The Architect Who Lost the Ability to Imagine: The Cerebral Basis of Visual Imagery
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Visual Imagery
2.3. Neuropsychological and Experimental Tests
2.3.1. Delayed Matching and Surprise Recognition of Words, Objects and Faces—The WOF Test
2.3.2. Familiarity Decisions
2.3.3. Naming of Familiar Items
2.4. Structural MRI: Lesions
3. Results
3.1. Visual Imagery
3.2. Neuropsychological and Experimental Tests
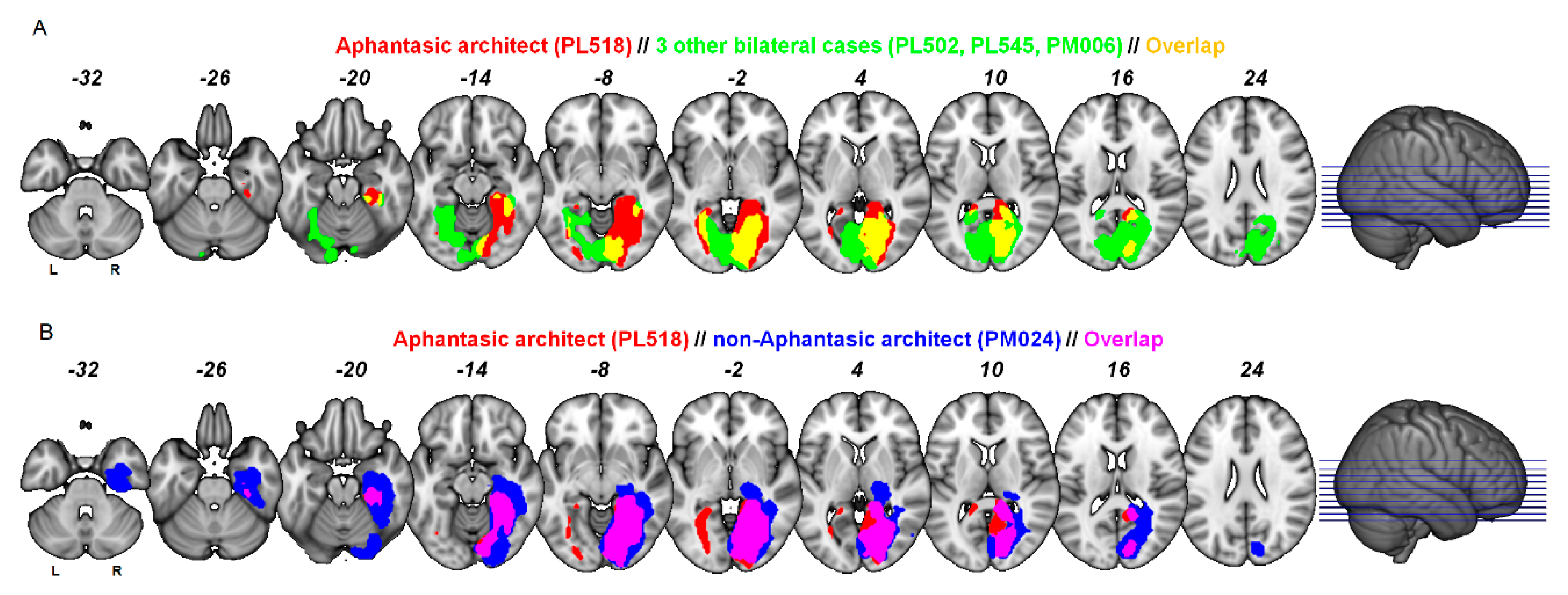
3.3. Lesion Localisation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| VVIQ Question | PL518 | PL502 | PL545 | PM006 | PM024 |
|---|---|---|---|---|---|
| 1 | 2 | 4 | 4 | 5 | 5 |
| 2 | 1 | 3 | 3 | 4 | 5 |
| 3 | 2 | 1 | 2 | 4 | 4 |
| 4 | 1 | 1 | 4 | 5 | 5 |
| 5 | 1 | 4 | 4 | 5 | 5 |
| 6 | 1 | 3 | 4 | 4 | 5 |
| 7 | 1 | 3 | 3 | 5 | 5 |
| 8 | 1 | 5 | 4 | 5 | 5 |
| 9 | 1 | 3 | 2 | 4 | 4 |
| 10 | 1 | 1 | 2 | 4 | 5 |
| 11 | 1 | 3 | 2 | 4 | 4 |
| 12 | 1 | 5 | 3 | 4 | 5 |
| 13 | 1 | 4 | 4 | 5 | 5 |
| 14 | 1 | 3 | 4 | 5 | 4 |
| 15 | 1 | 2 | 4 | 5 | 5 |
| 16 | 1 | 4 | 4 | 4 | 5 |
| Total | 18 | 49 | 53 | 72 | 76 |
| Mean | 1.13 | 3.06 | 3.31 | 4.50 | 4.75 |
References
- Pylyshyn, Z.W. What the mind’s eye tells the mind’s brain: A critique of mental imagery. Psychol. Bull. 1973, 80, 1–24. [Google Scholar] [CrossRef]
- Pylyshyn, Z.W. The imagery debate: Analogue media versus tacit knowledge. Psychol. Rev. 1981, 88, 16–45. [Google Scholar] [CrossRef]
- Kosslyn, S.M. The medium and the message in mental imagery: A theory. Psychol. Rev. 1981, 88, 46–66. [Google Scholar] [CrossRef]
- Zeman, A.Z.; Della Sala, S.; Torrens, L.A.; Gountouna, V.E.; McGonigle, D.J.; Logie, R.H. Loss of imagery phenomenology with intact visuo-spatial task performance: A case of ‘blind imagination’. Neuropsychologia 2010, 48, 145–155. [Google Scholar] [CrossRef]
- Charcot, J.M.; Bernard, D. Un cas de suppression brusque et isolée de la vision mentale des signes et des objets (formes et couleurs). Le Progrés Médical 1883, 11, 568–571. [Google Scholar]
- Zago, S.; Allegri, N.; Cristoffanini, M.; Ferrucci, R.; Porta, M.; Priori, A. Is the Charcot and Bernard case (1883) of loss of visual imagery really based on neurological impairment? Cogn. Neuropsychiatry 2011, 16, 481–504. [Google Scholar] [CrossRef]
- Farah, M.J. The neurological basis of mental imagery: A componential analysis. Cognition 1984, 18, 245–272. [Google Scholar] [CrossRef]
- Farah, M.J.; Hammond, K.M.; Levine, D.N.; Calvanio, R. Visual and spatial mental imagery: Dissociable systems of representation. Cogn. Psychol. 1988, 20, 439–462. [Google Scholar] [CrossRef]
- Levine, D.N.; Warach, J.; Farah, M. Two visual systems in mental imagery: Dissociation of “what” and “where” in imagery disorders due to bilateral posterior cerebral lesions. Neurology 1985, 35, 1010. [Google Scholar] [CrossRef]
- Kosslyn, S.M. Seeing and imagining in the cerebral hemispheres: A computational approach. Psychol. Rev. 1987, 94, 148–175. [Google Scholar] [CrossRef]
- Mazard, A.; Tzourio-Mazoyer, N.; Crivello, F.; Mazoyer, B.; Mellet, E. A PET meta-analysis of object and spatial mental imagery. Eur. J. Cogn. Psychol. 2004, 16, 673–695. [Google Scholar] [CrossRef]
- Kosslyn, S.M.; Ganis, G.; Thompson, W.L. Neural foundations of imagery. Nat. Rev. Neurosci. 2001, 2, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Kosslyn, S.M.; Thompson, W.L. When is early visual cortex activated during visual mental imagery? Psychol. Bull. 2003, 129, 723–746. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Kato, T.; Zhu, X.H.; Ogawa, S.; Tank, D.W.; Ugurbil, K. Human primary visual cortex and lateral geniculate nucleus activation during visual imagery. Neuroreport 1998, 9, 3669–3674. [Google Scholar] [CrossRef]
- Kosslyn, S.M.; Pascual-Leone, A.; Felician, O.; Camposano, S.; Keenan, J.P.; Ganis, G.; Sukel, K.E.; Alpert, N.M. The role of area 17 in visual imagery: Convergent evidence from PET and rTMS. Science 1999, 284, 167–170. [Google Scholar] [CrossRef]
- Klein, I.; Paradis, A.L.; Poline, J.B.; Kosslyn, S.M.; Le Bihan, D. Transient activity in the human calcarine cortex during visual-mental imagery: An event-related fMRI study. J. Cogn. Neurosci. 2000, 12, 15–23. [Google Scholar] [CrossRef]
- Ganis, G.; Thompson, W.L.; Kosslyn, S.M. Brain areas underlying visual mental imagery and visual perception: An fMRI study. Cogn. Brain Res. 2004, 20, 226–241. [Google Scholar] [CrossRef]
- Slotnick, S.D.; Thompson, W.L.; Kosslyn, S.M. Visual mental imagery induces retinotopically organized activation of early visual areas. Cereb. Cortex 2005, 15, 1570–1583. [Google Scholar] [CrossRef]
- Bergmann, J.; Genç, E.; Kohler, A.; Singer, W.; Pearson, J. Smaller primary visual cortex is associated with stronger, but less precise mental imagery. Cereb. Cortex 2016, 26, 3838–3850. [Google Scholar] [CrossRef]
- Dijkstra, N.; Zeidman, P.; Ondobaka, S.; van Gerven, M.A.; Friston, K. Distinct top-down and bottom-up brain connectivity during visual perception and imagery. Sci. Rep. 2017, 7, 5677. [Google Scholar] [CrossRef]
- Lee, S.H.; Kravitz, D.J.; Baker, C.I. Disentangling visual imagery and perception of real-world objects. Neuroimage 2012, 59, 4064–4073. [Google Scholar] [CrossRef] [PubMed]
- Moro, V.; Berlucchi, G.; Lerch, J.; Tomaiuolo, F.; Aglioti, S.M. Selective deficit of mental visual imagery with intact primary visual cortex and visual perception. Cortex 2008, 44, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Bridge, H.; Hicks, S.L.; Xie, J.; Okell, T.W.; Mannan, S.; Alexander, I.; Cowey, A.; Kennard, C. Visual activation of extra-striate cortex in the absence of V1 activation. Neuropsychologia 2010, 48, 4148–4154. [Google Scholar] [CrossRef] [PubMed]
- Bridge, H.; Harrold, S.; Holmes, E.A.; Stokes, M.; Kennard, C. Vivid visual mental imagery in the absence of the primary visual cortex. J. Neurol. 2012, 259, 1062–1070. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, A.; Southwood, M.H. Cortical blindness and visual imagery. Neurology 1995, 45, 2189–2195. [Google Scholar] [CrossRef]
- Bartolomeo, P. The relationship between visual perception and visual mental imagery: A reappraisal of the neuropsychological evidence. Cortex 2002, 38, 357–378. [Google Scholar] [CrossRef]
- Shuttleworth, E.C., Jr.; Syring, V.; Allen, N. Further observations on the nature of prosopagnosia. Brain Cogn. 1982, 1, 307–322. [Google Scholar] [CrossRef]
- OʹCraven, K.M.; Kanwisher, N. Mental imagery of faces and places activates corresponding stimulus-specific brain regions. J. Cogn. Neurosci. 2000, 12, 1013–1023. [Google Scholar] [CrossRef]
- Reddy, L.; Tsuchiya, N.; Serre, T. Reading the mind’s eye: Decoding category information during mental imagery. Neuroimage 2010, 50, 818–825. [Google Scholar] [CrossRef]
- Dijkstra, N.; Bosch, S.E.; van Gerven, M.A. Shared neural mechanisms of visual perception and imagery. Trends Cogn. Sci. 2019, 23, 423–434. [Google Scholar] [CrossRef]
- Pearson, J.; Kosslyn, S.M. The heterogeneity of mental representation: Ending the imagery debate. Proc. Natl. Acad. Sci. USA 2015, 112, 10089–10092. [Google Scholar] [CrossRef]
- Riddoch, M.J.; Humphreys, G.W. A case of integrative visual agnosia. Brain 1987, 110, 1431–1462. [Google Scholar] [CrossRef]
- Humphreys, G.W.; Riddoch, M.J. Routes to object constancy: Implications from neurological impairments of object constancy. Q. J. Exp. Psychol. 1984, 36, 385–415. [Google Scholar] [CrossRef] [PubMed]
- Riddoch, M.J.; Humphreys, G.W.; Gannon, T.; Blott, W.; Jones, V. Memories are made of this: The effects of time on stored visual knowledge in a case of visual agnosia. Brain 1999, 122, 537–559. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Behrmann, M.; Winocur, G.; Moscovitch, M. Dissociation between mental imagery and object recognition in a brain-damaged patient. Nature 1992, 359, 636–637. [Google Scholar] [CrossRef] [PubMed]
- Behrmann, M.; Moscovitch, M.; Winocur, G. Intact visual imagery and impaired visual perception in a patient with visual agnosia. J. Exp. Psychol. Hum. Percept. Perform. 1994, 20, 1068–1087. [Google Scholar] [CrossRef]
- Farah, M.J.; Levine, D.N.; Calvanio, R. A case study of mental imagery deficit. Brain Cogn. 1988, 8, 147–164. [Google Scholar] [CrossRef]
- Goldenberg, G. Loss of visual imagery and loss of visual knowledge—A case study. Neuropsychologia 1992, 30, 1081–1099. [Google Scholar] [CrossRef]
- Zeman, A.Z.; Dewar, M.; Della Sala, S. Lives without imagery—Congenital aphantasia. Cortex 2015, 73, 378–380. [Google Scholar] [CrossRef]
- Robotham, R.J. The Neuropsychology of Stroke in the Back of the Brain: Clinical and Cognitive Aspects. Ph.D. Dissertation, University of Copenhagen, Faculty of Social Science, Department of Psychology, Copenhagen, Denmark, 2018. [Google Scholar]
- Veale, J.F. Edinburgh handedness inventory–short form: A revised version based on confirmatory factor analysis. Laterality: Asymmetries of Body. Brain Cogn. 2014, 19, 164–177. [Google Scholar]
- Yesavage, J.A.; Sheikh, J.I. Geriatric Depression Scale (GDS): Recent evidence and development of a shorter version. Clin. Gerontol. 1986, 5, 165–173. [Google Scholar] [CrossRef]
- Demeyere, N.; Riddoch, M.J.; Slavkova, E.D.; Bickerton, W.L.; Humphreys, G.W. The Oxford Cognitive Screen (OCS): Validation of a stroke-specific short cognitive screening tool. Psychol. Assess. 2015, 27, 883. [Google Scholar] [CrossRef] [PubMed]
- Wechsler, D. Wechsler Adult Intelligence Scale Fourth UK Edition; The Psychology Corporation: London, UK, 2010. [Google Scholar]
- Linksz, A. The Farnsworth panel D-15 test. Am. J. Ophthalmol. 1966, 62, 27–37. [Google Scholar] [CrossRef]
- Torfs, K.; Vancleef, K.; Lafosse, C.; Wagemans, J.; de-Wit, L. The Leuven Perceptual Organization Screening Test. (L-POST), an online test to assess mid-level visual perception. Behav. Res. Methods 2014, 46, 472–487. [Google Scholar] [CrossRef] [PubMed]
- Martinaud, O.; Pouliquen, D.; Gérardin, E.; Loubeyre, M.; Hirsbein, D.; Hannequin, D.; Cohen, L. Visual agnosia and posterior cerebral artery infarcts: An anatomical-clinical study. PLoS ONE 2012, 7, e30433. [Google Scholar] [CrossRef]
- Duchaine, B.; Nakayama, K. The Cambridge Face Memory Test: Results for neurologically intact individuals and an investigation of its validity using inverted face stimuli and prosopagnosic participants. Neuropsychologia 2006, 44, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Rey, A. L’examen psychologique dans les cas d’encéphalopathie traumatique.(Les problems.). Arch. Psychol. 1941, 28, 215–285. [Google Scholar]
- Marks, D.F. Visual imagery differences in the recall of pictures. Br. J. Psychol. 1973, 64, 17–24. [Google Scholar] [CrossRef]
- Campos, A.; Pérez-Fabello, M.J. Psychometric quality of a revised version Vividness of Visual Imagery Questionnaire. Percept. Mot. Ski. 2009, 108, 798–802. [Google Scholar] [CrossRef]
- Campos, A. Internal consistency and construct validity of two versions of the Revised Vividness of Visual Imagery Questionnaire. Percept. Mot. Ski. 2011, 113, 454–460. [Google Scholar] [CrossRef]
- Morrison, R.G.; Wallace, B. Imagery vividness, creativity and the visual arts. J. Ment. Imag. N. Y. Int. Imag. Assoc. 2001, 25, 135–152. [Google Scholar]
- Rice, G.E.; Keryy, S.J.; Robotham, R.J.; Leff, A.P.; Lambon Ralph, M.A.; Starrfelt, R. Revealing the spectrum of visual perceptual function following posterior cerebral artery stroke. In preparation.
- Gerlach, C. Normal and abnormal category-effects in visual object recognition: A legacy of Glyn W. Humphreys. Vis. Cogn. 2017, 25, 60–78. [Google Scholar] [CrossRef]
- Seghier, M.L.; Ramlackhansingh, A.; Crinion, J.; Leff, A.P.; Price, C.J. Lesion identification using unified segmentation-normalisation models and fuzzy clustering. NeuroImage 2008, 41, 1253–1266. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, S.; Weiner, K.S.; Caspers, J.; Mohlberg, H.; Schleicher, A.; Bludau, S.; Eickhoff, S.B.; Grill-Spector, K.; Zilles, K.; Amunts, K. Two new cytoarchitectonic areas on the human mid-fusiform gyrus. Cereb. Cortex 2015, 27, 373–385. [Google Scholar] [CrossRef]
- Duvernoy, H.M. The Human Brain: Surface, Three-Dimensional Sectional Anatomy with MRI, and Blood Supply; Springer: Vienna, Austria, 1999. [Google Scholar]
- Bouyeure, A.; Germanaud, D.; Bekha, D.; Delattre, V.; Lefèvre, J.; Pinabiaux, C.; Mangin, J.F.; Rivière, D.; Fischer, C.; Chiron, C.; et al. Three-dimensional probabilistic maps of mesial temporal lobe structures in children and adolescents’ brains. Front. Neuroanat. 2018, 12, 98. [Google Scholar] [CrossRef]
- Bogousslavsky, J.; Miklossy, J.; Deruaz, J.P.; Assal, G.; Regli, F. Lingual and fusiform gyri in visual processing: A clinico-pathologic study of superior altitudinal hemianopia. J. Neurol. Neurosurg. Psychiatry 1987, 50, 607–614. [Google Scholar] [CrossRef]
- Bartolomeo, P. The neural correlates of visual mental imagery: An ongoing debate. Cortex 2008, 44, 107–108. [Google Scholar] [CrossRef]
- D’Esposito, M.; Detre, J.A.; Aguirre, G.K.; Stallcup, M.; Alsop, D.C.; Tippet, L.J.; Farah, M.J. A functional MRI study of mental image generation. Neuropsychologia 1997, 35, 725–730. [Google Scholar] [CrossRef]
- Farah, M.J. The laterality of mental image generation: A test with normal subjects. Neuropsychologia 1986, 24, 541–551. [Google Scholar] [CrossRef]
- Farah, M.J.; Gazzaniga, M.S.; Holtzman, J.D.; Kosslyn, S.M. A left hemisphere basis for visual mental imagery? Neuropsychologia 1985, 23, 115–118. [Google Scholar] [CrossRef]
- Fulford, J.; Milton, F.; Salas, D.; Smith, A.; Simler, A.; Winlove, C.; Zeman, A. The neural correlates of visual imagery vividness—An fMRI study and literature review. Cortex 2018, 105, 26–40. [Google Scholar] [CrossRef] [PubMed]
- Kosslyn, S.M.; Holtzman, J.D.; Farah, M.J.; Gazzaniga, M.S. A computational analysis of mental image generation: Evidence from functional dissociations in split-brain patients. J. Exp. Psychol. Gen. 1985, 114, 311–341. [Google Scholar] [CrossRef][Green Version]
- Trojano, L.; Grossi, D. A critical review of mental imagery defects. Brain Cogn. 1994, 24, 213–243. [Google Scholar] [CrossRef][Green Version]
- Winlove, C.I.; Milton, F.; Ranson, J.; Fulford, J.; MacKisack, M.; Macpherson, F.; Zeman, A. The neural correlates of visual imagery: A co-ordinate-based meta-analysis. Cortex 2018, 105, 4–25. [Google Scholar] [CrossRef] [PubMed]
- De Gelder, B.; Tamietto, M.; Pegna, A.J.; Van den Stock, J. Visual imagery influences brain responses to visual stimulation in bilateral cortical blindness. Cortex 2015, 72, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Gerlach, C.; Klargaard, S.K.; Alnæs, D.; Kolskår, K.K.; Karstoft, J.; Westlye, L.T.; Starrfelt, R. Left hemisphere abnormalities in developmental prosopagnosia when looking at faces but not words. Brain Commun. 2019, 1, fcz034. [Google Scholar] [CrossRef]
- Dinkelacker, V.; Grüter, M.; Klaver, P.; Grüter, T.; Specht, K.; Weis, S.; Kennerknecht, I.; Elger, C.E.; Fernandez, G. Congenital prosopagnosia: Multistage anatomical and functional deficits in face processing circuitry. J. Neurol. 2011, 258, 770–782. [Google Scholar] [CrossRef]
- Dobel, C.; Putsche, C.; Zwitserlood, P.; Junghöfer, M. Early left-hemispheric dysfunction of face processing in congenital prosopagnosia: An MEG study. PLoS ONE 2008, 3, e2326. [Google Scholar] [CrossRef]
- Grüter, T.; Grüter, M.; Bell, V.; Carbon, C.C. Visual mental imagery in congenital prosopagnosia. Neurosci. Lett. 2009, 453, 135–140. [Google Scholar] [CrossRef]
- Barton, J.J.; Cherkasova, M. Face imagery and its relation to perception and covert recognition in prosopagnosia. Neurology 2003, 61, 220–225. [Google Scholar] [CrossRef]
- Rossion, B.; Dricot, L.; Devolder, A.; Bodart, J.M.; Crommelinck, M.; Gelder, B.D.; Zoontjes, R. Hemispheric asymmetries for whole-based and part-based face processing in the human fusiform gyrus. J. Cogn. Neurosci. 2000, 12, 793–802. [Google Scholar] [CrossRef]
- Sack, A.T.; Camprodon, J.A.; Pascual-Leone, A.; Goebel, R. The dynamics of interhemispheric compensatory processes in mental imagery. Science 2005, 308, 702–704. [Google Scholar] [CrossRef]
- Palermo, L.; Nori, R.; Piccardi, L.; Giusberti, F.; Guariglia, C. Environment and object mental images in patients with representational neglect: Two case reports. J. Int. Neuropsychol. Soc. 2010, 16, 921–932. [Google Scholar] [CrossRef]
- Cui, X.; Jeter, C.B.; Yang, D.; Montague, P.R.; Eagleman, D.M. Vividness of mental imagery: Individual variability can be measured objectively. Vis. Res. 2007, 47, 474–478. [Google Scholar] [CrossRef]
- Dijkstra, N.; Bosch, S.E.; van Gerven, M.A. Vividness of visual imagery depends on the neural overlap with perception in visual areas. J. Neurosci. 2017, 37, 1367–1373. [Google Scholar] [CrossRef]
- Nanay, B. Multimodal mental imagery. Cortex 2018, 105, 125–134. [Google Scholar] [CrossRef]
- Grossi, D.; Modafferi, A.; Pelosi, L.; Trojano, L. On the different roles of the cerebral hemispheres in mental imagery: The “o’Clock Test.” in two clinical cases. Brain Cogn. 1989, 10, 18–27. [Google Scholar] [CrossRef]
- Paivio, A. Comparisons of mental clocks. J. Exp. Psychol. Hum. Percept. Perform. 1978, 4, 61–71. [Google Scholar] [CrossRef]
- El Haj, M.; Gallouj, K.; Antoine, P. Mental imagery and autobiographical memory in Alzheimer’s disease. Neuropsychology 2019, 33, 609–616. [Google Scholar] [CrossRef]
- Bartolomeo, P.; Bachoud-Lévi, A.C.; Chokron, S.; Degos, J.D. Visually- and motor-based knowledge of letters: Evidence from a pure alexic patient. Neuropsychologia 2002, 40, 1363–1371. [Google Scholar] [CrossRef]
- Ogden, J.A. Visual object agnosia, prosopagnosia, achromatopsia, loss of visual imagery, and autobiographical amnesia following recovery from cortical blindness: Case MH. Neuropsychologia 1993, 31, 571–589. [Google Scholar] [CrossRef]
- Greenberg, D.L.; Eacott, M.J.; Brechin, D.; Rubin, D.C. Visual memory loss and autobiographical amnesia: A case study. Neuropsychologia 2005, 43, 1493–1502. [Google Scholar] [CrossRef]
- Pearson, J.; Clifford, C.W.; Tong, F. The functional impact of mental imagery on conscious perception. Curr. Biol. 2008, 18, 982–986. [Google Scholar] [CrossRef]
- Pearson, J. New directions in mental-imagery research. Curr. Dir. Psychol. Sci. 2014, 23, 178–183. [Google Scholar] [CrossRef]
- Boccia, M.; Sulpizio, V.; Palermo, L.; Piccardi, L.; Guariglia, C.; Galati, G. I can see where you would be: Patterns of fMRI activity reveal imagined landmarks. Neuroimage 2017, 144, 174–182. [Google Scholar] [CrossRef]
- Dijkstra, N.; Ambrogioni, L.; van Gerven, M. Neural dynamics of perceptual inference and its reversal during imagery. BioRxiv 2019, 781294. [Google Scholar] [CrossRef]
- Lawrence, S.J.; Formisano, E.; Muckli, L.; de Lange, F.P. Laminar fMRI: Applications for cognitive neuroscience. Neuroimage 2019, 197, 785–791. [Google Scholar] [CrossRef]
- Bergmann, J.; Morgan, A.T.; Muckli, L. Two distinct feedback codes in V1 for ‘real’and ‘imaginary’internal experiences. BioRxiv 2019, 664870. [Google Scholar] [CrossRef]


| Participant | PL518 | PL502 | PL545 | PM006 | PM024 | Controls Mean (SD) |
|---|---|---|---|---|---|---|
| Lesion Laterality | Bilat | Bilat | Bilat | Bilat | R | n/a |
| Age | 52 | 55 | 62 | 67 | 66 | 62 (15) |
| Education (years) | 18 | 17 | 16 | 12 | 16 | 15 (2) |
| Gender | M | M | M | F | M | 24 F |
| Handedness (EHI) | −50 | 100 | 100 | 100 | 100 | 44 Right |
| Time Since Stroke (months) | 35 | 20 | 14 | 36 | 10 | n/a |
| Lesion Volume (cm3) | 52 | 23 | 57 | 24 | 112 | n/a |
| Geriatric Depression Scale (GDS-15) | 0 | 12 | 1 | 3 | 3 | n/a |
| OCS-Impaired Subtests | 0 | 1 | 6 | 1 | 1 | n/a |
| Digit Span Forward (WAIS-IV max = 16) | 10 | 13 | 15 | 11 | 11 | 11 (2) |
| Digit Span Backward (WAIS-IV max = 14) | 6 | 6 | 8 | 8 | 6 | 8 (2) |
| Basic Motor RT (ms) | 370 | 439 | 1195 | 665 | 686 | 398 (73) |
| Patient | PL518 | PL502 | PL545 | PM006 | PM024 | |
|---|---|---|---|---|---|---|
| Laterality | Bilateral | Bilateral | Bilateral | Bilateral | Right | |
| Left hemisphere | Occipital Pole | x | x | |||
| FG 1 | x | x | ||||
| FG 2 | x | |||||
| FG 3 | x | x | ||||
| FG 4 | ||||||
| Lingual Gyrus | x | x | x | x | ||
| Parahipp. Gyrus | x | |||||
| Right hemisphere | Occipital Pole | x | x | x | x | |
| FG 1 | x | x | ||||
| FG 2 | x | x | ||||
| FG 3 | x | x | x | |||
| FG 4 | x | x | x | |||
| Lingual Gyrus | x | x | x | x | ||
| Parahipp. Gyrus | x | x | x |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thorudottir, S.; Sigurdardottir, H.M.; Rice, G.E.; Kerry, S.J.; Robotham, R.J.; Leff, A.P.; Starrfelt, R. The Architect Who Lost the Ability to Imagine: The Cerebral Basis of Visual Imagery. Brain Sci. 2020, 10, 59. https://doi.org/10.3390/brainsci10020059
Thorudottir S, Sigurdardottir HM, Rice GE, Kerry SJ, Robotham RJ, Leff AP, Starrfelt R. The Architect Who Lost the Ability to Imagine: The Cerebral Basis of Visual Imagery. Brain Sciences. 2020; 10(2):59. https://doi.org/10.3390/brainsci10020059
Chicago/Turabian StyleThorudottir, Sandra, Heida M. Sigurdardottir, Grace E. Rice, Sheila J. Kerry, Ro J. Robotham, Alex P. Leff, and Randi Starrfelt. 2020. "The Architect Who Lost the Ability to Imagine: The Cerebral Basis of Visual Imagery" Brain Sciences 10, no. 2: 59. https://doi.org/10.3390/brainsci10020059
APA StyleThorudottir, S., Sigurdardottir, H. M., Rice, G. E., Kerry, S. J., Robotham, R. J., Leff, A. P., & Starrfelt, R. (2020). The Architect Who Lost the Ability to Imagine: The Cerebral Basis of Visual Imagery. Brain Sciences, 10(2), 59. https://doi.org/10.3390/brainsci10020059






