Surgical Strategies and Clinical Outcome of Large to Giant Sphenoid Wing Meningiomas: A Case Series Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion and Exclusion Criteria
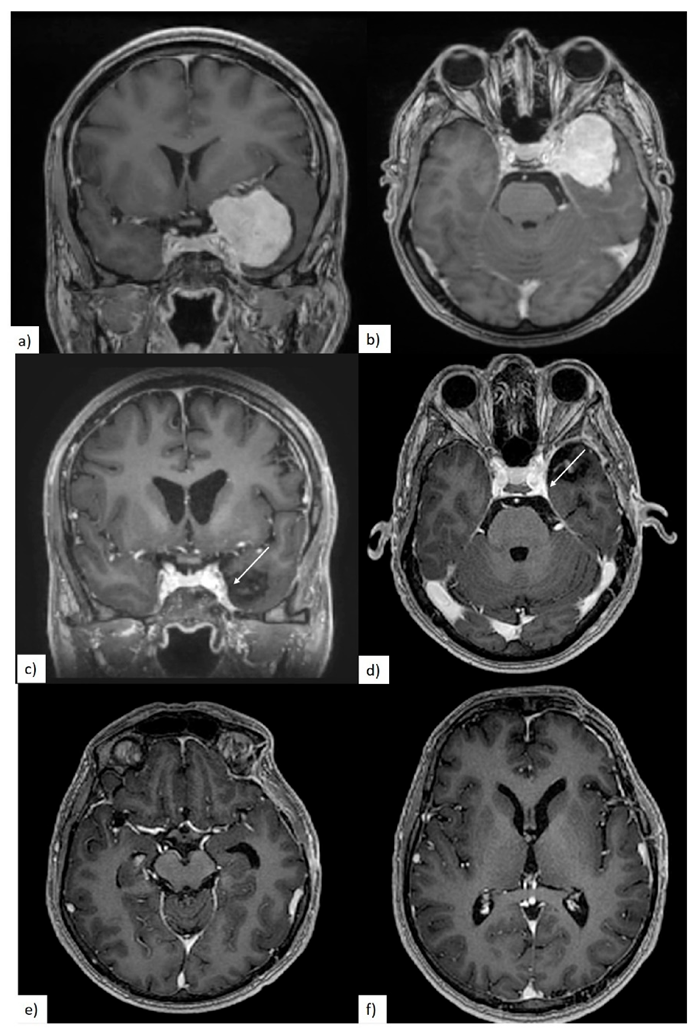
2.2. Clinical and Radiological Evaluation
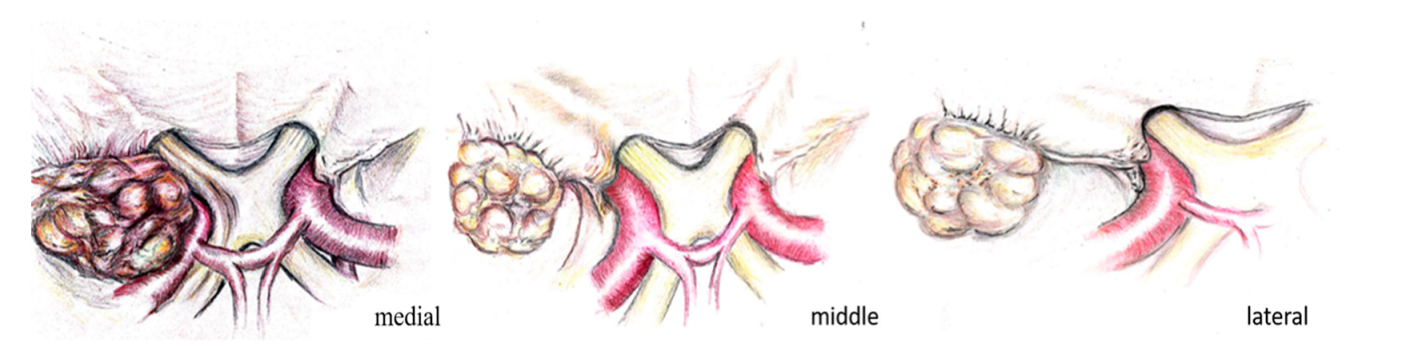
2.3. Classification of Meningiomas
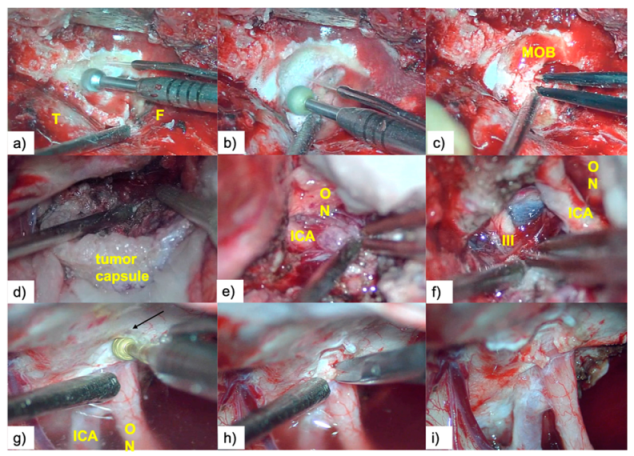
2.4. Description of Surgical Technique
2.5. Resection Quality
3. Results
3.1. Patient Population
3.2. Preoperative Clinical and Radiological Data
3.3. Quality of Resection
3.4. Postoperative Clinical Evolution and Follow-up
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Buerki, R.A.; Horbinski, C.M.; Kruser, T.; Horowitz, P.M.; James, C.D.; Lukas, R.V. An overview of meningiomas. Future Oncol. 2018, 14, 2161–2177. [Google Scholar] [CrossRef] [PubMed]
- Goto, T.; Ohata, K. Surgical resectability of skull base meningiomas. Neurol. Med. Chir. 2016, 56, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Champagne, P.; Lemoine, E.; Bojanowski, M.W. Surgical management of giant sphenoid wing meningiomas encasing major cerebral arteries. Neurosurg. Focus 2018, 44, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Osswald, M. Meningiomas: Overview and New Directions in Therapy. Semin. Neurol. 2018, 38, 112–120. [Google Scholar] [CrossRef]
- Fogh, S.E.; Johnson, D.R.; Barker, F.G.; Brastianos, P.K.; Clarke, J.L.; Kaufmann, T.J.; Oberndorfer, S.; Preusser, M.; Raghunathan, A.; Santagata, S.; et al. Case-based review: Meningiomaa. Neuro-Oncol. Pract. 2016, 3, 120–134. [Google Scholar] [CrossRef]
- Rogers, L.; Barani, I.; Chamberlain, M.; Kaley, T.J.; McDermott, M.; Raizer, J.; Schiff, D.; Weber, D.C.; Wen, P.Y.; Vogelbaum, M.A. Meningiomas: Knowledge base, treatment outcomes, and uncertainties. A RANO review. J. Neurosurg. 2015, 122, 4–23. [Google Scholar] [CrossRef]
- Han, X.Y.; Wang, W.; Wang, L.L.; Wang, X.R.; Li, G. Genetic variants and increased risk of meningioma: An updated meta-analysis. Onco Targets Ther. 2017, 10, 1875–1888. [Google Scholar] [CrossRef]
- Van Alkemade, H.; De Leau, M.; Dieleman, E.M.T.; Kardaun, J.W.P.F.; Van Os, R.; Vandertop, W.P.; Van Furth, W.R.; Stalpers, L.J.A. Impaired survival and long-term neurological problems in benign meningioma. Neuro-Oncology 2012, 14, 658–666. [Google Scholar] [CrossRef]
- Huang, R.Y.; Bi, W.L.; Griffith, B.; Kaufmann, T.J.; La Fougère, C.; Schmidt, N.O.; Tonn, J.C.; Vogelbaum, M.A.; Wen, P.Y.; Aldape, K.; et al. Imaging and diagnostic advances for intracranial meningiomas. Neuro-Oncology 2019, 21, I44–I61. [Google Scholar] [CrossRef]
- Bălașa, A.; Șerban, G.; Chinezu, R.; Hurghiș, C.; Tămaș, F.; Manu, D. The involvement of exosomes in glioblastoma development, diagnosis, prognosis, and treatment. Brain Sci. 2020, 10, 1–16. [Google Scholar] [CrossRef]
- Frič, R.; Hald, J.K.; Antal, E. Benign Sphenoid Wing Meningioma Presenting with an Acute Intracerebral Hemorrhage—A Case Report. J. Cent. Nerv. Syst. Dis. 2016, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Jang, W.Y.; Jung, T.Y.; Kim, I.Y.; Lee, K.H.; Kang, W.D.; Kim, S.K.; Moon, K.S.; Jung, S. Predictive factors for surgical outcome in anterior clinoidal meningiomas. Medicine 2017, 96. [Google Scholar] [CrossRef] [PubMed]
- Zamanipoor Najafabadi, A.H.; Peeters, M.C.M.; Dirven, L.; Lobatto, D.J.; Groen, J.L.; Broekman, M.L.D.; Peerdeman, S.M.; Peul, W.C.; Taphoorn, M.J.B.; Van Furth, W.R. Impaired health-related quality of life in meningioma patients—A systematic review. Neuro-Oncology 2017, 19, 897–907. [Google Scholar] [CrossRef] [PubMed]
- Harvey Cushing, M.D.C.C.T. Meningiomas. Their Classification, Regional Behaviour, Life History, and Surgical End Results. Bull. Med. Libr. Assoc. 1938, 27, 185. [Google Scholar]
- Behari, S.; Giri, P.J.; Shukla, D.; Jain, V.K.; Banerji, D. Surgical strategies for giant medial sphenoid wing meningiomas: A new scoring system for predicting extent of resection. Acta Neurochir. 2008, 150, 865–877. [Google Scholar] [CrossRef]
- Park, H.H.; Yoo, J.; Yun, I.; Hong, C. Comparative Analysis of Endoscopic Transorbital Approach and Extended Mini-Pterional Approach for Sphenoid Wing Meningiomas with Osseous Involvement: Preliminary Surgical Results. World Neurosurg. 2020, 139, e1–e12. [Google Scholar] [CrossRef]
- Bălaşa, A.F.; Chinezu, R.; Teleanu, D.M.; Paşcanu, M.I.; Chinezu, L.; Borda, A. Ectopic intracavernous corticotroph microadenoma: Case report of an extremely rare pathology. Rom. J. Morphol. Embryol. 2017, 58, 1447–1451. [Google Scholar]
- Güdük, M.; Özduman, K.; Pamir, M.N. Sphenoid Wing Meningiomas: Surgical Outcomes in a Series of 141 Cases and Proposal of a Scoring System Predicting Extent of Resection. World Neurosurg. 2019, 125, e48–e59. [Google Scholar] [CrossRef]
- Yu, J.; Guo, Y.; Xu, B.; Xu, K. Clinical importance of the middle meningeal artery: A review of the literature. Int. J. Med. Sci. 2016, 13, 790–799. [Google Scholar] [CrossRef]
- Chaichana, K.L.; Jackson, C.; Patel, A.; Miller, N.R.; Subramanian, P.; Lim, M.; Gallia, G.; Olivi, A.; Weingart, J.; Brem, H.; et al. Predictors of visual outcome following surgical resection of medial sphenoid wing meningiomas. J. Neurol. Surg. Part B Skull Base 2012, 73, 321–326. [Google Scholar] [CrossRef][Green Version]
- Chotai, S.; Liu, Y.; Qi, S. Review of surgical anatomy of the tumors involving cavernous sinus. Asian J. Neurosurg. 2018, 13, 1. [Google Scholar] [CrossRef] [PubMed]
- Islim, A.I.; Mohan, M.; Moon, R.D.C.; Srikandarajah, N.; Mills, S.J.; Brodbelt, A.R.; Jenkinson, M.D. Incidental intracranial meningiomas: A systematic review and meta- analysis of prognostic factors and outcomes. J. Neuro-Oncol. 2019, 142, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Aldahak, N.; El Tantowy, M.; Dupre, D.; Yu, A.; Keller, J.T.; Froelich, S.; Aziz, K.M. Drilling of the marginal tubercle to enhance exposure via mini pterional approach: An anatomical study and clinical series of 25 sphenoid wing meningiomas. Surg. Neurol. Int. 2016, 7, S989–S994. [Google Scholar] [PubMed]
- Giammalva, G.R.; Iacopino, D.G.; Graziano, F.; Gulì, C.; Pino, M.A.; Maugeri, R. Clinical and radiological features of Forestier’s disease presenting with dysphagia. Surg. Neurol. Int. 2018, 9, 236. [Google Scholar] [CrossRef]
- Bălașa, A.F.; Chircov, C.; Grumezescu, A.M. Marine biocompounds for neuroprotection—A review. Mar. Drugs 2020, 18, 290. [Google Scholar] [CrossRef]
- Ivan, M.E.; Cheng, J.S.; Kaur, G.; Sughrue, M.E.; Clark, A.; Kane, A.J.; Aranda, D.; McDermott, M.; Barani, I.J.; Parsa, A.T. Association of morbidity with extent of resection and cavernous sinus invasion in sphenoid wing meningiomas. Skull Base 2011, 21, 76–83. [Google Scholar] [CrossRef]
- Sumkovski, R.; Micunovic, M.; Kocevski, I.; Ilievski, B.; Petrov, I. Surgical treatment of meningiomas-outcome associated with type of resection, recurrence, karnofsky performance score, mitotic count. Open Access Maced. J. Med. Sci. 2019, 7, 56–64. [Google Scholar] [CrossRef]
- Fariselli, L.; Biroli, A.; Signorelli, A.; Broggi, M.; Marchetti, M.; Biroli, F. The cavernous sinus meningiomas’ dilemma: Surgery or stereotactic radiosurgery? Rep. Pract. Oncol. Radiother. 2016, 21, 379–385. [Google Scholar] [CrossRef]
- Chivoret, N.; Fontaine, D.; Lachaud, S.; Chau, Y.; Sedat, J. Endovascular angioplasty before resection of a sphenoidal meningioma with vascular encasement. Interv. Neuroradiol. 2011, 17, 391–394. [Google Scholar] [CrossRef]
- Ehresman, J.S.; Garzon-Muvdi, T.; Rogers, D.; Lim, M.; Gallia, G.L.; Weingart, J.; Brem, H.; Bettegowda, C.; Chaichana, K.L. Risk of Developing Postoperative Deficits Based on Tumor Location after Surgical Resection of an Intracranial Meningioma. J. Neurol. Surg. Part B Skull Base 2018, 80, 59–66. [Google Scholar] [CrossRef]
- Dautzenberg, G.; Lijmer, J.; Beekman, A. Diagnostic accuracy of the Montreal Cognitive Assessment (MoCA) for cognitive screening in old age psychiatry: Determining cutoff scores in clinical practice. Avoiding spectrum bias caused by healthy controls. Int. J. Geriatr. Psychiatry 2020, 35, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Paternostro-Sluga, T.; Grim-Stieger, M.; Posch, M.; Schuhfried, O.; Vacariu, G.; Mittermaier, C.; Bittner, C.; Fialka-Moser, V. Reliability and validity of the Medical Research Council (MRC) scale and a modified scale for testing muscle strength in patients with radial palsy. J. Rehabil. Med. 2008, 40, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Nouwens, F.; Visch-Brink, E.G.; El Hachioui, H.; Lingsma, H.F.; Van De Sandt-Koenderman, M.W.M.E.; Dippel, D.W.J.; Koudstaal, P.J.; De Lau, L.M.L. Validation of a prediction model for long-term outcome of aphasia after stroke. BMC Neurol. 2018, 18, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Nanda, A.; Bir, S.C.; Maiti, T.K.; Konar, S.K.; Missios, S.; Guthikonda, B. Relevance of Simpson grading system and recurrence-free survival after surgery for World Health Organization Grade I meningioma. J. Neurosurg. 2017, 126, 201–211. [Google Scholar] [CrossRef]
- Kattner, K.A.; Fukushima, T. Management of vascular invasion during radical resection of medial sphenoid wing meningiomas. Skull Base 2001, 11, 99–104. [Google Scholar] [CrossRef]
- El Badry, A.; Abdelazeez, A. Outcome of medial sphenoidal wing meningioma surgery. Rom. Neurosurg. 2018, 32, 40–55. [Google Scholar] [CrossRef]
- Lee, J.H.; Jeun, S.S.; Evans, J.; Kosmorsky, G. Surgical management of clinoidal meningiomas. Neurosurgery 2001, 48, 1012–1021. [Google Scholar] [CrossRef] [PubMed]
- Bassiouni, H.; Asgari, S.; Erol Sandalcioglu, I.; Seifert, V.; Stolke, D.; Marquardt, G. Anterior clinoidal meningiomas: Functional outcome after microsurgical resection in a consecutive series of 106 patients—Clinical article. J. Neurosurg. 2009, 111, 1078–1090. [Google Scholar] [CrossRef] [PubMed]
- Romani, R.; Laakso, A.; Kangasniemi, M.; Niemelä, M.; Hernesniemi, J. Lateral supraorbital approach applied to tuberculum sellae meningiomas: Experience with 52 consecutive patients. Neurosurgery 2012, 70, 1504–1518. [Google Scholar] [CrossRef] [PubMed]
- Mariniello, G.; De Divitiis, O.; Seneca, V.; Maiuri, F. Classical pterional compared to the extended skull base approach for the removal of clinoidal meningiomas. J. Clin. Neurosci. 2012, 19, 1646–1650. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Roser, F.; Jacobs, C.; Vorkapic, P.; Samii, M. Medial sphenoid wing meningiomas: Clinical outcome and recurrence rate. Neurosurgery 2006, 58, 626–638. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, K.; Nakamizo, A.; Sasaki, T. Surgical techniques for the dissection of encased perforators in giant clinoidal meningiomas. Acta Neurochir. 2013, 155, 1409–1412. [Google Scholar] [CrossRef] [PubMed]
- Maier, S.; Motataianu, A.; Bajko, Z.; Romaniuc, A.; Balasa, A. Pontine cavernoma haemorrhage at 24 weeks of pregnancy that resulted in eight-and-a-half syndrome. Acta Neurol. Belg. 2019, 119, 471–474. [Google Scholar] [CrossRef] [PubMed]
- McCracken, D.J.; Higginbotham, R.A.; Boulter, J.H.; Liu, Y.; Wells, J.A.; Halani, S.H.; Saindane, A.M.; Oyesiku, N.M.; Barrow, D.L.; Olson, J.J. Degree of vascular encasement in sphenoid wing meningiomas predicts postoperative ischemic complications. Clin. Neurosurg. 2017, 80, 957–966. [Google Scholar] [CrossRef]
- Al-Mefty, O.; Smith, R.R. Surgery of tumors invading the cavernous sinus. Surg. Neurol. 1988, 30, 370–381. [Google Scholar] [CrossRef]
- Sekhar, L.N.; Moller, A.R. Operative management of tumors involving the cavernous sinus. J. Neurosurg. 1986, 64, 879–889. [Google Scholar] [CrossRef]
- Samii, M.; Tatagiba, M. Experience with 36 surgical cases of petroclival meningiomas. Acta Neurochir. 1992, 118, 27–32. [Google Scholar] [CrossRef]
- Sughrue, M.E.; Kane, A.J.; Shangari, G.; Rutkowski, M.J.; McDermott, M.W.; Berger, M.S.; Parsa, A.T. The relevance of Simpson grade I and II resection in modern neurosurgical treatment of World Health Organization grade I meningiomas. J. Neurosurg. 2010, 113, 1029–1035. [Google Scholar] [CrossRef]
- Walsh, M.T.; Couldwell, W.T. Management options for cavernous sinus meningiomas. J. Neuro-Oncol. 2009, 92, 307–316. [Google Scholar] [CrossRef]



| Preoperative Clinical Data | Preoperative Radiological Evaluation | ||||
|---|---|---|---|---|---|
| Clinical Signs | n (%) | MRI Characteristics | n (%) | ||
| cognitive decline | mild | 6 (46%) | sphenoidal ridge tumor origin | medial | 9 (43%) |
| medium | 5 (38.5%) | middle and medial | 6 (28.5%) | ||
| severe | 2 (15.5%) | lateral | 6 (28.5%) | ||
| visual disfunctions | visual field | 6 (54.5) | cavernous sinus invasion | 5 (24%) | |
| visual acuity | 5 (45.5) | optic canal invasion | 6 (28.5%) | ||
| headaches | 9 (43%) | major arterial encasement | total | 11 (52%) | |
| aphasia | 6 (28.5) | partial | 10 (48%) | ||
| motor deficits | 6 (28.5) | cerebral edema | 21 (100%) | ||
| oculomotor nerve palsy | 3 (14%) | average diameter | 6.3 cm ranging between 5–7.4 cm | ||
| seizures | 2 (9.5%) | ||||
| Immediate Postoperative Clinical Evolution | Quality of Resection and Tumoral Progression | ||||
|---|---|---|---|---|---|
| Clinical Data | n (%) | Radiological Data | n (%) | ||
| unchanged | 14 (67%) | GTR | 14 (67%) | ||
| worsened | total | 7 (33%) | |||
| visual disfunctions | 4 (19%) | STR | 7 (33%) | ||
| motor deficits | 2 (9.5%) | location of residual tumor | total | 7 | |
| aphasia | 1 (4.7%) | cavernous sinus | 4 (57%) | ||
| long term clinical follow-up | ICA/MCA | 2 (29%) | |||
| improved | 17 (81%) | sphenoidal ridge | 1 (14%) | ||
| worsened | 2 (9.5%) | site of residual tumor which required surgical reintervention | cavernous sinus | 2 (9.5%) | |
| deaths | 2 (9.5%) | ||||
| surgical reintervention | 2 (9.5%) | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balasa, A.; Hurghis, C.; Tamas, F.; Chinezu, R. Surgical Strategies and Clinical Outcome of Large to Giant Sphenoid Wing Meningiomas: A Case Series Study. Brain Sci. 2020, 10, 957. https://doi.org/10.3390/brainsci10120957
Balasa A, Hurghis C, Tamas F, Chinezu R. Surgical Strategies and Clinical Outcome of Large to Giant Sphenoid Wing Meningiomas: A Case Series Study. Brain Sciences. 2020; 10(12):957. https://doi.org/10.3390/brainsci10120957
Chicago/Turabian StyleBalasa, Adrian, Corina Hurghis, Flaviu Tamas, and Rares Chinezu. 2020. "Surgical Strategies and Clinical Outcome of Large to Giant Sphenoid Wing Meningiomas: A Case Series Study" Brain Sciences 10, no. 12: 957. https://doi.org/10.3390/brainsci10120957
APA StyleBalasa, A., Hurghis, C., Tamas, F., & Chinezu, R. (2020). Surgical Strategies and Clinical Outcome of Large to Giant Sphenoid Wing Meningiomas: A Case Series Study. Brain Sciences, 10(12), 957. https://doi.org/10.3390/brainsci10120957






