Pathophysiology, Biomarkers, and Therapeutic Modalities Associated with Skeletal Muscle Loss Following Spinal Cord Injury
Abstract
1. Introduction
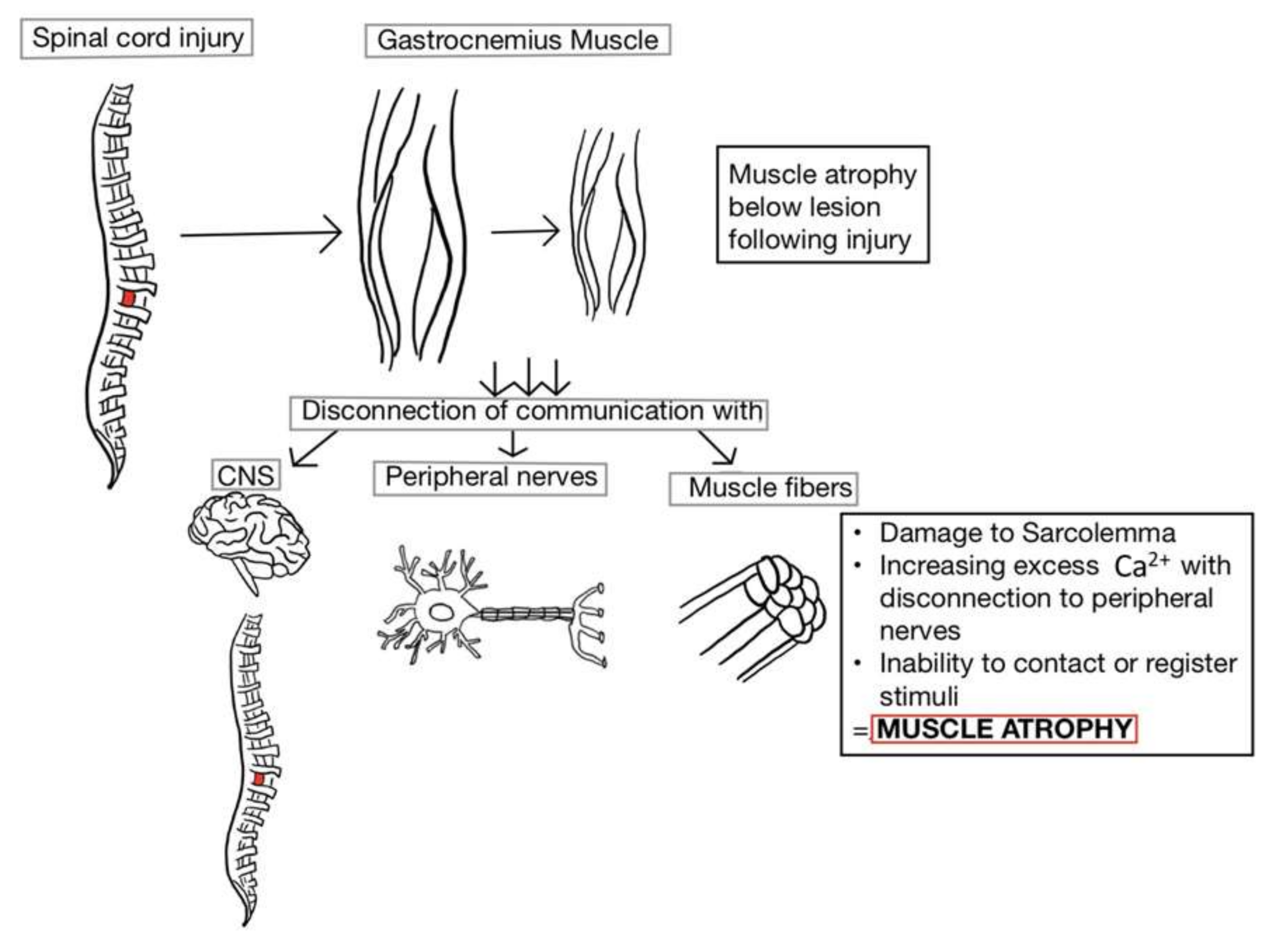
2. Muscle Atrophy
Muscle Fiber Transformation
3. Molecular Pathways and Biomarkers after SCI
3.1. Muscle Hypertrophy Biomarkers
3.2. Inflammatory Biomarkers
3.3. Muscle Atrophy Biomarkers
3.4. Calcium-Activated Proteases
4. Treatment Paradigms to Mitigate Muscle Atrophy
4.1. Clinical Rehabilitation
4.2. Muscle Stimulation
4.3. Ursolic Acid
4.4. Acetoside Injection
4.5. Pyruvate Kinase Muscle Isoform 2 (PKM2)
4.6. Effects of IFN-γ and Calpeptin
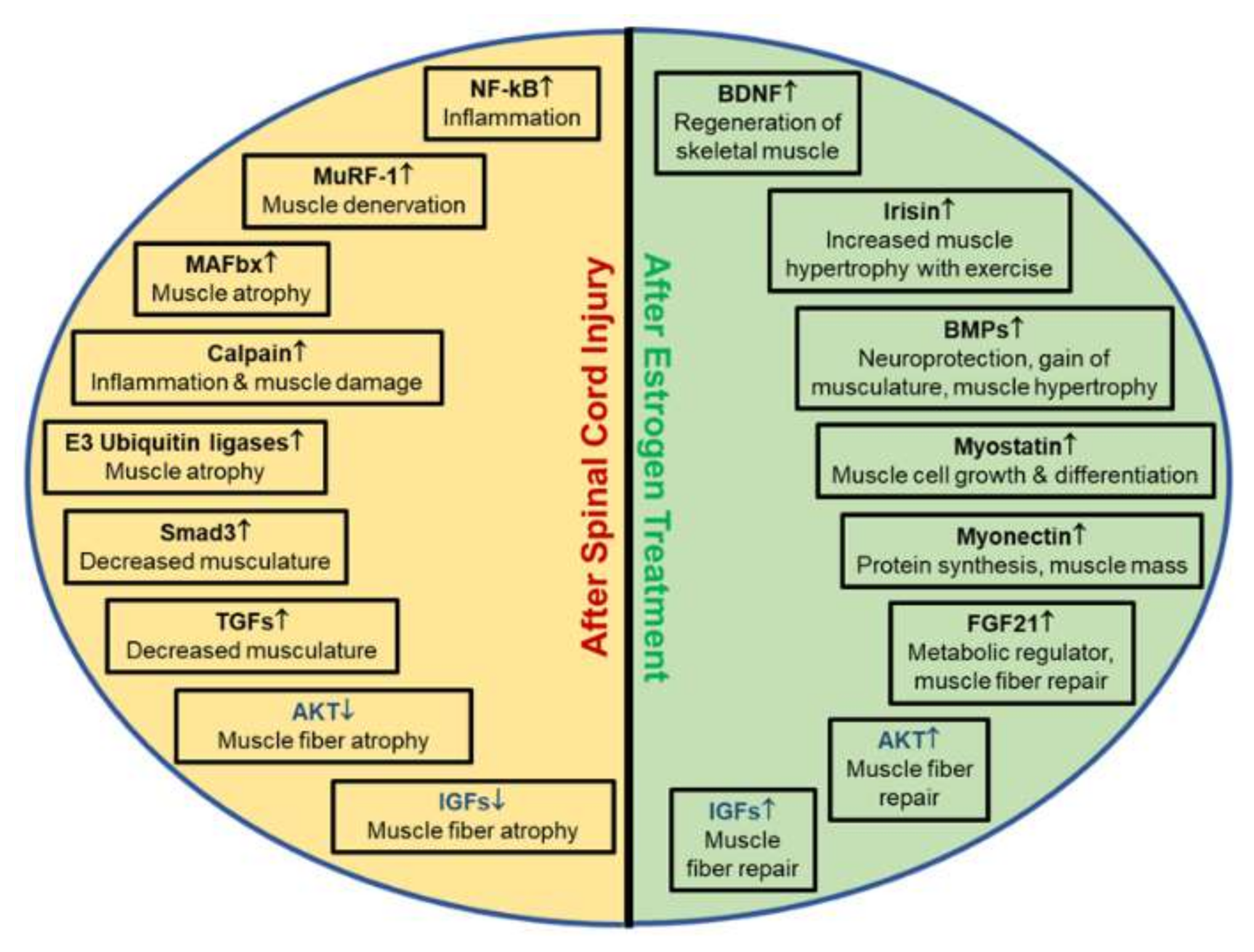
4.7. Estrogen Effects
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lasfargues, J.E.; Custis, D.; Morrone, F.; Well, J.C.; Nguyen, T. A model for estimating spinal cord injury prevalence in the United States. Spinal Cord 1995, 33, 62–68. [Google Scholar] [CrossRef]
- Jain, N.B.; Ayers, G.D.; Peterson, E.N.; Harris, M.B.; Morse, L.R.; O’Connor, K.C.; Garshick, E. Traumatic Spinal Cord Injury in the United States, 1993–2012. JAMA 2015, 313, 2236–2243. [Google Scholar] [CrossRef]
- Thakore, N.P.; Samantaray, S.; Park, S.; Nozaki, K.; Smith, J.A.; Cox, A.; Krause, J.; Banik, N.L. Molecular Changes in Sub-lesional Muscle Following Acute Phase of Spinal Cord Injury. Neurochem. Res. 2015, 41, 44–52. [Google Scholar] [CrossRef]
- Baligand, C.; Chen, Y.-W.; Ye, F.; Pandey, S.N.; Lai, S.-H.; Liu, M.; Vandenborne, K. Transcriptional Pathways Associated with Skeletal Muscle Changes after Spinal Cord Injury and Treadmill Locomotor Training. BioMed Res. Int. 2015, 2015, 1–13. [Google Scholar] [CrossRef]
- Park, E.; Velumian, A.A.; Fehlings, M.G. The Role of Excitotoxicity in Secondary Mechanisms of Spinal Cord Injury: A Review with an Emphasis on the Implications for White Matter Degeneration. J. Neurotrauma 2004, 21, 754–774. [Google Scholar] [CrossRef]
- Nash, M.S.; Groah, S.L.; Gater, D.R.; Dyson-Hudson, T.A.; Lieberman, J.A.; Myers, J.; Sabharwal, S.; Taylor, A.J.; Consortium for Spinal Cord Medicine. Identification and Management of Cardiometabolic Risk after Spinal Cord Injury: Clinical Practice Guideline for Health Care Providers. Top. Spinal Cord Inj. Rehabil. 2018, 24, 379–423. [Google Scholar] [CrossRef]
- Sribnick, E.A.; Wingrave, J.M.; Matzelle, D.D.; Ray, S.K.; Banik, N.L. Estrogen as a Neuroprotective Agent in the Treatment of Spinal Cord Injury. Ann. N. Y. Acad. Sci. 2003, 993, 125–133. [Google Scholar] [CrossRef]
- Sribnick, E.A.; Wingrave, J.M.; Matzelle, D.D.; Wilford, G.G.; Ray, S.K.; Banik, N.L. Estrogen attenuated markers of inflammation and decreased lesion volume in acute spinal cord injury in rats. J. Neurosci. Res. 2005, 82, 283–293. [Google Scholar] [CrossRef]
- Kodani, A.; Kikuchi, T.; Tohda, C. Acteoside Improves Muscle Atrophy and Motor Function by Inducing New Myokine Secretion in Chronic Spinal Cord Injury. J. Neurotrauma 2019, 36, 1935–1948. [Google Scholar] [CrossRef]
- Schwab, J.M.; Zhang, Y.; Kopp, M.A.; Brommer, B.; Popovich, P.G. The paradox of chronic neuroinflammation, systemic immune suppression, autoimmunity after traumatic chronic spinal cord injury. Exp. Neurol. 2014, 258, 121–129. [Google Scholar] [CrossRef]
- Alizadeh, A.; Dyck, S.M.; Karimi-Abdolrezaee, S. Traumatic Spinal Cord Injury: An Overview of Pathophysiology, Models and Acute Injury Mechanisms. Front. Neurol. 2019, 10, 282. [Google Scholar] [CrossRef]
- Tran, A.P.; Warren, P.M.; Silver, J. The Biology of Regeneration Failure and Success after Spinal Cord Injury. Physiol. Rev. 2018, 98, 881–917. [Google Scholar] [CrossRef]
- Pukos, N.; Goodus, M.T.; Sahinkaya, F.R.; McTigue, D.M. Myelin status and oligodendrocyte lineage cells over time after spinal cord injury: What do we know and what still needs to be unwrapped? Glia 2019, 67, 2178–2202. [Google Scholar] [CrossRef]
- Orr, M.B.; Gensel, J.C. Spinal Cord Injury Scarring and Inflammation: Therapies Targeting Glial and Inflammatory Responses. Neurotherapeutics 2018, 15, 541–553. [Google Scholar] [CrossRef]
- Chen, K.; Deng, S.; Lu, H.; Zheng, Y.; Yang, G.; Kim, D.; Cao, K.; Wu, J.Q. RNA-seq characterization of spinal cord injury transcriptome in acute/subacute phases: A resource for understanding the pathology at the systems level. PLoS ONE 2013, 8, e72567. [Google Scholar] [CrossRef]
- Otzel, D.M.; Lee, J.; Ye, F.; Borst, S.E.; Yarrow, J.F. Activity-Based Physical Rehabilitation with Adjuvant Testosterone to Promote Neuromuscular Recovery after Spinal Cord Injury. Int. J. Mol. Sci. 2018, 19, 1701. [Google Scholar] [CrossRef]
- Galea, M.P. Spinal cord injury and physical activity: Preservation of the body. Spinal Cord 2011, 50, 344–351. [Google Scholar] [CrossRef]
- Gorgey, A.S.; Witt, O.; O’Brien, L.; Cardozo, C.; Chen, Q.; Lesnefsky, E.J.; Graham, Z.A. Mitochondrial health and muscle plasticity after spinal cord injury. Graefe’s Arch. Clin. Exp. Ophthalmol. 2018, 119, 315–331. [Google Scholar] [CrossRef]
- Sribnick, E.A.; Samantaray, S.; Das, A.; Smith, J.; Matzelle, D.D.; Ray, S.K.; Banik, N.L. Postinjury estrogen treatment of chronic spinal cord injury improves locomotor function in rats. J. Neurosci. Res. 2010, 88, 1738–1750. [Google Scholar] [CrossRef]
- Yang, J.; Weimer, R.M.; Kallop, D.; Olsen, O.; Wu, Z.; Renier, N.; Uryu, K.; Tessier-Lavigne, M. Regulation of Axon Degeneration after Injury and in Development by the Endogenous Calpain Inhibitor Calpastatin. Neuron 2013, 80, 1175–1189. [Google Scholar] [CrossRef]
- Lee, J.H.; Jun, H.-S. Role of Myokines in Regulating Skeletal Muscle Mass and Function. Front. Physiol. 2019, 10, 42. [Google Scholar] [CrossRef]
- Giangregorio, L.; McCartney, N. Bone Loss and Muscle Atrophy in Spinal Cord Injury: Epidemiology, Fracture Prediction, and Rehabilitation Strategies. J. Spinal Cord Med. 2006, 29, 489–500. [Google Scholar] [CrossRef]
- Cartee, G.D.; Hepple, R.T.; Bamman, M.M.; Zierath, J.R. Exercise Promotes Healthy Aging of Skeletal Muscle. Cell Metab. 2016, 23, 1034–1047. [Google Scholar] [CrossRef]
- Graham, Z.A.; Siedlik, J.A.; Harlow, L.; Sahbani, K.; Bauman, W.A.; Tawfeek, H.A.; Cardozo, C.P. Key Glycolytic Metabolites in Paralyzed Skeletal Muscle Are Altered Seven Days after Spinal Cord Injury in Mice. J. Neurotrauma 2019, 36, 2722–2731. [Google Scholar] [CrossRef]
- Baldi, J.C.; Jackson, R.D.; Moraille, R.; Mysiw, W.J. Muscle atrophy is prevented in patients with acute spinal cord injury using functional electrical stimulation. Spinal Cord 1998, 36, 463–469. [Google Scholar] [CrossRef]
- Anwar, M.A.; Al Shehabi, T.S.; Eid, A.H. Inflammogenesis of Secondary Spinal Cord Injury. Front. Cell. Neurosci. 2016, 10, 98. [Google Scholar] [CrossRef]
- Visavadiya, N.P.; Patel, S.P.; VanRooyen, J.L.; Sullivan, P.G.; Rabchevsky, A.G. Cellular and subcellular oxidative stress parameters following severe spinal cord injury. Redox Biol. 2016, 8, 59–67. [Google Scholar] [CrossRef]
- Kennedy, P.R. Corticospinal, rubrospinal and rubro-olivary projections: A unifying hypothesis. Trends Neurosci. 1990, 13, 474–479. [Google Scholar] [CrossRef]
- Tetzlaff, W.; Kobayashi, N.; Giehl, K.; Tsui, B.; Cassar, S.; Bedard, A. Chapter 22 Response of Rubrospinal and Corticospinal Neurons to Injury and Neurotrophins; Elsevier BV: Amsterdam, The Netherlands, 1994; Volume 103, pp. 271–286. [Google Scholar]
- Mousavi, K.; Parry, D.J.; Jasmin, B.J. BDNF rescues myosin heavy chain IIB muscle fibers after neonatal nerve injury. Am. J. Physiol. Physiol. 2004, 287, C22–C29. [Google Scholar] [CrossRef]
- Hill, M.A.; Bennett, M.R. Cholinergic growth factor from skeletal muscle elevated following denervation. Neurosci. Lett. 1983, 35, 31–35. [Google Scholar] [CrossRef]
- Edwards, C. The effects of innervation on the properties of acetylcholine receptors in muscle. Neuroscience 1979, 4, 565–584. [Google Scholar] [CrossRef]
- Lund, D.; Ruggiero, A.M.; Ferguson, S.M.; Wright, J.; English, B.A.; Reisz, P.A.; Whitaker, S.M.; Peltier, A.C.; Blakely, R.D. Motor neuron-specific overexpression of the presynaptic choline transporter: Impact on motor endurance and evoked muscle activity. Neuroscience 2010, 171, 1041–1053. [Google Scholar] [CrossRef]
- Longo, M.; Zatterale, F.; Naderi, J.; Parrillo, L.; Formisano, P.; Raciti, G.A.; Beguinot, F.; Miele, C.; Longo, M.; Zatterale, F.; et al. Adipose Tissue Dysfunction as Determinant of Obesity-Associated Metabolic Complications. Int. J. Mol. Sci. 2019, 20, 2358. [Google Scholar] [CrossRef]
- Xu, X.M.; Guénard, V.; Kleitman, N.; Aebischer, P.; Bunge, M.B. A Combination of BDNF and NT-3 Promotes Supraspinal Axonal Regeneration into Schwann Cell Grafts in Adult Rat Thoracic Spinal Cord. Exp. Neurol. 1995, 134, 261–272. [Google Scholar] [CrossRef]
- Shenkman, B.S.; Belova, S.P.; Lomonosova, Y.N.; Kostrominova, T.Y.; Nemirovskaya, T.L. Calpain-dependent regulation of the skeletal muscle atrophy following unloading. Arch. Biochem. Biophys. 2015, 584, 36–41. [Google Scholar] [CrossRef]
- Marshall, A.D.; Salerno, M.S.; Thomas, M.; Davies, T.; Berry, C.; Dyer, K.; Bracegirdle, J.; Watson, T.; Dziadek, M.; Kambadur, R.; et al. Mighty is a novel promyogenic factor in skeletal myogenesis. Exp. Cell Res. 2008, 314, 1013–1029. [Google Scholar] [CrossRef]
- Seldin, M.M.; Peterson, J.M.; Byerly, M.S.; Wei, Z.; Wong, G.W. Myonectin (CTRP15), a Novel Myokine That Links Skeletal Muscle to Systemic Lipid Homeostasis. J. Biol. Chem. 2012, 287, 11968–11980. [Google Scholar] [CrossRef]
- Nozaki, K.; Das, A.; Ray, S.K.; Banik, N.L. Calpain inhibition attenuates intracellular changes in muscle cells in response to extracellular inflammatory stimulation. Exp. Neurol. 2010, 225, 430–435. [Google Scholar] [CrossRef]
- Zendedel, R.; Schouten, B.C.; Van Weert, J.C.M.; Putte, B.V.D. Informal interpreting in general practice: The migrant patient’s voice. Ethn. Health 2016, 23, 158–173. [Google Scholar] [CrossRef]
- Nozaki, K.; Das, A.; Ray, S.K.; Banik, N.L. Calpeptin attenuated apoptosis and intracellular inflammatory changes in muscle cells. J. Neurosci. Res. 2011, 89, 536–543. [Google Scholar] [CrossRef]
- McClung, J.M.; Davis, J.M.; Wilson, M.A.; Goldsmith, E.C.; Carson, J.A. Estrogen status and skeletal muscle recovery from disuse atrophy. J. Appl. Physiol. 2006, 100, 2012–2023. [Google Scholar] [CrossRef]
- Mochalova, E.P.; Belova, S.P.; Mirzoev, T.M.; Shenkman, B.S.; Nemirovskaya, T.L. Atrogin-1/MAFbx mRNA expression is regulated by histone deacetylase 1 in rat soleus muscle under hindlimb unloading. Sci. Rep. 2019, 9, 1–8. [Google Scholar] [CrossRef]
- Okada, A.; Ono, Y.; Nagatomi, R.; Kishimoto, K.N.; Itoi, E. Decreased muscle atrophy F-box (MAFbx) expression in regenerating muscle after muscle-damaging exercise. Muscle Nerve 2008, 38, 1246–1253. [Google Scholar] [CrossRef]
- Ray, S.K.; Hogan, E.L.; Banik, N.L. Calpain in the pathophysiology of spinal cord injury: Neuroprotection with calpain inhibitors. Brain Res. Brain Res. Rev. 2003, 42, 169–185. [Google Scholar] [CrossRef]
- Springer, J.E.; Azbill, D.; Kennedy, S.E.; George, J.; Geddes, J.W. Rapid calpain I activation and cytoskeletal protein degradation following traumatic spinal cord injury: Attenuation with riluzole pretreatment. J. Neurochem. 1997, 69, 1592–1600. [Google Scholar] [CrossRef]
- Yu, C.-G.; Li, Y.; Raza, K.; Yu, X.X.; Ghoshal, S.; Geddes, J.W. Calpain 1 Knockdown Improves Tissue Sparing and Functional Outcomes after Spinal Cord Injury in Rats. J. Neurotrauma 2013, 30, 427–433. [Google Scholar] [CrossRef]
- Blomgren, K.; Zhu, C.; Wang, X.; Karlsson, J.-O. Synergistic activation of caspase-3 by m-calpain after neonatal hypoxia-ischemia: A mechanism of pathological apoptosis? J. Biol. Chem. 2001, 276, 10191–10198. [Google Scholar] [CrossRef]
- Pantoja-Melendez, C.A.; Miranda-Duarte, A.; Roque-Ramirez, B.; Zenteno, J.C. Epidemiological and Molecular Characterization of a Mexican Population Isolate with High Prevalence of Limb-Girdle Muscular Dystrophy Type 2A Due to a Novel Calpain-3 Mutation. PLoS ONE 2017, 12, e0170280. [Google Scholar] [CrossRef]
- Hayes, P.; Varga, V.; Olego-Fernandez, S.; Sunter, J.; Ginger, M.L.; Gull, K. Modulation of a cytoskeletal calpain-like protein induces major transitions in trypanosome morphology. J. Cell Biol. 2014, 206, 377–384. [Google Scholar] [CrossRef]
- Richard, I.; Broux, O.; Allamand, V.; Fougerousse, F.; Chiannilkulchai, N.; Bourg, N.; Brenguier, L.; Devaud, C.; Pasturaud, P.; Roudaut, C.; et al. Mutations in the proteolytic enzyme calpain 3 cause limb-girdle muscular dystrophy type 2A. Cell 1995, 81, 27–40. [Google Scholar] [CrossRef]
- Burns, A.S.; Marino, R.J.; Kalsi-Ryan, S.; Middleton, J.W.; Tetreault, L.A.; Dettori, J.R.; Mihalovich, K.E.; Fehlings, M.G. Type and Timing of Rehabilitation Following Acute and Subacute Spinal Cord Injury: A Systematic Review. Glob. Spine J. 2017, 7, 175S–194S. [Google Scholar] [CrossRef] [PubMed]
- Bryce, T.N.; Budh, C.N.; Cardenas, D.D.; Dijkers, M.; Felix, E.R.; Finnerup, N.B.; Kennedy, P.; Lundeberg, T.; Richards, J.S.; Rintala, D.H.; et al. Pain After Spinal Cord Injury: An Evidence-based Review for Clinical Practice and Research. J. Spinal Cord Med. 2007, 30, 421–440. [Google Scholar] [CrossRef]
- Ethier, C.; Brizzi, L.; Giguère, D.; Capaday, C. Corticospinal control of antagonistic muscles in the cat. Eur. J. Neurosci. 2007, 26, 1632–1641. [Google Scholar] [CrossRef]
- Hanna-Boutros, B.; Sangari, S.; Giboin, L.-S.; El Mendili, M.-M.; Lackmy-Vallee, A.; Marchand-Pauvert, V.; Knikou, M. Corticospinal and reciprocal inhibition actions on human soleus motoneuron activity during standing and walking. Physiol. Rep. 2015, 3, e12276. [Google Scholar] [CrossRef]
- Thomas, C.; Zaidner, E.; Calancie, B.; Broton, J.; Bigland-Ritchie, B. Muscle Weakness, Paralysis, and Atrophy after Human Cervical Spinal Cord Injury. Exp. Neurol. 1997, 148, 414–423. [Google Scholar] [CrossRef]
- Larson, C.A.; Dension, P.M. Effectiveness of intense, activity-based physical therapy for individuals with spinal cord injury in promoting motor and sensory recovery: Is olfactory mucosa autograft a factor? J. Spinal Cord Med. 2013, 36, 44–57. [Google Scholar] [CrossRef][Green Version]
- Kim, J.C.; Kang, Y.S.; Noh, E.B.; Seo, B.W.; Seo, D.Y.; Park, G.D.; Kim, S.H. Concurrent treatment with ursolic acid and low-intensity treadmill exercise improves muscle atrophy and related outcomes in rats. Korean J. Physiol. Pharmacol. 2018, 22, 427–436. [Google Scholar] [CrossRef]
- Bigford, G.; Darr, A.J.; Bracchi-Ricard, V.C.; Gao, H.; Nash, M.S.; Bethea, J.R. Effects of ursolic acid on sub-lesional muscle pathology in a contusion model of spinal cord injury. PLoS ONE 2018, 13, e0203042. [Google Scholar] [CrossRef]
- Sribnick, E.; Ray, S.; Banik, N.L. Estrogen prevents glutamate-induced apoptosis in C6 glioma cells by a receptor-mediated mechanism. Neuroscience 2006, 137, 197–209. [Google Scholar] [CrossRef]
- Tiidus, P.M.; Lowe, D.A.; Brown, M. Estrogen replacement and skeletal muscle: Mechanisms and population health. J. Appl. Physiol. 2013, 115, 569–578. [Google Scholar] [CrossRef]
- Cui, J.; Shen, Y.; Li, R. Estrogen synthesis and signaling pathways during aging: From periphery to brain. Trends Mol. Med. 2013, 19, 197–209. [Google Scholar] [CrossRef]
- Kahlert, S.; Grohé, C.; Karas, R.H.; Löbbert, K.; Neyses, L.; Vetter, H. Effects of Estrogen on Skeletal Myoblast Growth. Biochem. Biophys. Res. Commun. 1997, 232, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Samantaray, S.; Sribnick, E.A.; Das, A.; Thakore, N.P.; Matzelle, D.; Yu, S.P.; Ray, S.K.; Wei, L.; Banik, N.L. Neuroprotective efficacy of estrogen in experimental spinal cord injury in rats. Ann. N. Y. Acad. Sci. 2010, 1199, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Samantaray, S.; Das, A.; Matzelle, D.C.; Yu, S.P.; Wei, L.; Varma, A.; Ray, S.K.; Banik, N.L. Administration of low dose estrogen attenuates gliosis and protects neurons in acute spinal cord injury in rats. J. Neurochem. 2016, 136, 1064–1073. [Google Scholar] [CrossRef]
- Samantaray, S.; Das, A.; Matzelle, D.C.; Yu, S.P.; Wei, L.; Varma, A.; Ray, S.K.; Banik, N.L. Administration of low dose estrogen attenuates persistent inflammation, promotes angiogenesis, and improves locomotor function following chronic spinal cord injury in rats. J. Neurochem. 2016, 137, 604–617. [Google Scholar] [CrossRef]
- Douketis, J.D.; Julian, J.A.; Kearon, C.; Anderson, D.R.; Crowther, M.A.; Bates, S.M.; Barone, M.; Piovella, F.; Turpie, A.G.; Middeldorp, S.; et al. Does the type of hormone replacement therapy influence the risk of deep vein thrombosis? A prospective case-control study. J. Thromb. Haemost. 2005, 3, 943–948. [Google Scholar] [CrossRef]
- Greenblatt, R.B.; Stoddard, L.D. The Estrogen-Cancer Controversy. J. Am. Geriatr. Soc. 1978, 26, 1–8. [Google Scholar] [CrossRef]
- Sola, B.; Renoir, J.M. Estrogenic or antiestrogenic therapies for multiple myeloma? Mol. Cancer 2007, 6, 59. [Google Scholar] [CrossRef]
- Pomerantz, M.; Manola, J.; Taplin, M.-E.; Bubley, G.; Inman, M.; Lowell, J.; Beard, C.; Kantoff, P.W.; Oh, W.K. Phase II Study of Low Dose and High Dose Conjugated Estrogen for Androgen Independent Prostate Cancer. J. Urol. 2007, 177, 2146–2150. [Google Scholar] [CrossRef]
- Cao, G.; Xing, J.; Xiao, X.; Liou, A.K.F.; Gao, Y.; Yin, X.-M.; Clark, R.S.B.; Graham, S.H.; Chen, J. Critical Role of Calpain I in Mitochondrial Release of Apoptosis-Inducing Factor in Ischemic Neuronal Injury. J. Neurosci. 2007, 27, 9278–9293. [Google Scholar] [CrossRef]
- Cox, A.; Varma, A.; Barry, J.; Vertegel, A.; Banik, N. Nanoparticle Estrogen in Rat Spinal Cord Injury Elicits Rapid Anti-Inflammatory Effects in Plasma, Cerebrospinal Fluid, and Tissue. J. Neurotrauma 2015, 32, 1413–1421. [Google Scholar] [CrossRef] [PubMed]
- Samantaray, S.; Matzelle, D.D.; Ray, S.K.; Banik, N.L. Physiological low dose of estrogen-protected neurons in experimental spinal cord injury. Ann. N. Y. Acad. Sci. 2010, 1199, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Cox, A.; Capone, M.; Matzelle, D.; Vertegel, A.A.; Bredikhin, M.; Varma, A.K.; Haque, A.; Shields, D.; Banik, N.L. Nanoparticle based estrogen delivery to spinal cord injury site reduces local parenchymal destruction and improves functional recovery. J. Neurotrauma 2020. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
P. Drasites, K.; Shams, R.; Zaman, V.; Matzelle, D.; C. Shields, D.; P. Garner, D.; J. Sole, C.; Haque, A.; Banik, N.L. Pathophysiology, Biomarkers, and Therapeutic Modalities Associated with Skeletal Muscle Loss Following Spinal Cord Injury. Brain Sci. 2020, 10, 933. https://doi.org/10.3390/brainsci10120933
P. Drasites K, Shams R, Zaman V, Matzelle D, C. Shields D, P. Garner D, J. Sole C, Haque A, Banik NL. Pathophysiology, Biomarkers, and Therapeutic Modalities Associated with Skeletal Muscle Loss Following Spinal Cord Injury. Brain Sciences. 2020; 10(12):933. https://doi.org/10.3390/brainsci10120933
Chicago/Turabian StyleP. Drasites, Kelsey, Ramsha Shams, Vandana Zaman, Denise Matzelle, Donald C. Shields, Dena P. Garner, Christopher J. Sole, Azizul Haque, and Narendra L. Banik. 2020. "Pathophysiology, Biomarkers, and Therapeutic Modalities Associated with Skeletal Muscle Loss Following Spinal Cord Injury" Brain Sciences 10, no. 12: 933. https://doi.org/10.3390/brainsci10120933
APA StyleP. Drasites, K., Shams, R., Zaman, V., Matzelle, D., C. Shields, D., P. Garner, D., J. Sole, C., Haque, A., & Banik, N. L. (2020). Pathophysiology, Biomarkers, and Therapeutic Modalities Associated with Skeletal Muscle Loss Following Spinal Cord Injury. Brain Sciences, 10(12), 933. https://doi.org/10.3390/brainsci10120933








