Age-Related Decline of Sensorimotor Integration Influences Resting-State Functional Brain Connectivity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
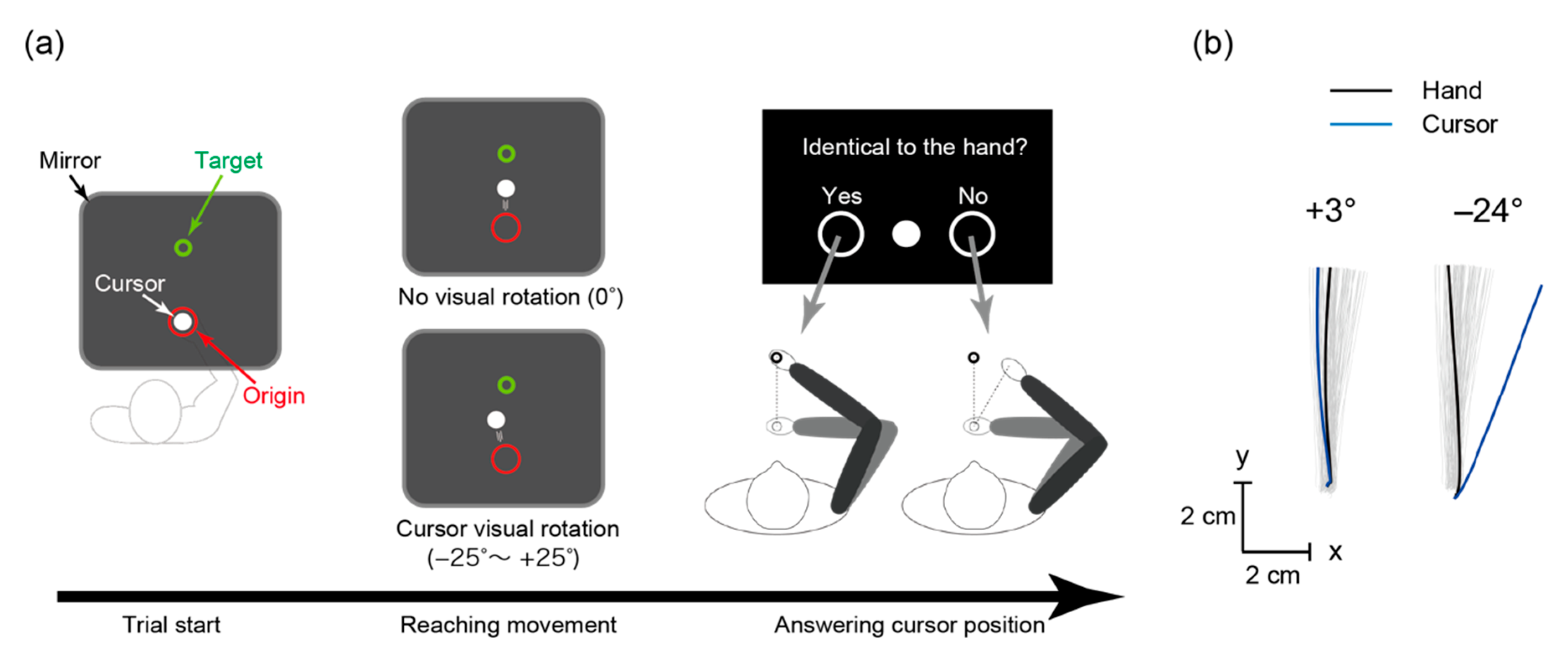
2.2. Arm-Reaching Task
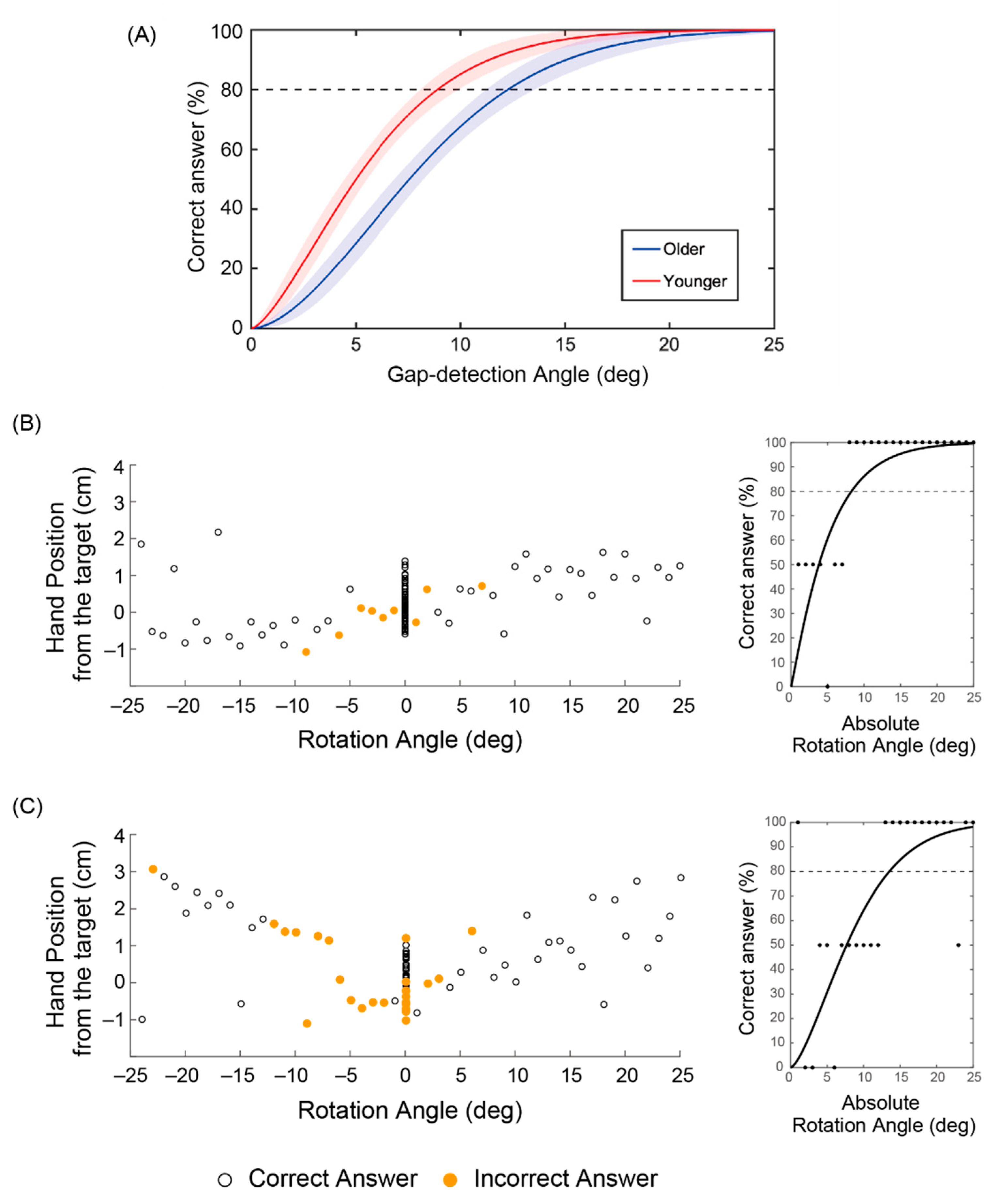
2.3. Behavioral Data Analyses
2.4. MRI Acquisition
2.5. Regions of Interest (ROIs)
2.6. Functional and Effective Connectivity Analyses
2.7. Structural Analyses
3. Results
3.1. Behavioral Difference between Younger and Older Adults
3.2. Functional Connectivity Reflecting Age and Gap Detection Ability
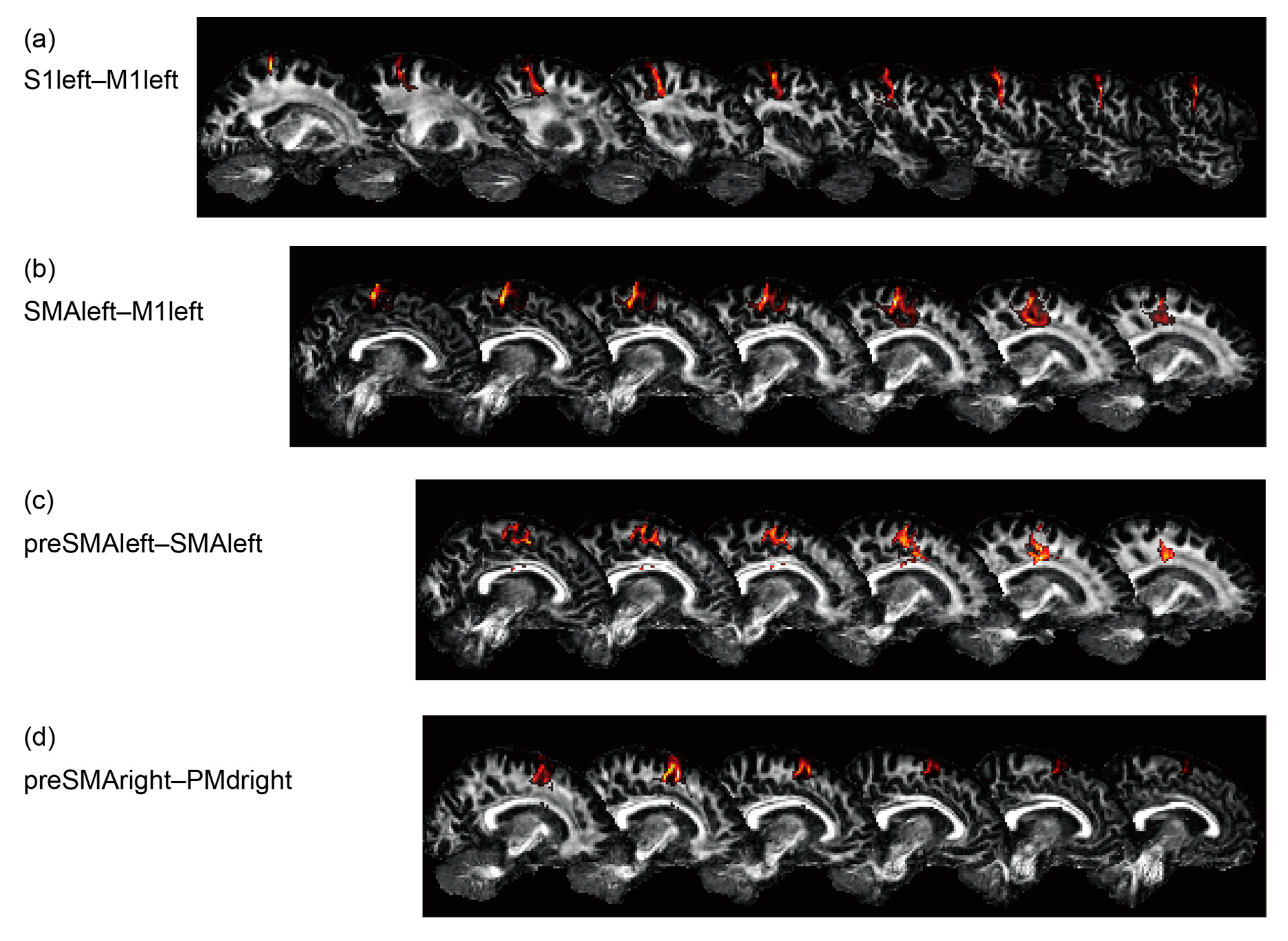
3.3. Effective Connectivity Reflecting Age and Gap Detection Ability
3.4. Structural Aspects Reflecting Age and Gap Detection Ability
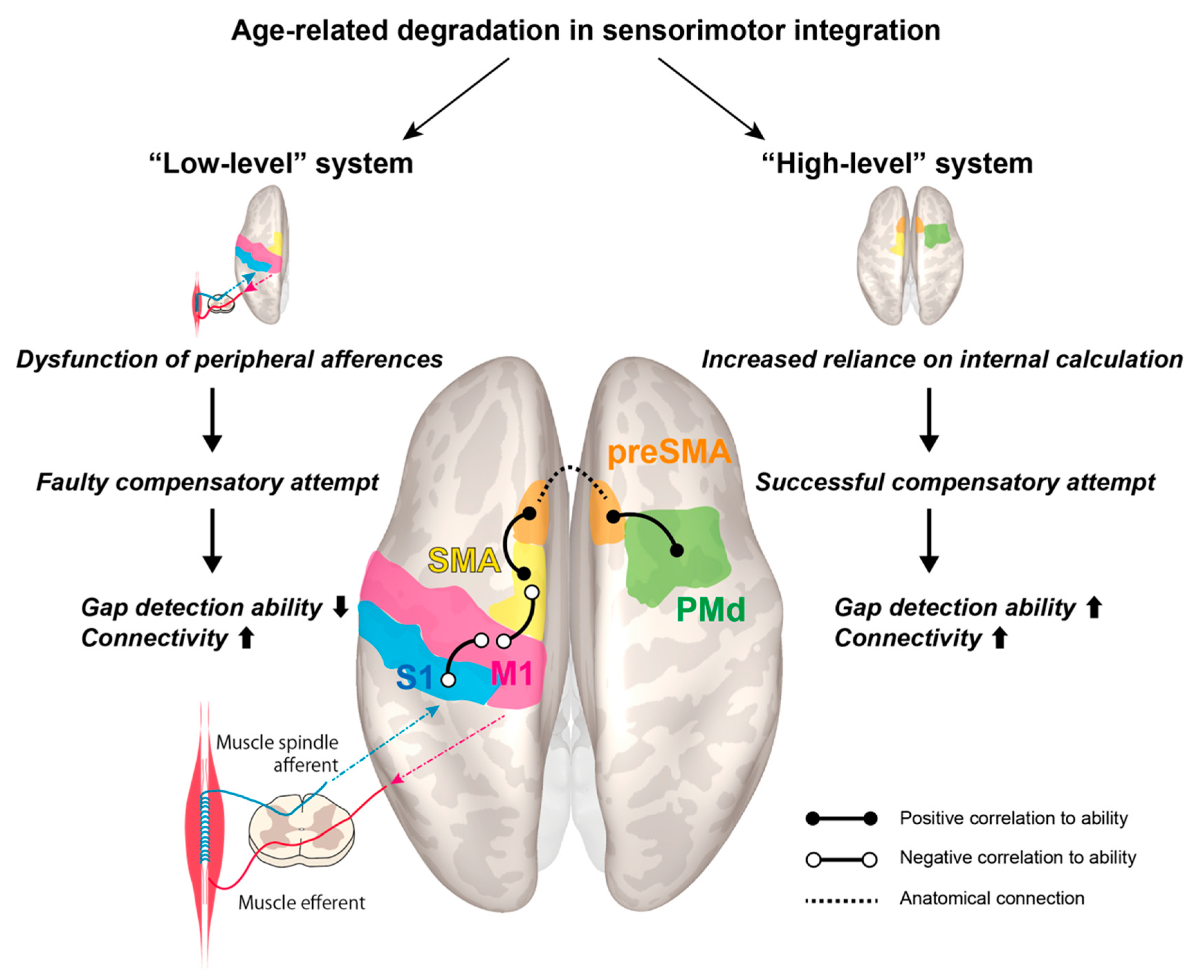
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- VanSwearingen, J.M.; Studenski, S.A. Aging, Motor Skill, and the Energy Cost of Walking: Implications for the Prevention and Treatment of Mobility Decline in Older Persons. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2014, 69, 1429–1436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hunter, S.K.; Pereira, H.M.; Keenan, K.G. The aging neuromuscular system and motor performance. J. Appl. Physiol. 2016, 121, 982–995. [Google Scholar] [CrossRef] [PubMed]
- Khow, K.S.; Visvanathan, R. Falls in the Aging Population. Clin. Geriatr. Med. 2017, 33, 357–368. [Google Scholar] [CrossRef] [PubMed]
- DiPietro, L.; Campbell, W.W.; Buchner, D.M.; Erickson, K.I.; Powell, K.E.; Bloodgood, B.; Hughes, T.; Day, K.R.; Piercy, K.L.; Vaux-Bjerke, A.; et al. Physical Activity, Injurious Falls, and Physical Function in Aging: An Umbrella Review. Med. Sci. Sports Exerc. 2019, 51, 1303–1313. [Google Scholar] [CrossRef]
- Manini, T.M.; Hong, S.L.; Clark, B.C. Aging and muscle: A neuron’s perspective. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16. [Google Scholar] [CrossRef]
- Plow, E.B.; Cunningham, D.A.; Bonnett, C.; Gohar, D.; Bayram, M.; Wyant, A.; Varnerin, N.; Mamone, B.; Siemionow, V.; Hou, J.; et al. Neurophysiological correlates of aging-related muscle weakness. J. Neurophysiol. 2013, 110, 2563–2573. [Google Scholar] [CrossRef] [Green Version]
- Correa-De-Araujo, R.; Hadley, E. Skeletal Muscle Function Deficit: A New Terminology to Embrace the Evolving Concepts of Sarcopenia and Age-Related Muscle Dysfunction. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2014, 69, 591–594. [Google Scholar] [CrossRef] [Green Version]
- Hepple, R.T. Mitochondrial Involvement and Impact in Aging Skeletal Muscle. Front. Aging Neurosci. 2014, 6, 211. [Google Scholar] [CrossRef] [Green Version]
- Ambrose, C. Muscle weakness during aging: A deficiency state involving declining angiogenesis. Ageing Res. Rev. 2015, 23, 139–153. [Google Scholar] [CrossRef] [Green Version]
- Bayram, M.B.; Siemionow, V.; Yue, G.H. Weakening of Corticomuscular Signal Coupling During Voluntary Motor Action in Aging. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2015, 70, 1037–1043. [Google Scholar] [CrossRef]
- Clark, B.C.; Law, T.D.; Ehong, S.L. Editorial: “From brain to body: The impact of nervous system declines on muscle performance in aging”. Front. Aging Neurosci. 2015, 7, 66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, K.H. Mechanisms of Muscle Denervation in Aging: Insights from a Mouse Model of Amyotrophic Lateral Sclerosis. Aging Dis. 2015, 6, 380–389. [Google Scholar] [CrossRef] [PubMed]
- Frontera, W.R. Physiologic Changes of the Musculoskeletal System with Aging: A Brief Review. Phys. Med. Rehabil. Clin. N. Am. 2017, 28, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Willadt, S.; Nash, M.; Slater, C.R. Age-related changes in the structure and function of mammalian neuromuscular junctions. Ann. N. Y. Acad. Sci. 2017, 1412, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Proske, U.; Gandevia, S.C. The Proprioceptive Senses: Their Roles in Signaling Body Shape, Body Position and Movement, and Muscle Force. Physiol. Rev. 2012, 92, 1651–1697. [Google Scholar] [CrossRef] [PubMed]
- Guzzetta, A.; Staudt, M.; Petacchi, E.; Ehlers, J.; Erb, M.; Wilke, M.; Krägeloh-Mann, I.; Cioni, G. Brain Representation of Active and Passive Hand Movements in Children. Pediatr. Res. 2007, 61, 485–490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goble, D.J.; Coxon, J.P.; Van Impe, A.; De Vos, J.; Wenderoth, N.; Swinnen, S.P. The neural control of bimanual movements in the elderly: Brain regions exhibiting age-related increases in activity, frequency-induced neural modulation, and task-specific compensatory recruitment. Hum. Brain Mapp. 2010, 31, 1281–1295. [Google Scholar] [CrossRef] [PubMed]
- Goble, D.J.; Coxon, J.P.; Van Impe, A.; Geurts, M.; Doumas, M.; Wenderoth, N.; Swinnen, S.P. Brain Activity during Ankle Proprioceptive Stimulation Predicts Balance Performance in Young and Older Adults. J. Neurosci. 2011, 31, 16344–16352. [Google Scholar] [CrossRef] [Green Version]
- Piitulainen, H.; Seipäjärvi, S.; Avela, J.; Parviainen, T.; Walker, S. Cortical Proprioceptive Processing Is Altered by Aging. Front. Aging Neurosci. 2018, 10, 147. [Google Scholar] [CrossRef]
- Skinner, H.B.; Barrack, R.L.; Cook, S.D. Age-related Decline in Proprioception. Clin. Orthop. Relat. Res. 1984, 208–211. [Google Scholar] [CrossRef]
- Goble, D.J.; Coxon, J.P.; Wenderoth, N.; Van Impe, A.; Swinnen, S.P. Proprioceptive sensibility in the elderly: Degeneration, functional consequences and plastic-adaptive processes. Neurosci. Biobehav. Rev. 2009, 33, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Ben-Shabat, E.; Matyas, T.A.; Pell, G.S.; Brodtmann, A.; Carey, L.M. The Right Supramarginal Gyrus Is Important for Proprioception in Healthy and Stroke-Affected Participants: A Functional MRI Study. Front. Neurol. 2015, 6, 248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, T.; Zang, Y.; Wang, L.; Long, X.; Hallett, M.; Chen, Y.; Li, K.; Chan, P. Aging influence on functional connectivity of the motor network in the resting state. Neurosci. Lett. 2007, 422, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, M.; Kawato, M.; Imamizu, H. Predicting learning plateau of working memory from whole-brain intrinsic network connectivity patterns. Sci. Rep. 2015, 5, 7622. [Google Scholar] [CrossRef] [PubMed]
- Ventura-Campos, N.; Sanjuán, A.; González, J.; Palomar-García, M.-Á.; Rodríguez-Pujadas, A.; Sebastián-Gallés, N.; Deco, G.; Ávila, C. Spontaneous Brain Activity Predicts Learning Ability of Foreign Sounds. J. Neurosci. 2013, 33, 9295–9305. [Google Scholar] [CrossRef]
- Baldassarre, A.; Lewis, C.M.; Committeri, G.; Snyder, A.Z.; Romani, G.L.; Corbetta, M. Individual variability in functional connectivity predicts performance of a perceptual task. Proc. Natl. Acad. Sci. USA 2012, 109, 3516–3521. [Google Scholar] [CrossRef] [Green Version]
- Palva, J.M.; Monto, S.; Kulashekhar, S.; Palva, S. Neuronal synchrony reveals working memory networks and predicts individual memory capacity. Proc. Natl. Acad. Sci. USA 2010, 107, 7580–7585. [Google Scholar] [CrossRef] [Green Version]
- Lemée, J.-M.; Berro, D.H.; Bernard, F.; Chinier, E.; Leiber, L.; Menei, P.; Ter Minassian, A. Resting-state functional magnetic resonance imaging versus task-based activity for language mapping and correlation with perioperative cortical mapping. Brain Behav. 2019, 9, e01362. [Google Scholar] [CrossRef] [Green Version]
- Rosazza, C.; Aquino, D.; D’Incerti, L.; Cordella, R.; Andronache, A.; Zaca, D.; Bruzzone, M.G.; Tringali, G.; Minati, L. Preoperative Mapping of the Sensorimotor Cortex: Comparative Assessment of Task-Based and Resting-State fMRI. PLoS ONE 2014, 9, e98860. [Google Scholar] [CrossRef] [Green Version]
- Rand, M.K.; Wang, L.; Müsseler, J.; Heuer, H. Vision and proprioception in action monitoring by young and older adults. Neurobiol. Aging 2013, 34, 1864–1872. [Google Scholar] [CrossRef]
- Eikema, D.J.A.; Hatzitaki, V.; Tzovaras, D.; Papaxanthis, C. Age-dependent modulation of sensory reweighting for controlling posture in a dynamic virtual environment. Age 2011, 34, 1381–1392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pasma, J.H.; Engelhart, D.; Maier, A.B.; Schouten, A.C.; Van Der Kooij, H.; Meskers, C.G.M. Changes in sensory reweighting of proprioceptive information during standing balance with age and disease. J. Neurophysiol. 2015, 114, 3220–3233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolpert, D.M.; Ghahramani, Z.; I Jordan, M. An internal model for sensorimotor integration. Science 1995, 269, 1880–1882. [Google Scholar] [CrossRef] [PubMed]
- Wolpert, D.M. Computational approaches to motor control. Trends Cogn. Sci. 1997, 1, 209–216. [Google Scholar] [CrossRef]
- Yamashita, A.; Yahata, N.; Itahashi, T.; Lisi, G.; Yamada, T.; Ichikawa, N.; Takamura, M.; Yoshihara, Y.; Kunimatsu, A.; Okada, N.; et al. Harmonization of resting-state functional MRI data across multiple imaging sites via the separation of site differences into sampling bias and measurement bias. PLoS Biol. 2019, 17, e3000042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jovicich, J.; Marizzoni, M.; Bosch, B.; Bartrés-Faz, D.; Arnold, J.; Benninghoff, J.; Wiltfang, J.; Roccatagliata, L.; Picco, A.; Nobili, F.; et al. Multisite longitudinal reliability of tract-based spatial statistics in diffusion tensor imaging of healthy elderly subjects. NeuroImage 2014, 101, 390–403. [Google Scholar] [CrossRef] [PubMed]
- Jovicich, J.; Marizzoni, M.; Sala-Llonch, R.; Bosch, B.; Bartrés-Faz, D.; Arnold, J.; Benninghoff, J.; Wiltfang, J.; Roccatagliata, L.; Nobili, F.; et al. Brain morphometry reproducibility in multi-center 3T MRI studies: A comparison of cross-sectional and longitudinal segmentations. NeuroImage 2013, 83, 472–484. [Google Scholar] [CrossRef]
- Jovicich, J.; Minati, L.; Marizzoni, M.; Marchitelli, R.; Sala-Llonch, R.; Bartrés-Faz, D.; Arnold, J.; Benninghoff, J.; Fiedler, U.; Roccatagliata, L.; et al. Longitudinal reproducibility of default-mode network connectivity in healthy elderly participants: A multicentric resting-state fMRI study. NeuroImage 2016, 124, 442–454. [Google Scholar] [CrossRef]
- Mayka, M.A.; Corcos, D.M.; Leurgans, S.E.; Vaillancourt, D.E. Three-dimensional locations and boundaries of motor and premotor cortices as defined by functional brain imaging: A meta-analysis. NeuroImage 2006, 31, 1453–1474. [Google Scholar] [CrossRef] [Green Version]
- Carp, J.; Park, J.; Hebrank, A.; Park, D.C.; Polk, T.A. Age-Related Neural Dedifferentiation in the Motor System. PLoS ONE 2011, 6, e29411. [Google Scholar] [CrossRef]
- Blumen, H.M.; Holtzer, R.; Brown, L.L.; Gazes, Y.; Verghese, J. Behavioral and neural correlates of imagined walking and walking-while-talking in the elderly. Hum. Brain Mapp. 2014, 35, 4090–4104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crockett, R.A.; Hsu, C.L.; Best, J.R.; Liu-Ambrose, T. Resting State Default Mode Network Connectivity, Dual Task Performance, Gait Speed, and Postural Sway in Older Adults with Mild Cognitive Impairment. Front. Aging Neurosci. 2017, 9, 423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loehrer, P.A.; Nettersheim, F.S.; Jung, F.; Weber, I.; Huber, C.; Dembek, T.A.; Pelzer, E.A.; Fink, G.R.; Tittgemeyer, M.; Timmermann, L. Ageing changes effective connectivity of motor networks during bimanual finger coordination. NeuroImage 2016, 143, 325–342. [Google Scholar] [CrossRef] [PubMed]
- Michels, L.; Dietz, V.; Schättin, A.; Schrafl-Altermatt, M. Neuroplastic Changes in Older Adults Performing Cooperative Hand Movements. Front. Hum. Neurosci. 2018, 12, 488. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, R.; Fujiwara, Y.; Yasunaga, M.; Suzuki, H.; Kanosue, K.; Montero-Odasso, M.; Ishii, K. Association between Hypometabolism in the Supplementary Motor Area and Fear of Falling in Older Adults. Front. Aging Neurosci. 2017, 9, 251. [Google Scholar] [CrossRef] [Green Version]
- Whitfield-Gabrieli, S.; Nieto-Castanon, A. Conn: A Functional Connectivity Toolbox for Correlated and Anticorrelated Brain Networks. Brain Connect. 2012, 2, 125–141. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Daunizeau, J.; Stephan, K.E.; Penny, W.; Hu, D.; Friston, K.; Penny, W.D. Generalised filtering and stochastic DCM for fMRI. NeuroImage 2011, 58, 442–457. [Google Scholar] [CrossRef] [Green Version]
- Behrens, T.E.J.; Woolrich, M.W.; Jenkinson, M.; Johansen-Berg, H.; Nunes, R.; Clare, S.; Matthews, P.; Brady, J.; Smith, S. Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magn. Reson. Med. 2003, 50, 1077–1088. [Google Scholar] [CrossRef]
- Costello, M.C.; Bloesch, E.K. Are Older Adults Less Embodied? A Review of Age Effects through the Lens of Embodied Cognition. Front. Psychol. 2017, 8, 267. [Google Scholar] [CrossRef] [Green Version]
- Uithol, S.; Van Rooij, I.; Bekkering, H.; Haselager, P. Hierarchies in Action and Motor Control. J. Cogn. Neurosci. 2012, 24, 1077–1086. [Google Scholar] [CrossRef] [Green Version]
- Merel, J.; Botvinick, M.M.; Wayne, G. Hierarchical motor control in mammals and machines. Nat. Commun. 2019, 10, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Kim, G.H.; Suzuki, S.; Kanda, K. Age-related physiological and morphological changes of muscle spindles in rats. J. Physiol. 2007, 582, 525–538. [Google Scholar] [CrossRef] [PubMed]
- Nachev, P.; Kennard, C.; Husain, M. Functional role of the supplementary and pre-supplementary motor areas. Nat. Rev. Neurosci. 2008, 9, 856–869. [Google Scholar] [CrossRef] [PubMed]
- Roski, C.; Caspers, S.; Langner, R.; Laird, A.R.; Fox, P.T.; Zilles, K.; Amunts, K.; Eickhoff, S.B. Adult age-dependent differences in resting-state connectivity within and between visual-attention and sensorimotor networks. Front. Aging Neurosci. 2013, 5, 67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vergani, F.; Lacerda, L.; Martino, J.; Attems, J.; Morris, C.; Mitchell, P.; De Schotten, M.T.; Dell’Acqua, F. White matter connections of the supplementary motor area in humans. J. Neurol. Neurosurg. Psychiatry 2014, 85, 1377–1385. [Google Scholar] [CrossRef] [PubMed]
- Wolpe, N.; Ingram, J.N.; Tsvetanov, K.A.; Geerligs, L.; Kievit, R.A.; Henson, R.N.; Wolpert, D.M.; Rowe, J.B.; Tyler, L.K.; Brayne, C.; et al. Ageing increases reliance on sensorimotor prediction through structural and functional differences in frontostriatal circuits. Nat. Commun. 2016, 7, 13034. [Google Scholar] [CrossRef]
- Green, P.E.; Ridding, M.C.; Hill, K.D.; Semmler, J.G.; Drummond, P.D.; Vallence, A.-M. Supplementary motor area—Primary motor cortex facilitation in younger but not older adults. Neurobiol. Aging 2018, 64, 85–91. [Google Scholar] [CrossRef]
- Wang, L.; Qiu, M.; Liu, C.; Yan, R.; Yang, J.; Zhang, J.; Zhang, Y.; Sang, L.; Qiu, M. Age-specific activation of cerebral areas in motor imagery—A fMRI study. Neuroradiology 2014, 56, 339–348. [Google Scholar] [CrossRef]
- Cassady, K.; Ruitenberg, M.F.L.; A Reuter-Lorenz, P.; Tommerdahl, M.; Seidler, R.D. Neural Dedifferentiation across the Lifespan in the Motor and Somatosensory Systems. Cereb. Cortex 2020, 30, 3704–3716. [Google Scholar] [CrossRef]
- Yuan, J.; Blumen, H.M.; Verghese, J.; Holtzer, R. Functional connectivity associated with gait velocity during walking and walking-while-talking in aging: A resting-state fMRI study. Hum. Brain Mapp. 2015, 36, 1484–1493. [Google Scholar] [CrossRef] [Green Version]
- Hsu, C.L.; Best, J.R.; Voss, M.W.; Handy, T.C.; Beauchet, O.; Lim, B.C.; Liu-Ambrose, T. Functional Neural Correlates of Slower Gait Among Older Adults with Mild Cognitive Impairment. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2018, 74, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Marcori, A.J.; Okazaki, V.H.A. Motor repertoire and gray matter plasticity: Is there a link? Med. Hypotheses 2019, 130, 109261. [Google Scholar] [CrossRef] [PubMed]
- Schulz, R.; Zimerman, M.; Timmermann, J.; Wessel, M.J.; Gerloff, C.; Hummel, F.C. White matter integrity of motor connections related to training gains in healthy aging. Neurobiol. Aging 2014, 35, 1404–1411. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshimura, N.; Tsuda, H.; Aquino, D.; Takagi, A.; Ogata, Y.; Koike, Y.; Minati, L. Age-Related Decline of Sensorimotor Integration Influences Resting-State Functional Brain Connectivity. Brain Sci. 2020, 10, 966. https://doi.org/10.3390/brainsci10120966
Yoshimura N, Tsuda H, Aquino D, Takagi A, Ogata Y, Koike Y, Minati L. Age-Related Decline of Sensorimotor Integration Influences Resting-State Functional Brain Connectivity. Brain Sciences. 2020; 10(12):966. https://doi.org/10.3390/brainsci10120966
Chicago/Turabian StyleYoshimura, Natsue, Hayato Tsuda, Domenico Aquino, Atsushi Takagi, Yousuke Ogata, Yasuharu Koike, and Ludovico Minati. 2020. "Age-Related Decline of Sensorimotor Integration Influences Resting-State Functional Brain Connectivity" Brain Sciences 10, no. 12: 966. https://doi.org/10.3390/brainsci10120966




