005, 23, 341–353. [Google Scholar] [CrossRef]
- Cheung, C.H.M.; McLoughlin, G.; Brandeis, D.; Banaschewski, T.; Asherson, P.; Kuntsi, J. Neurophysiological Correlates of Attentional Fluctuation in Attention-Deficit/Hyperactivity Disorder. Brain Topogr. 2017, 30, 320–332. [Google Scholar] [CrossRef] [PubMed]
- Poo, M.M.; Pignatelli, M.; Ryan, T.J.; Tonegawa, S.; Bonhoeffer, T.; Martin, K.C.; Rudenko, A.; Tsai, L.H.; Tsien, R.W.; Fishell, G.; et al. What is memory? The present state of the engram. BMC Biol. 2016, 14, 40. [Google Scholar] [CrossRef] [PubMed]
- Sugar, J.; Moser, M.B. Episodic memory: Neuronal codes for what, where, and when. Hippocampus 2019, 29, 1190–1205. [Google Scholar] [CrossRef] [PubMed]
- Takeda, M. Brain mechanisms of visual long-term memory retrieval in primates. Neurosci. Res. 2019, 142, 7–15. [Google Scholar] [CrossRef]
- Lu, Y.; Christian, K.; Lu, B. BDNF: A key regulator for protein synthesis-dependent LTP and long-term memory? Neurobiol. Learn. Mem. 2008, 89, 312–323. [Google Scholar] [CrossRef]
- Kida, S. A Functional Role for CREB as a Positive Regulator of Memory Formation and LTP. Exp. Neurobiol. 2012, 21, 136–140. [Google Scholar] [CrossRef]
- Deak, F.; Sonntag, W.E. Aging, synaptic dysfunction, and insulin-like growth factor (IGF)-1. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2012, 67, 611–625. [Google Scholar] [CrossRef]
- Lisman, J.; Yasuda, R.; Raghavachari, S. Mechanisms of CaMKII action in long-term potentiation. Nat. Rev. Neurosci. 2012, 13, 169–182. [Google Scholar] [CrossRef]
- Ehrlich, I.; Malinow, R. Postsynaptic density 95 controls AMPA receptor incorporation during long-term potentiation and experience-driven synaptic plasticity. J. Neurosci. Off. J. Soc. Neurosci. 2004, 24, 916–927. [Google Scholar] [CrossRef]
- Muller, D.; Buchs, P.A.; Stoppini, L.; Boddeke, H. Long-term potentiation, protein kinase C, and glutamate receptors. Mol. Neurobiol. 1991, 5, 277–288. [Google Scholar] [CrossRef]
- Luscher, C.; Malenka, R.C. NMDA receptor-dependent long-term potentiation and long-term depression (LTP/LTD). Cold Spring Harb. Perspect. Biol. 2012, 4. [Google Scholar] [CrossRef] [PubMed]
- Minichiello, L. TrkB signalling pathways in LTP and learning. Nat. Rev. Neurosci. 2009, 10, 850–860. [Google Scholar] [CrossRef] [PubMed]
- Jankowsky, J.L.; Derrick, B.E.; Patterson, P.H. Cytokine responses to LTP induction in the rat hippocampus: A comparison of in vitro and in vivo techniques. Learn. Mem. 2000, 7, 400–412. [Google Scholar] [CrossRef] [PubMed]
- Jankowsky, J.L.; Patterson, P.H. Cytokine and growth factor involvement in long-term potentiation. Mol. Cell. Neurosci. 1999, 14, 273–286. [Google Scholar] [CrossRef]
- Li, A.J.; Katafuchi, T.; Oda, S.; Hori, T.; Oomura, Y. Interleukin-6 inhibits long-term potentiation in rat hippocampal slices. Brain Res. 1997, 748, 30–38. [Google Scholar] [CrossRef]
- Kim, K.B. Effect of different training mode on Interleukin-6 (IL-6) and C-reactive protein (CRP) in type 2 diabetes mellitus (T2DM) patients. J. Exerc. Nutr. Biochem. 2014, 18, 371–378. [Google Scholar] [CrossRef][Green Version]
- Liu, Z.; Chen, Z.; Shang, C.; Yan, F.; Shi, Y.; Zhang, J.; Qu, B.; Han, H.; Wang, Y.; Li, D.; et al. IGF1-Dependent Synaptic Plasticity of Mitral Cells in Olfactory Memory during Social Learning. Neuron 2017, 95, 106–122.e105. [Google Scholar] [CrossRef]
- Kopec, A.M.; Carew, T.J. Growth factor signaling and memory formation: Temporal and spatial integration of a molecular network. Learn. Mem. 2013, 20, 531–539. [Google Scholar] [CrossRef][Green Version]
- Llorens-Martin, M.; Torres-Aleman, I.; Trejo, J.L. Mechanisms mediating brain plasticity: IGF1 and adult hippocampal neurogenesis. Neurosci. Rev. J. Bringing Neurobiol. Neurol. Psychiatry 2009, 15, 134–148. [Google Scholar] [CrossRef]
- Le Greves, M.; Steensland, P.; Le Greves, P.; Nyberg, F. Growth hormone induces age-dependent alteration in the expression of hippocampal growth hormone receptor and N-methyl-D-aspartate receptor subunits gene transcripts in male rats. Proc. Natl. Acad. Sci. USA 2002, 99, 7119–7123. [Google Scholar] [CrossRef]
- Zheng, W.H.; Quirion, R. Insulin-like growth factor-1 (IGF-1) induces the activation/phosphorylation of Akt kinase and cAMP response element-binding protein (CREB) by activating different signaling pathways in PC12 cells. BMC Neurosci. 2006, 7, 51. [Google Scholar] [CrossRef] [PubMed]
- Arazi, H.; Khanmohammadi, A.; Asadi, A.; Haff, G.G. The effect of resistance training set configuration on strength, power, and hormonal adaptation in female volleyball players. Appl. Physiol. Nutr. Metab. 2017, 43, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.-L.; Wang, C.-H.; Pan, C.-Y.; Chen, F.-C. The effects of long-term resistance exercise on the relationship between neurocognitive performance and GH, IGF-1, and homocysteine levels in the elderly. Front. Behav. Neurosci. 2015, 9, 23. [Google Scholar] [CrossRef] [PubMed]
- Strickland, J.C.; Smith, M.A. Animal models of resistance exercise and their application to neuroscience research. J. Neurosci. Methods 2016, 273, 191–200. [Google Scholar] [CrossRef]
- Ari, Z.; Kutlu, N.; Uyanik, B.S.; Taneli, F.; Buyukyazi, G.; Tavli, T. Serum testosterone, growth hormone, and insulin-like growth factor-1 levels, mental reaction time, and maximal aerobic exercise in sedentary and long-term physically trained elderly males. Int. J. Neurosci. 2004, 114, 623–637. [Google Scholar] [CrossRef]
- Gordon, S.E.; Kraemer, W.J.; Looney, D.P.; Flanagan, S.D.; Comstock, B.A.; Hymer, W.C. The influence of age and exercise modality on growth hormone bioactivity in women. Growth Horm. IGF Res. 2014, 24, 95–103. [Google Scholar] [CrossRef]
- Arikawa, A.Y.; Kurzer, M.S.; Thomas, W.; Schmitz, K.H. No effect of exercise on insulin-like growth factor-I, insulin, and glucose in young women participating in a 16-week randomized controlled trial. Cancer Epidemiol. Biomark. Prev. 2010, 19, 2987–2990. [Google Scholar] [CrossRef][Green Version]
- Borst, S.E.; De Hoyos, D.V.; Garzarella, L.; Vincent, K.; Pollock, B.H.; Lowenthal, D.T.; Pollock, M.L. Effects of resistance training on insulin-like growth factor-I and IGF binding proteins. Med. Sci. Sports Exerc. 2001, 33, 648–653. [Google Scholar] [CrossRef]
- Cassilhas, R.C.; Antunes, H.K.; Tufik, S.; de Mello, M.T. Mood, anxiety, and serum IGF-1 in elderly men given 24 weeks of high resistance exercise. Percept. Mot. Ski. 2010, 110, 265–276. [Google Scholar] [CrossRef]
- Correia, P.R.; Pansani, A.; Machado, F.; Andrade, M.; Silva, A.C.; Scorza, F.A.; Cavalheiro, E.A.; Arida, R.M. Acute strength exercise and the involvement of small or large muscle mass on plasma brain-derived neurotrophic factor levels. Clinics 2010, 65, 1123–1126. [Google Scholar] [CrossRef]
- Goekint, M.; De Pauw, K.; Roelands, B.; Njemini, R.; Bautmans, I.; Mets, T.; Meeusen, R. Strength training does not influence serum brain-derived neurotrophic factor. Eur. J. Appl. Physiol. 2010, 110, 285–293. [Google Scholar] [CrossRef] [PubMed]
- McTiernan, A.; Sorensen, B.; Yasui, Y.; Tworoger, S.S.; Ulrich, C.M.; Irwin, M.L.; Rudolph, R.E.; Stanczyk, F.Z.; Schwartz, R.S.; Potter, J.D. No effect of exercise on insulin-like growth factor 1 and insulin-like growth factor binding protein 3 in postmenopausal women: A 12-month randomized clinical trial. Cancer Epidemiol. Biomark. Prev. 2005, 14, 1020–1021. [Google Scholar] [CrossRef][Green Version]
- Vale, R.G.; de Oliveira, R.D.; Pernambuco, C.S.; de Meneses, Y.P.; Novaes Jda, S.; de Andrade Ade, F. Effects of muscle strength and aerobic training on basal serum levels of IGF-1 and cortisol in elderly women. Arch. Gerontol. Geriatr. 2009, 49, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Dinoff, A.; Herrmann, N.; Swardfager, W.; Liu, C.S.; Sherman, C.; Chan, S.; Lanctot, K.L. The Effect of Exercise Training on Resting Concentrations of Peripheral Brain-Derived Neurotrophic Factor (BDNF): A Meta-Analysis. PLoS ONE 2016, 11, e0163037. [Google Scholar] [CrossRef] [PubMed]
- Knaepen, K.; Goekint, M.; Heyman, E.M.; Meeusen, R. Neuroplasticity-exercise-induced response of peripheral brain-derived neurotrophic factor: A systematic review of experimental studies in human subjects. Sports Med. 2010, 40, 765–801. [Google Scholar] [CrossRef] [PubMed]
- Church, D.D.; Hoffman, J.R.; Mangine, G.T.; Jajtner, A.R.; Townsend, J.R.; Beyer, K.S.; Wang, R.; La Monica, M.B.; Fukuda, D.H.; Stout, J.R. Comparison of high-intensity vs. high-volume resistance training on the BDNF response to exercise. J. Appl. Physiol. (1985) 2016, 121, 123–128. [Google Scholar] [CrossRef] [PubMed]
- De Meireles, L.C.F.; Galvao, F., Jr.; Walker, D.M.; Cechinel, L.R.; de Souza Grefenhagen, A.I.; Andrade, G.; Palazzo, R.P.; Lovatel, G.A.; Basso, C.G.; Nestler, E.J.; et al. Exercise Modalities Improve Aversive Memory and Survival Rate in Aged Rats: Role of Hippocampal Epigenetic Modifications. Mol. Neurobiol. 2019, 56, 8408–8419. [Google Scholar] [CrossRef]
- Yarrow, J.F.; White, L.J.; McCoy, S.C.; Borst, S.E. Training augments resistance exercise induced elevation of circulating brain derived neurotrophic factor (BDNF). Neurosci. Lett. 2010, 479, 161–165. [Google Scholar] [CrossRef]
- Frystyk, J. Exercise and the growth hormone-insulin-like growth factor axis. Med. Sci. Sports Exerc. 2010, 42, 58–66. [Google Scholar] [CrossRef]
- Carro, E.; Nunez, A.; Busiguina, S.; Torres-Aleman, I. Circulating insulin-like growth factor I mediates effects of exercise on the brain. J. Neurosci. 2000, 20, 2926–2933. [Google Scholar] [CrossRef]
- Cassilhas, R.C.; Lee, K.S.; Fernandes, J.; Oliveira, M.G.; Tufik, S.; Meeusen, R.; de Mello, M.T. Spatial memory is improved by aerobic and resistance exercise through divergent molecular mechanisms. Neuroscience 2012, 202, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Cassilhas, R.C.; Tufik, S.; de Mello, M.T. Physical exercise, neuroplasticity, spatial learning and memory. Cell. Mol. Life Sci. 2016, 73, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.C.; Okamoto, M.; Liu, Y.F.; Inoue, K.; Matsui, T.; Nogami, H.; Soya, H. Voluntary resistance running with short distance enhances spatial memory related to hippocampal BDNF signaling. J. Appl. Physiol. 2012, 113, 1260–1266. [Google Scholar] [CrossRef] [PubMed]
- Vilela, T.C.; Muller, A.P.; Damiani, A.P.; Macan, T.P.; da Silva, S.; Canteiro, P.B.; de Sena Casagrande, A.; Pedroso, G.D.S.; Nesi, R.T.; de Andrade, V.M.; et al. Strength and Aerobic Exercises Improve Spatial Memory in Aging Rats through Stimulating Distinct Neuroplasticity Mechanisms. Mol. Neurobiol. 2017, 54, 7928–7937. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Kang, Y.T.; Yin, B.; Sun, L.J.; Fan, X.S. Effects of weight-bearing ladder and aerobic treadmill exercise on learning and memory ability of diabetic rats and its mechanism. Zhongguo Ying Yong Sheng Li Xue Za Zhi 2017, 33, 436–440. [Google Scholar] [CrossRef] [PubMed]
- Voss, M.W.; Vivar, C.; Kramer, A.F.; van Praag, H. Bridging animal and human models of exercise-induced brain plasticity. Trends Cogn. Sci. 2013, 17, 525–544. [Google Scholar] [CrossRef]
- Zentall, T.R. Animal Cognition: The Bridge Between Animal Learning and Human Cognition. Psychol. Sci. 1999, 10, 206–208. [Google Scholar] [CrossRef]
- Sejnowski, T.J.; Paulsen, O. Network oscillations: Emerging computational principles. J. Neurosci. Off. J. Soc. Neurosci. 2006, 26, 1673–1676. [Google Scholar] [CrossRef]
- Capocchi, G.; Zampolini, M.; Larson, J. Theta burst stimulation is optimal for induction of LTP at both apical and basal dendritic synapses on hippocampal CA1 neurons. Brain Res. 1992, 591, 332–336. [Google Scholar] [CrossRef]
- Buzsaki, G. Theta oscillations in the hippocampus. Neuron 2002, 33, 325–340. [Google Scholar] [CrossRef]
- McFarland, W.L.; Teitelbaum, H.; Hedges, E.K. Relationship between hippocampal theta activity and running speed in the rat. J. Comp. Physiol. Psychol. 1975, 88, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Vanderwolf, C.H. Hippocampal electrical activity and voluntary movement in the rat. Electroencephalogr. Clin. Neurophysiol. 1969, 26, 407–418. [Google Scholar] [CrossRef]
- Feter, N.; Spanevello, R.M.; Soares, M.S.P.; Spohr, L.; Pedra, N.S.; Bona, N.P.; Freitas, M.P.; Gonzales, N.G.; Ito, L.; Stefanello, F.M.; et al. How does physical activity and different models of exercise training affect oxidative parameters and memory? Physiol. Behav. 2018. [Google Scholar] [CrossRef] [PubMed]
- Handayaningsih, A.E.; Iguchi, G.; Fukuoka, H.; Nishizawa, H.; Takahashi, M.; Yamamoto, M.; Herningtyas, E.H.; Okimura, Y.; Kaji, H.; Chihara, K.; et al. Reactive oxygen species play an essential role in IGF-I signaling and IGF-I-induced myocyte hypertrophy in C2C12 myocytes. Endocrinology 2011, 152, 912–921. [Google Scholar] [CrossRef] [PubMed]


| Protein | Role in Influencing LTP | Does Blocking This Parameter Influence Memory? | References |
|---|---|---|---|
| BDNF | Facilitates function and structural changes at the synapse. Induces the transformation of E-LTP to L-LTP by, for example, activating PI3K/AKT (protein transcription) and ERK (regulates dendritic and spine morphology) pathways and phosphorylation of CREB. | Yes | [48] |
| CREB | L-LTP via transcription of regulatory proteins. | Yes | [49] |
| IGF-1 | Phosphorylation of voltage-gated calcium channels (increasing calcium influx and neurotransmitter release) and activates PI3K-AKT pathway. | Yes | [50] |
| β-CaMKII | Phosphorylation of AMPA receptors and exocytosis of AMPA receptors. | Yes | [51] |
| PSD-95 | Receptor (e.g., AMPA-R incorporation) and synapse stabilization. | Yes | [52] |
| PKC | LTP induction mechanisms: increased release of pre-synaptic neurotransmitters; closure of dendritic chloride conductances. | Yes | [53] |
| Receptor | |||
| NMDA | Calcium influx, redistribution of AMPA receptors, downstream activation of proteins to maintain L-LTP. | Yes | [54] |
| TrkB | Activation of MAPK, PI3K, and PLCγ pathways. MAPK and PI3K pathways ultimately have effects on neuronal survival and protein transcription; PLCγ activation increases release of intracellular calcium. | Yes | [55] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loprinzi, P.D.; Moore, D.; Loenneke, J.P. Does Aerobic and Resistance Exercise Influence Episodic Memory through Unique Mechanisms? Brain Sci. 2020, 10, 913. https://doi.org/10.3390/brainsci10120913
Loprinzi PD, Moore D, Loenneke JP. Does Aerobic and Resistance Exercise Influence Episodic Memory through Unique Mechanisms? Brain Sciences. 2020; 10(12):913. https://doi.org/10.3390/brainsci10120913
Chicago/Turabian StyleLoprinzi, Paul D., Damien Moore, and Jeremy P. Loenneke. 2020. "Does Aerobic and Resistance Exercise Influence Episodic Memory through Unique Mechanisms?" Brain Sciences 10, no. 12: 913. https://doi.org/10.3390/brainsci10120913
APA StyleLoprinzi, P. D., Moore, D., & Loenneke, J. P. (2020). Does Aerobic and Resistance Exercise Influence Episodic Memory through Unique Mechanisms? Brain Sciences, 10(12), 913. https://doi.org/10.3390/brainsci10120913





