Dorsal Root Ganglion Stimulation Modulates Cortical Gamma Activity in the Cognitive Dimension of Chronic Pain
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
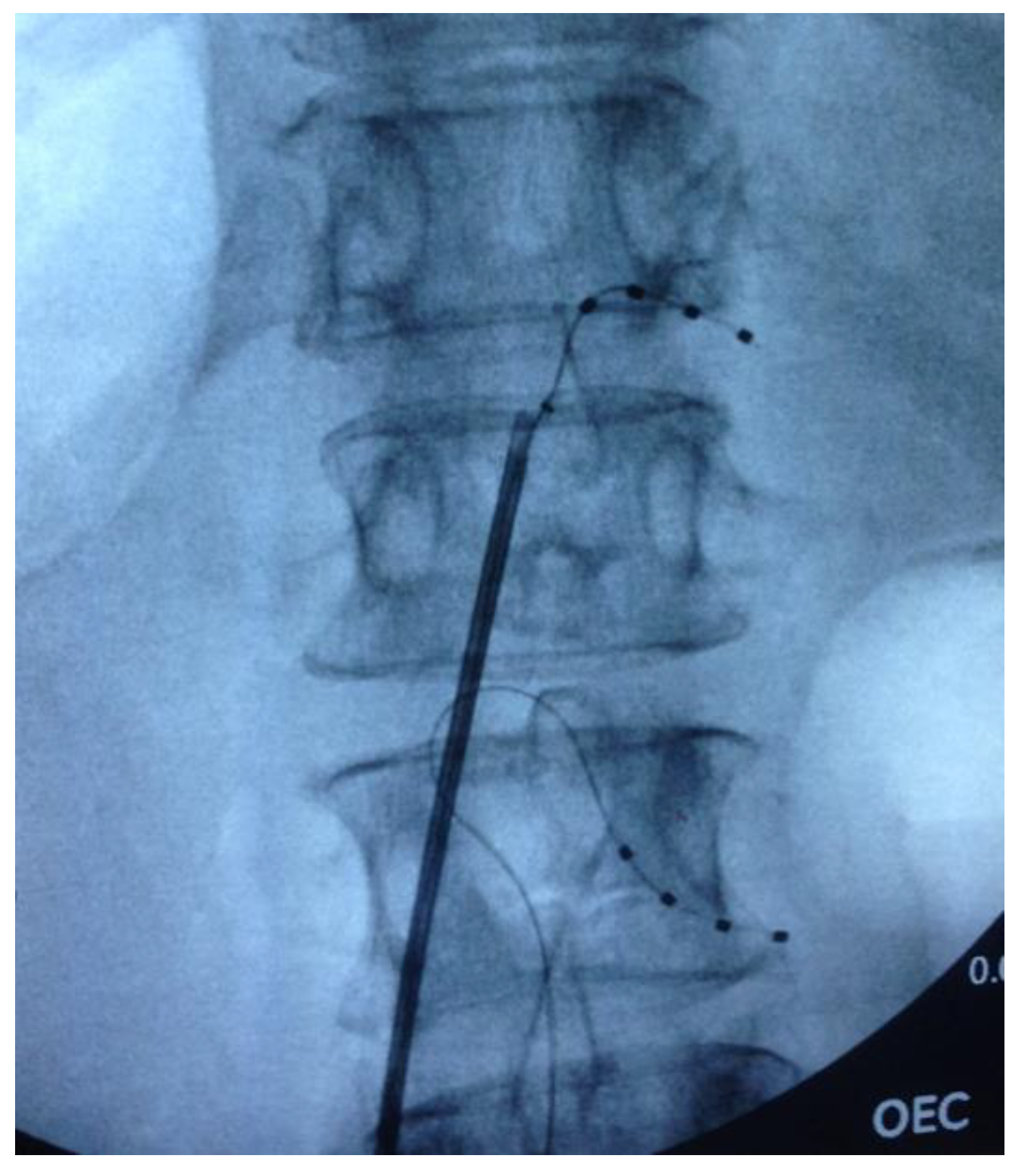
2.2. Surgical Procedure
2.3. Attentional Task
2.4. Magnetoencephalography
2.5. Spectral and Source Analysis
2.6. Statistical Analysis
2.7. Mediation Analysis
3. Results
3.1. Task Performance
3.2. Gamma Band Activity
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Melzack, R.; Casey, K. Sensory, Motivational, and Central Control Determinants of Pain. In The Skin Senses: Proceedings; Thomas, C.C., Ed.; Springfield: Illinois, IL, USA, 1968; pp. 423–439. [Google Scholar]
- Bushnell, M.C.; Čeko, M.; Low, L.A. Cognitive and emotional control of pain and its disruption in chronic pain. Nat. Rev. Neurosci. 2013, 14, 502–511. [Google Scholar] [CrossRef] [PubMed]
- Torta, D.M.; Legrain, V.; Mouraux, A.; Valentini, E. Attention to pain! A neurocognitive perspective on attentional modulation of pain in neuroimaging studies. Cortex 2017, 89, 120–134. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, K.L.; Campbell, C.; Martel, M.O.; Greenbaum, S.; Wasan, A.D.; Borsook, D.; Jamison, R.N.; Edwards, R.R. Distraction analgesia in chronic pain patients the impact of catastrophizing. Anesthesiology 2014, 121, 1292–1301. [Google Scholar] [CrossRef] [PubMed]
- Valet, M.; Sprenger, T.; Boecker, H.; Willoch, F.; Rummeny, E.; Conrad, B.; Erhard, P.; Tolle, T.R. Distraction modulates connectivity of the cingulo-frontal cortex and the midbrain during pain—An fMRI analysis. Pain 2004, 109, 399–408. [Google Scholar] [CrossRef]
- Berryman, C.; Stanton, T.R.; Jane Bowering, K.; Tabor, A.; McFarlane, A.; Lorimer Moseley, G. Evidence for working memory deficits in chronic pain: A systematic review and meta-analysis. Pain 2013, 154, 1181–1196. [Google Scholar] [CrossRef]
- Eccleston, C.; Crombez, G. Pain demands attention: A cognitive-affective model of the interruptive function of pain. Psychol. Bull. 1999, 125, 356–366. [Google Scholar] [CrossRef]
- Gruber, T.; Müller, M.M.; Keil, A.; Elbert, T. Selective visual-spatial attention alters induced gamma band responses in the human EEG. Clin. Neurophysiol. 1999, 110, 2074–2085. [Google Scholar] [CrossRef]
- Tallon-Baudry, C.; Bertrand, O.; Hénaff, M.-A.; Isnard, J.; Fischer, C. Attention Modulates Gamma-band Oscillations Differently in the Human Lateral Occipital Cortex and Fusiform Gyrus. Cereb. Cortex 2005, 15, 654–662. [Google Scholar] [CrossRef]
- De Pascalis, V.; Cacace, I.; Massicolle, F. Perception and modulation of pain in waking and hypnosis: Functional significance of phase-ordered gamma oscillations. Pain 2004, 112, 27–36. [Google Scholar] [CrossRef]
- Tan, L.L.; Oswald, M.J.; Heinl, C.; Retana Romero, O.A.; Kaushalya, S.K.; Monyer, H.; Kuner, R. Gamma oscillations in somatosensory cortex recruit prefrontal and descending serotonergic pathways in aversion and nociception. Nat. Commun. 2019, 10, 983. [Google Scholar] [CrossRef]
- Bai, Y.; Xia, X.; Liang, Z.; Wang, Y.; Yang, Y.; He, J.; Li, X. Frontal Connectivity in EEG Gamma (30–45 Hz) Respond to Spinal Cord Stimulation in Minimally Conscious State Patients. Front. Cell. Neurosci. 2017, 11, 177. [Google Scholar] [CrossRef] [PubMed]
- Deer, T.R.; Levy, R.M.; Kramer, J.; Poree, L.; Amirdelfan, K.; Grigsby, E.; Staats, P.; Burton, A.W.; Burgher, A.H.; Obray, J.; et al. Dorsal root ganglion stimulation yielded higher treatment success rate for complex regional pain syndrome and causalgia at 3 and 12 months: A randomized comparative trial. Pain 2017, 158, 669–681. [Google Scholar] [CrossRef] [PubMed]
- Bantick, S.J.; Wise, R.G.; Ploghaus, A.; Clare, S.; Smith, S.M.; Tracey, I. Imaging how attention modulates pain in humans using functional MRI. Brain 2002, 125, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Tracey, I.; Ploghaus, A.; Gati, J.S.; Clare, S.; Smith, S.; Menon, R.S.; Matthews, P.M. Imaging Attentional Modulation of Pain in the Periaqueductal Gray in Humans. J. Neurosci. 2002, 22, 2748–2752. [Google Scholar] [CrossRef]
- Seminowicz, D.A.; Davis, K.D. Interactions of pain intensity and cognitive load: The brain stays on task. Cereb. Cortex 2007, 17, 1412–1422. [Google Scholar] [CrossRef]
- Sprenger, C.; Eippert, F.; Finsterbusch, J.; Bingel, U.; Rose, M.; Büchel, C. Attention Modulates Spinal Cord Responses to Pain. Curr. Biol. 2012, 22, 1019–1022. [Google Scholar] [CrossRef]
- Moore, D.J.; Keogh, E.; Eccleston, C. The effect of threat on attentional interruption by pain. Pain 2013, 154, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Attridge, N.; Noonan, D.; Eccleston, C.; Keogh, E. The disruptive effects of pain on n-back task performance in a large general population sample. Pain 2015, 156, 1885–1891. [Google Scholar] [CrossRef]
- Parker, T.; Green, A.; Aziz, T. Rapid onset and short washout periods of dorsal root ganglion stimulation facilitate multiphase crossover study designs. Brain Stimul. 2019, 12, 1617–1618. [Google Scholar] [CrossRef]
- Taulu, S.; Simola, J. Spatiotemporal signal space separation method for rejecting nearby interference in MEG measurements. Phys. Med. Biol. 2006, 51, 1759–1768. [Google Scholar] [CrossRef]
- Medvedovsky, M.; Taulu, S.; Bikmullina, R.; Ahonen, A.; Paetau, R. Fine tuning the correlation limit of spatio-temporal signal space separation for magnetoencephalography. J. Neurosci. Methods 2009, 177, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Carrette, E.; De Tiège, X.; Op De Beeck, M.; De Herdt, V.; Meurs, A.; Legros, B.; Raedt, R.; Deblaere, K.; Van Roost, D.; Bourguignon, M.; et al. Magnetoencephalography in epilepsy patients carrying a vagus nerve stimulator. Epilepsy Res. 2011, 93, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Oostenveld, R.; Fries, P.; Maris, E.; Schoffelen, J.M. FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput. Intell. Neurosci. 2011. [Google Scholar] [CrossRef] [PubMed]
- Tadel, F.; Baillet, S.; Mosher, J.C.; Pantazis, D.; Leahy, R.M. Brainstorm: A user-friendly application for MEG/EEG analysis. Comput. Intell. Neurosci. 2011, 879716. [Google Scholar] [CrossRef]
- Gohel, B.; Lim, S.; Kim, M.-Y.; Kwon, H.; Kim, K. Approximate Subject Specific Pseudo MRI from an Available MRI Dataset for MEG Source Imaging. Front. Neuroinform. 2017, 11, 50. [Google Scholar] [CrossRef]
- Maris, E.; Oostenveld, R. Nonparametric statistical testing of EEG- and MEG-data. J. Neurosci. Methods 2007, 164, 177–190. [Google Scholar] [CrossRef]
- MacKinnon, D.P.; Lockwood, C.M.; Hoffman, J.M.; West, S.G.; Sheets, V. A comparison of methods to test mediation and other intervening variable effects. Psychol. Methods 2002, 7, 83–104. [Google Scholar] [CrossRef]
- Meule, A. Reporting and interpreting task performance in Go/no-go affective shifting tasks. Front. Psychol. 2017, 8, 701. [Google Scholar] [CrossRef]
- Buhle, J.; Wager, T.D. Performance-dependent inhibition of pain by an executive working memory task. Pain 2010, 149, 19–26. [Google Scholar] [CrossRef]
- Goubert, L.; Crombez, G.; Eccleston, C.; Devulder, J. Distraction from chronic pain during a pain-inducing activity is associated with greater post-activity pain. Pain 2004, 110, 220–227. [Google Scholar] [CrossRef]
- McCaul, K.D.; Malott, J.M. Distraction and coping with pain. Psychol. Bull. 1984, 95, 516–533. [Google Scholar] [CrossRef] [PubMed]
- Kahneman, D. Attention and Effort; Prentice-Hall: Englewood Cliffs, NJ, USA, 1973. [Google Scholar]
- Broadbent, D. Perception and Communication; Elsevier: Amsterdam, The Netherlands, 1958. [Google Scholar]
- Eccleston, C. Chronic pain and distraction: An experimental investigation into the role of sustained and shifting attention in the processing of chronic persistent pain. Behav. Res. Ther. 1995, 33, 391–405. [Google Scholar] [CrossRef]
- Roa Romero, Y.; Straube, T.; Nitsch, A.; Miltner, W.H.R.; Weiss, T. Interaction between stimulus intensity and perceptual load in the attentional control of pain. Pain 2013, 154, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Moore, D.J.; Keogh, E.; Eccleston, C. Headache impairs attentional performance. Pain 2013, 154, 1840–1845. [Google Scholar] [CrossRef]
- Keogh, E.; Cavill, R.; Moore, D.J.; Eccleston, C. The effects of menstrual-related pain on attentional interference. Pain 2014, 155, 821–827. [Google Scholar] [CrossRef]
- Attridge, N.; Keogh, E.; Eccleston, C. The effect of pain on task switching: Pain reduces accuracy and increases reaction times across multiple switching paradigms. Pain 2016, 157, 2179–2193. [Google Scholar] [CrossRef]
- Nadar, M.S.; Jasem, Z.; Manee, F.S. The cognitive functions in adults with chronic pain: A comparative study. Pain Res. Manag. 2016, 2016, 5719380. [Google Scholar] [CrossRef]
- Moriarty, O.; Ruane, N.; O’Gorman, D.; Maharaj, C.H.; Mitchell, C.; Sarma, K.M.; Finn, D.P.; McGuire, B.E. Cognitive impairment in patients with chronic neuropathic or radicular pain: An interaction of pain and age. Front. Behav. Neurosci. 2017, 11, 100. [Google Scholar] [CrossRef]
- Moore, D.J.; Keogh, E.; Eccleston, C. The Interruptive Effect of Pain on Attention. Q. J. Exp. Psychol. 2012, 65, 565–586. [Google Scholar] [CrossRef]
- Moore, D.J.; Eccleston, C.; Keogh, E. Cognitive load selectively influences the interruptive effect of pain on attention. Pain 2017, 158, 2035–2041. [Google Scholar] [CrossRef]
- Hauck, M.; Lorenz, J.; Engel, A.K. Attention to painful stimulation enhances γ-band activity and synchronization in human sensorimotor cortex. J. Neurosci. 2007, 27, 9270–9277. [Google Scholar] [CrossRef]
- Jensen, O.; Kaiser, J.; Lachaux, J.P. Human gamma-frequency oscillations associated with attention and memory. Trends Neurosci. 2007, 30, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.; Niebur, E.; Hsiao, S.S.; Sinai, A.; Crone, N.E. High-frequency gamma activity (80–150 Hz) is increased in human cortex during selective attention. Clin. Neurophysiol. 2008, 119, 116–133. [Google Scholar] [CrossRef] [PubMed]
- Schulman, J.J.; Ramirez, R.R.; Zonenshayn, M.; Ribary, U.; Llinas, R. Thalamocortical dysrhythmia syndrome: MEG imaging of neuropathic pain. Thalamus Relat. Syst. 2005, 3, 33–39. [Google Scholar] [CrossRef]
- Petrovic, P.; Ingvar, M.; Stone-Elander, S.; Petersson, K.M.; Hansson, P. A PET activation study of dynamic mechanical allodynia in patients with mononeuropathy. Pain 1999, 83, 459–470. [Google Scholar] [CrossRef]
- Petrovic, P.; Petersson, K.M.; Ghatan, P.H.; Stone-Elander, S.; Ingvar, M. Pain-related cerebral activation is altered by a distracting cognitive task. Pain 2000, 85, 19–30. [Google Scholar] [CrossRef]
- Davis, K.D.; Taylor, S.J.; Crawley, A.P.; Wood, M.L.; Mikulis, D.J. Functional MRI of pain- and attention-related activations in the human cingulate cortex. J. Neurophysiol. 1997, 77, 3370–3380. [Google Scholar] [CrossRef]
- Schmidt-Wilcke, T.; Kairys, A.; Ichesco, E.; Fernandez-Sanchez, M.L.; Barjola, P.; Heitzeg, M.; Harris, R.E.; Clauw, D.J.; Glass, J.; Williams, D.A. Changes in clinical pain in fibromyalgia patients correlate with changes in brain activation in the cingulate cortex in a response inhibition task. Pain Med. (USA) 2014, 15, 1346–1358. [Google Scholar] [CrossRef]
- Legrain, V.; Van Damme, S.; Eccleston, C.; Davis, K.D.; Seminowicz, D.A.; Crombez, G. A neurocognitive model of attention to pain: Behavioral and neuroimaging evidence. Pain 2009, 144, 230–232. [Google Scholar] [CrossRef]
- Schulz, E.; May, E.S.; Postorino, M.; Tiemann, L.; Nickel, M.M.; Witkovsky, V.; Schmidt, P.; Gross, J.; Ploner, M. Prefrontal Gamma Oscillations Encode Tonic Pain in Humans. Cereb. Cortex 2015, 25, 4407–4414. [Google Scholar] [CrossRef]
- May, E.S.; Nickel, M.M.; Ta Dinh, S.; Tiemann, L.; Heitmann, H.; Voth, I.; Tölle, T.R.; Gross, J.; Ploner, M. Prefrontal gamma oscillations reflect ongoing pain intensity in chronic back pain patients. Hum. Brain Mapp. 2019, 40, 293–305. [Google Scholar] [CrossRef] [PubMed]
- Pawela, C.P.; Kramer, J.M.; Hogan, Q.H. Dorsal root ganglion stimulation attenuates the BOLD signal response to noxious sensory input in specific brain regions: Insights into a possible mechanism for analgesia. Neuroimage 2017, 147, 10–18. [Google Scholar] [CrossRef] [PubMed]
- De Ridder, D.; Plazier, M.; Kamerling, N.; Menovsky, T.; Vanneste, S. Burst spinal cord stimulation for limb and back pain. World Neurosurg. 2013, 80, 642–649. [Google Scholar] [CrossRef] [PubMed]





| Patient | Age | Gender | Diagnosis | Electrode Location | Stimulation Parameters (Frequency (Hz)/Amplitude (mA)/Pulse Width (μs)) | |
|---|---|---|---|---|---|---|
| 1 | 49 | Female | Postherpetic neuralgia | Right L5 | 20/1.6/400 | |
| 2 | 53 | Female | Meralgia paresthetica | Right L2 | 20/0.6/300 | |
| 3 | 29 | Male | Post-traumatic compressive neuropathy | Left L2 | 20/0.7/250 | |
| 4 | 78 | Male | Diabetic neuropathy | Bilateral L5 | Right - 20/1.025/450 Left - 20/0.775/480 | |
| 5 | 46 | Male | CRPS | Right L3 | 20/0.7/410 | |
| 6 | 52 | Male | Post-operative nerve entrapment | Left L1 | 28/1.3/250 | |
| 7 | 58 | Female | CRPS | Right L2/L3 | 20/2.1/250 | |
| 8 | 61 | Male | Post-operative mononeuropathy | Left L3 | 20/2.1/140 | |
| 9 | 47 | Male | CRPS | Left L4 | 20/6/350 | |
| 10 | 55 | Male | Nerve entrapment | Right C7/C8 | 20/0.425/300 | |
| 11 | 29 | Male | Post-operative radiculopathy | Bilateral L5 | Right - 20/2.25/700, Left - 20/650/800 | |
| 12 | 52 | Female | CRPS | Right L5 | 30/0.7/500 | |
| 13 | 77 | Female | Postherpetic neuralgia | Right T1 | 30/0.4/300 | |
| 14 | 22 | Female | Dystonic pain | Right L2/L3 | 20/2.4/300 | |
| 15 | 52 | Male | Post-operative mononeuropathy | Right L1 | 30/0.525/400 | |
| 16 | 54 | Male | Post-operative radiculopathy | Right L3/L4 | 20/0.475/360 |
| Standardized β | Standard Error | p-value | |
|---|---|---|---|
| Frontal | |||
| Pain → Gamma | 0.398 | 0.008 | 0.044 |
| Cognition → Gamma | −0.332 | 0.00 | 0.082 |
| Somatosensoy cortex | |||
| Pain → Gamma | 0.93 | 0.014 | 0.63 |
| Cognition → Gamma | −0.447 | 0.00 | 0.028 |
| Dorsolateral Prefrontal cortex | |||
| Pain → Gamma | 0.179 | 0.019 | 0.4 |
| Cognition → Gamma | −0.134 | 0.00 | 0.53 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parker, T.; Huang, Y.; Raghu, A.L.B.; FitzGerald, J.J.; Green, A.L.; Aziz, T.Z. Dorsal Root Ganglion Stimulation Modulates Cortical Gamma Activity in the Cognitive Dimension of Chronic Pain. Brain Sci. 2020, 10, 95. https://doi.org/10.3390/brainsci10020095
Parker T, Huang Y, Raghu ALB, FitzGerald JJ, Green AL, Aziz TZ. Dorsal Root Ganglion Stimulation Modulates Cortical Gamma Activity in the Cognitive Dimension of Chronic Pain. Brain Sciences. 2020; 10(2):95. https://doi.org/10.3390/brainsci10020095
Chicago/Turabian StyleParker, Tariq, Yongzhi Huang, Ashley L.B. Raghu, James J. FitzGerald, Alexander L. Green, and Tipu Z. Aziz. 2020. "Dorsal Root Ganglion Stimulation Modulates Cortical Gamma Activity in the Cognitive Dimension of Chronic Pain" Brain Sciences 10, no. 2: 95. https://doi.org/10.3390/brainsci10020095
APA StyleParker, T., Huang, Y., Raghu, A. L. B., FitzGerald, J. J., Green, A. L., & Aziz, T. Z. (2020). Dorsal Root Ganglion Stimulation Modulates Cortical Gamma Activity in the Cognitive Dimension of Chronic Pain. Brain Sciences, 10(2), 95. https://doi.org/10.3390/brainsci10020095






