The Role of Sartans in the Treatment of Stroke and Subarachnoid Hemorrhage: A Narrative Review of Preclinical and Clinical Studies
Abstract
:1. Introduction
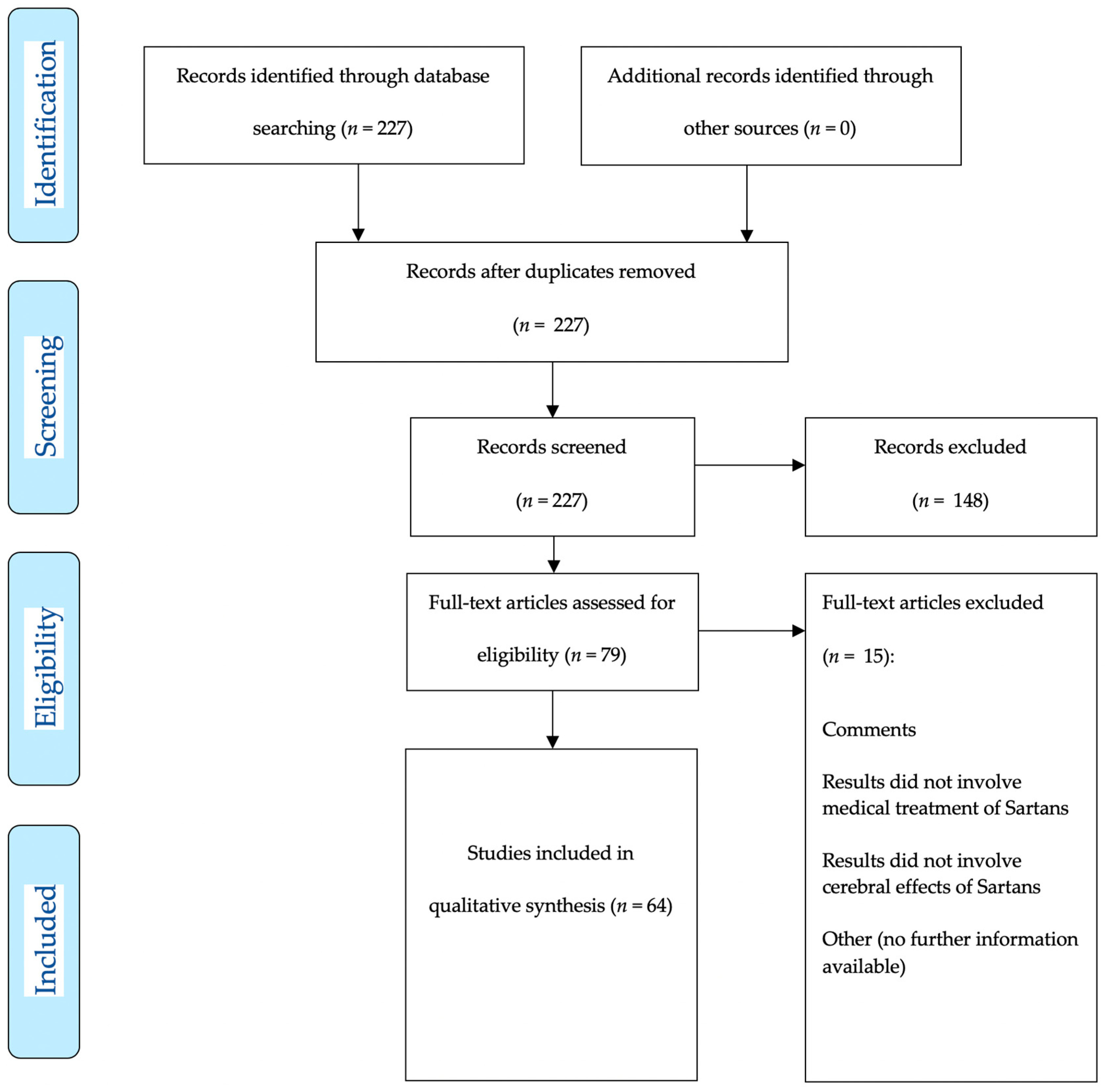
2. Materials and Methods
3. Results
3.1. Preclinical Studies on Sartans in Animal Models of Ischemic Stroke
3.2. Clinical Studies on Sartans in Ischemic Stroke
3.3. Therapeutic Interventions After aSAH
3.4. Effects of Losartan Following aSAH
4. Discussions
4.1. Translational Aspects
4.2. Synopsis and Forecast
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Vatter, H.; Konczalla, J.; Weidauer, S.; Preibisch, C.; Zimmermann, M.; Raabe, A.; Seifert, V. Effect of delayed cerebral vasospasm on cerebrovascular endothelin A receptor expression and function. J. Neurosurg. 2007, 107, 121–127. [Google Scholar] [CrossRef]
- Brandt, L.; Ljunggren, B.; Andersson, K.E.; Hindfelt, B.; Uski, T. Prostaglandin metabolism and prostacyclin in cerebral vasospasm. Gen. Pharmacol. 1983, 14, 141–143. [Google Scholar] [CrossRef]
- Egg, D.; Herold, M.; Rumpl, E.; Gunther, R. Prostaglandin F2 alpha levels in human cerebrospinal fluid in normal and pathological conditions. J. Neurol. 1980, 222, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Fujii, M.; Yan, J.; Rolland, W.B.; Soejima, Y.; Caner, B.; Zhang, J.H. Early brain injury, an evolving frontier in subarachnoid hemorrhage research. Transl. Stroke Res. 2013, 4, 432–446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dreier, J.P.; Drenckhahn, C.; Woitzik, J.; Major, S.; Offenhauser, N.; Weber-Carstens, S.; Wolf, S.; Strong, A.J.; Vajkoczy, P.; Hartings, J.A.; et al. Spreading ischemia after aneurysmal subarachnoid hemorrhage. Acta Neurochir. Suppl. 2013, 115, 125–129. [Google Scholar] [PubMed]
- Vergouwen, M.D.; Vermeulen, M.; van Gijn, J.; Rinkel, G.J.; Wijdicks, E.F.; Muizelaar, J.P.; Mendelow, A.D.; Juvela, S.; Yonas, H.; Terbrugge, K.G.; et al. Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies: Proposal of a multidisciplinary research group. Stroke 2010, 41, 2391–2395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Budohoski, K.P.; Czosnyka, M.; Smielewski, P.; Kasprowicz, M.; Helmy, A.; Bulters, D.; Pickard, J.D.; Kirkpatrick, P.J. Impairment of cerebral autoregulation predicts delayed cerebral ischemia after subarachnoid hemorrhage: A prospective observational study. Stroke 2012, 43, 3230–3237. [Google Scholar] [CrossRef] [Green Version]
- Bederson, J.B.; Connolly, E.S.; Batjer, H.H., Jr.; Dacey, R.G.; Dion, J.E.; Diringer, M.N.; Duldner, J.E., Jr.; Harbaugh, R.E.; Patel, A.B.; Rosenwasser, R.H.; et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: A statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke 2009, 40, 994–1025. [Google Scholar] [CrossRef] [Green Version]
- Salom, J.B.; Torregrosa, G.; Alborch, E. Endothelins and the cerebral circulation. Cerebrovasc. Brain Metab. Rev. 1995, 7, 131–152. [Google Scholar]
- Vatter, H.; Zimmermann, M.; Seifert, V.; Schilling, L. Experimental approaches to evaluate endothelin-A receptor antagonists. Methods Find. Exp. Clin. Pharmacol. 2004, 26, 277–286. [Google Scholar]
- Zimmermann, M.; Seifert, V. Endothelin and subarachnoid hemorrhage: An overview. Neurosurgery 1998, 43, 863–875. [Google Scholar] [CrossRef] [PubMed]
- Neuschmelting, V.; Marbacher, S.; Fathi, A.R.; Jakob, S.M.; Fandino, J. Elevated level of endothelin-1 in cerebrospinal fluid and lack of nitric oxide in basilar arterial plasma associated with cerebral vasospasm after subarachnoid haemorrhage in rabbits. Acta Neurochir. 2009, 151, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Josko, J.; Hendryk, S.; Jedrzejowska-Szypulka, H.; Slowinski, J.; Gwozdz, B.; Lange, D.; Harabin-Slowinska, M. Effect of endothelin-1 receptor antagonist BQ-123 on basilar artery diameter after subarachnoid hemorrhage (SAH) in rats. J. Physiol. Pharmacol. 2000, 51, 241–249. [Google Scholar] [PubMed]
- Nishizawa, S.; Chen, D.; Yokoyama, T.; Yokota, N.; Otha, S. Endothelin-1 initiates the development of vasospasm after subarachnoid haemorrhage through protein kinase C activation, but does not contribute to prolonged vasospasm. Acta Neurochir. 2000, 142, 1409–1415. [Google Scholar] [CrossRef] [PubMed]
- Hansen-Schwartz, J.; Hoel, N.L.; Zhou, M.; Xu, C.B.; Svendgaard, N.A.; Edvinsson, L. Subarachnoid hemorrhage enhances endothelin receptor expression and function in rat cerebral arteries. Neurosurgery 2003, 52, 1188–1194. [Google Scholar] [PubMed]
- Lei, Q.; Li, S.; Zheng, R.; Xu, K.; Li, S. Endothelin-1 expression and alterations of cerebral microcirculation after experimental subarachnoid hemorrhage. Neuroradiology 2015, 57, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Josko, J.; Hendryk, S.; Jedrzejowska-Szypulka, H.; Slowinski, J.; Gwozdz, B.; Lange, D.; Snietura, M.; Zwirska-Korczala, K.; Jochem, J. Cerebral angiogenesis after subarachnoid hemorrhage (SAH) and endothelin receptor blockage with BQ-123 antagonist in rats. J. Physiol. Pharmacol. 2001, 52, 237–248. [Google Scholar]
- Xie, A.; Aihara, Y.; Bouryi, V.A.; Nikitina, E.; Jahromi, B.S.; Zhang, Z.D.; Takahashi, M.; Macdonald, R.L. Novel mechanism of endothelin-1-induced vasospasm after subarachnoid hemorrhage. J. Cereb. Blood Flow Metab. 2007, 27, 1692–1701. [Google Scholar] [CrossRef] [Green Version]
- Kim, C.Y.; Paek, S.H.; Seo, B.G.; Kim, J.H.; Han, D.H. Changes in vascular responses of the basilar artery to acetylcholine and endothelin-1 in an experimental rabbit vasospasm model. Acta Neurochir. 2003, 145, 571–577. [Google Scholar] [CrossRef]
- Chow, M.; Dumont, A.S.; Kassell, N.F. Endothelin receptor antagonists and cerebral vasospasm: An update. Neurosurgery 2002, 51, 1333–1341. [Google Scholar] [CrossRef]
- Macdonald, R.L.; Higashida, R.T.; Keller, E.; Mayer, S.A.; Molyneux, A.; Raabe, A.; Vajkoczy, P.; Wanke, I.; Bach, D.; Frey, A.; et al. Randomised trial of clazosentan, an endothelin receptor antagonist, in patients with aneurysmal subarachnoid hemorrhage undergoing surgical clipping (CONSCIOUS-2). Acta Neurochir. Suppl. 2013, 115, 27–31. [Google Scholar] [PubMed]
- Higashida, R.T.; Bruder, N.; Gupta, R.; Guzman, R.; Hmissi, A.; Marr, A.; Mayer, S.A.; Roux, S.; Weidauer, S.; Aldrich, E.F. Reversal of Vasospasm with Clazosentan After Aneurysmal Subarachnoid Hemorrhage: A Pilot Study. World Neurosurg. 2019, 128, e639–e648. [Google Scholar] [CrossRef] [PubMed]
- Konczalla, J.; Wanderer, S.; Mrosek, J.; Schuss, P.; Platz, J.; Güresir, E.; Seifert, V.; Vatter, H. Crosstalk between the angiotensin and endothelin-system in the cerebrovasculature. Curr. Neurovasc. Res. 2013, 10, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [Green Version]
- Justin, A.; Divakar, S.; Ramanathan, M. Cerebral ischemia induced inflammatory response and altered glutaminergic function mediated through brain AT1 and not AT2 receptor. Biomed. Pharmacother. 2018, 102, 947–958. [Google Scholar] [CrossRef]
- Iwanami, J.; Mogi, M.; Tsukuda, K.; Min, L.J.; Sakata, A.; Jing, F.; Iwai, M.; Horiuchi, M. Low dose of telmisartan prevents ischemic brain damage with peroxisome proliferator-activated receptor-gamma activation in diabetic mice. J. Hypertens. 2010, 28, 1730–1737. [Google Scholar] [CrossRef]
- Kasahara, Y.; Taguchi, A.; Uno, H.; Nakano, A.; Nakagomi, T.; Hirose, H.; Stern, D.M.; Matsuyama, T. Telmisartan suppresses cerebral injury in a murine model of transient focal ischemia. Brain Res. 2010, 1340, 70–80. [Google Scholar] [CrossRef]
- Iwai, M.; Inaba, S.; Tomono, Y.; Kanno, H.; Iwanami, J.; Mogi, M.; Horiuchi, M. Attenuation of focal brain ischemia by telmisartan, an angiotensin II type 1 receptor blocker, in atherosclerotic apolipoprotein E-deficient mice. Hypertens. Res. 2008, 31, 161–168. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Zhang, Y.; Zhu, B.; Wu, C.; Chen, Y. Telmisartan regulates the development of cerebral ischemia by alleviating endoplasmic reticulum stress. Pharmazie 2018, 73, 585–588. [Google Scholar]
- Gao, Y.; Li, W.; Liu, Y.; Wang, Y.; Zhang, J.; Li, M.; Bu, M. Effect of Telmisartan on Preventing Learning and Memory Deficits Via Peroxisome Proliferator-Activated Receptor-gamma in Vascular Dementia Spontaneously Hypertensive Rats. J. Stroke Cerebrovasc. Dis. 2018, 27, 277–285. [Google Scholar] [CrossRef]
- Ramanathan, M.; Justin, A.; Sudheer, A.; Shanthakumari, S. Comparison of pre- and post-ischemic treatment of telmisartan and nimodipine combination in experimentally induced cerebral ischemia. Indian J. Exp. Biol. 2016, 54, 560–568. [Google Scholar]
- Kono, S.; Kurata, T.; Sato, K.; Omote, Y.; Hishikawa, N.; Yamashita, T.; Deguchi, K.; Abe, K. Neurovascular protection by telmisartan via reducing neuroinflammation in stroke-resistant spontaneously hypertensive rat brain after ischemic stroke. J. Stroke Cerebrovasc. Dis. 2015, 24, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Dupuis, F.; Vincent, J.M.; Liminana, P.; Chillon, J.M.; Capdeville-Atkinson, C.; Atkinson, J. Effects of suboptimal doses of the AT1 receptor blocker, telmisartan, with the angiotensin-converting enzyme inhibitor, ramipril, on cerebral arterioles in spontaneously hypertensive rat. J. Hypertens. 2010, 28, 1566–1573. [Google Scholar] [CrossRef] [PubMed]
- Deguchi, K.; Kurata, T.; Fukui, Y.; Liu, W.; Yun, Z.; Omote, Y.; Sato, K.; Kono, S.; Hishikawa, N.; Yamashita, T.; et al. Long-term amelioration of telmisartan on metabolic syndrome-related molecules in stroke-resistant spontaneously hypertensive rat after transient middle cerebral artery occlusion. J. Stroke Cerebrovasc. Dis. 2014, 23, 2646–2653. [Google Scholar] [CrossRef]
- Thoene-Reineke, C.; Rumschussel, K.; Schmerbach, K.; Krikov, M.; Wengenmayer, C.; Godes, M.; Mueller, S.; Villringer, A.; Steckelings, U.; Namsolleck, P.; et al. Prevention and intervention studies with telmisartan, ramipril and their combination in different rat stroke models. PLoS ONE 2011, 6, e23646. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, T.; Kawamata, T.; Shibata, N.; Okada, Y.; Kobayashi, M.; Hori, T. Angiotensin II type 1 receptor blocker telmisartan reduces cerebral infarct volume and peri-infarct cytosolic phospholipase A(2) level in experimental stroke. J. Neurotrauma. 2009, 26, 2355–2364. [Google Scholar] [CrossRef]
- Jung, K.H.; Chu, K.; Lee, S.T.; Kim, S.J.; Song, E.C.; Kim, E.H.; Park, D.K.; Sinn, D.I.; Kim, J.M.; Kim, M. Blockade of AT1 receptor reduces apoptosis, inflammation, and oxidative stress in normotensive rats with intracerebral hemorrhage. J. Pharmacol. Exp. Ther. 2007, 322, 1051–1058. [Google Scholar] [CrossRef] [Green Version]
- Fukui, Y.; Yamashita, T.; Kurata, T.; Sato, K.; Lukic, V.; Hishikawa, N.; Deguchi, K.; Abe, K. Protective effect of telmisartan against progressive oxidative brain damage and synuclein phosphorylation in stroke-resistant spontaneously hypertensive rats. J. Stroke Cerebrovasc. Dis. 2014, 23, 1545–1553. [Google Scholar] [CrossRef]
- Hamai, M.; Iwai, M.; Ide, A.; Tomochika, H.; Tomono, Y.; Mogi, M.; Horiuchi, M. Comparison of inhibitory action of candesartan and enalapril on brain ischemia through inhibition of oxidative stress. Neuropharmacology 2006, 51, 822–828. [Google Scholar] [CrossRef]
- Awad, A.S. Effect of combined treatment with curcumin and candesartan on ischemic brain damage in mice. J. Stroke Cerebrovasc. Dis. 2011, 20, 541–548. [Google Scholar] [CrossRef]
- Soliman, S.; Ishrat, T.; Fouda, A.Y.; Patel, A.; Pillai, B.; Fagan, S.C. Sequential Therapy with Minocycline and Candesartan Improves Long-Term Recovery After Experimental Stroke. Transl. Stroke Res. 2015, 6, 309–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishrat, T.; Soliman, S.; Eldahshan, W.; Pillai, B.; Ergul, A.; Fagan, S.C. Silencing VEGF-B Diminishes the Neuroprotective Effect of Candesartan Treatment After Experimental Focal Cerebral Ischemia. Neurochem. Res. 2018, 43, 1869–1878. [Google Scholar] [CrossRef] [PubMed]
- Culman, J.; Jacob, T.; Schuster, S.O.; Brolund-Spaether, K.; Brolund, L.; Cascorbi, I.; Zhao, Y.; Gohlke, P. Neuroprotective effects of AT1 receptor antagonists after experimental ischemic stroke: What is important? Naunyn. Schmiedebergs Arch. Pharmacol. 2017, 390, 949–959. [Google Scholar] [CrossRef] [PubMed]
- Alhusban, A.; Kozak, A.; Pillai, B.; Ahmed, H.; Sayed, M.A.; Johnson, M.H.; Ishrat, T.; Ergul, A.; Fagan, S.C. Mechanisms of acute neurovascular protection with AT1 blockade after stroke: Effect of prestroke hypertension. PLoS ONE 2017, 12, e0178867. [Google Scholar] [CrossRef] [Green Version]
- Fouda, A.Y.; Alhusban, A.; Ishrat, T.; Pillai, B.; Eldahshan, W.; Waller, J.L.; Ergul, A.; Fagan, S.C. Brain-Derived Neurotrophic Factor Knockdown Blocks the Angiogenic and Protective Effects of Angiotensin Modulation After Experimental Stroke. Mol. Neurobiol. 2017, 54, 661–670. [Google Scholar] [CrossRef] [Green Version]
- Soliman, S.; Ishrat, T.; Pillai, A.; Somanath, P.R.; Ergul, A.; El-Remessy, A.B.; Fagan, S.C. Candesartan induces a prolonged proangiogenic effect and augments endothelium-mediated neuroprotection after oxygen and glucose deprivation: Role of vascular endothelial growth factors A and B. J. Pharmacol. Exp. Ther. 2014, 349, 444–457. [Google Scholar] [CrossRef] [Green Version]
- Alhusban, A.; Kozak, A.; Ergul, A.; Fagan, S.C. AT1 receptor antagonism is proangiogenic in the brain: BDNF a novel mediator. J. Pharmacol. Exp. Ther. 2013, 344, 348–359. [Google Scholar] [CrossRef] [Green Version]
- So, G.; Nakagawa, S.; Morofuji, Y.; Hiu, T.; Hayashi, K.; Tanaka, K.; Suyama, K.; Deli, M.A.; Nagata, I.; Matsuo, T.; et al. Candesartan improves ischemia-induced impairment of the blood-brain barrier in vitro. Cell Mol. Neurobiol. 2015, 35, 563–572. [Google Scholar] [CrossRef]
- Panahpour, H.; Nekooeian, A.A.; Dehghani, G.A. Candesartan attenuates ischemic brain edema and protects the blood-brain barrier integrity from ischemia/reperfusion injury in rats. Iran. Biomed. J. 2014, 18, 232–238. [Google Scholar]
- Ishrat, T.; Pillai, B.; Soliman, S.; Fouda, A.Y.; Kozak, A.; Johnson, M.H.; Ergul, A.; Fagan, S.C. Low-dose candesartan enhances molecular mediators of neuroplasticity and subsequent functional recovery after ischemic stroke in rats. Mol. Neurobiol. 2015, 51, 1542–1553. [Google Scholar] [CrossRef]
- Guan, W.; Kozak, A.; Fagan, S.C. Drug repurposing for vascular protection after acute ischemic stroke. Acta Neurochir. Suppl. 2011, 111, 295–298. [Google Scholar] [PubMed] [Green Version]
- Schmerbach, K.; Pfab, T.; Zhao, Y.; Culman, J.; Mueller, S.; Villringer, A.; Muller, D.N.; Hocher, B.; Unger, T.; Thoene-Reineke, C. Effects of aliskiren on stroke in rats expressing human renin and angiotensinogen genes. PLoS ONE 2010, 5, e15052. [Google Scholar] [CrossRef] [PubMed]
- Omura-Matsuoka, E.; Yagita, Y.; Sasaki, T.; Terasaki, Y.; Oyama, N.; Sugiyama, Y.; Okazaki, S.; Ssakoda, S.; Kitagawa, K. Postischemic administration of angiotensin II type 1 receptor blocker reduces cerebral infarction size in hypertensive rats. Hypertens. Res. 2009, 32, 548–553. [Google Scholar] [CrossRef]
- Kozak, W.; Kozak, A.; Johnson, M.H.; Elewa, H.F.; Fagan, S.C. Vascular protection with candesartan after experimental acute stroke in hypertensive rats: A dose-response study. J. Pharmacol. Exp. Ther. 2008, 326, 773–782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krikov, M.; Thone-Reineke, C.; Muller, S.; Villringer, A.; Unger, T. Candesartan but not ramipril pretreatment improves outcome after stroke and stimulates neurotrophin BNDF/TrkB system in rats. J. Hypertens. 2008, 26, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Fagan, S.C.; Kozak, A.; Hill, W.D.; Pollock, D.M.; Xu, L.; Johnson, M.H.; Ergul, A.; Hess, D.C. Hypertension after experimental cerebral ischemia: Candesartan provides neurovascular protection. J. Hypertens. 2006, 24, 535–539. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Zhu, Y.Z.; Wong, P.T. Neuroprotective effects of candesartan against cerebral ischemia in spontaneously hypertensive rats. Neuroreport 2005, 16, 1963–1967. [Google Scholar] [CrossRef]
- Kusaka, I.; Kusaka, G.; Zhou, C.; Ishikawa, M.; Nanda, A.; Granger, D.N.; Zhang, J.H.; Tang, J. Role of AT1 receptors and NAD(P)H oxidase in diabetes-aggravated ischemic brain injury. Am. J. Physiol. Heart Circ. Physiol. 2004, 286, H2442–H2451. [Google Scholar] [CrossRef]
- Groth, W.; Blume, A.; Gohlke, P.; Unger, T.; Culman, J. Chronic pretreatment with candesartan improves recovery from focal cerebral ischaemia in rats. J. Hypertens. 2003, 21, 2175–2182. [Google Scholar] [CrossRef]
- Nishimura, Y.; Ito, T.; Saavedra, J.M. Angiotensin II AT(1) blockade normalizes cerebrovascular autoregulation and reduces cerebral ischemia in spontaneously hypertensive rats. Stroke 2000, 31, 2478–2486. [Google Scholar] [CrossRef] [Green Version]
- Gaur, V.; Kumar, A. Neuroprotective potentials of candesartan, atorvastatin and their combination against stroke induced motor dysfunction. Inflammopharmacology 2011, 19, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Engelhorn, T.; Doerfler, A.; Heusch, G.; Schulz, R. Reduction of cerebral infarct size by the AT1-receptor blocker candesartan, the HMG-CoA reductase inhibitor rosuvastatin and their combination. An experimental study in rats. Neurosci. Lett. 2006, 406, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Mecca, A.P.; O’Connor, T.E.; Katovich, M.J.; Sumners, C. Candesartan pretreatment is cerebroprotective in a rat model of endothelin-1-induced middle cerebral artery occlusion. Exp. Physiol. 2009, 94, 937–946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmerbach, K.; Schefe, J.H.; Krikov, M.; Müller, S.; Villringer, A.; Kintscher, U.; Unger, T.; Thoene-Reineke, C. Comparison between single and combined treatment with candesartan and pioglitazone following transient focal ischemia in rat brain. Brain Res. 2008, 1208, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Pavel, J.; Macova, M.; Yu, Z.X.; Imboden, H.; Ge, L.; Nishioku, T.; Dou, J.; Delgiacco, E.; Saavedra, J.M. AT1 receptor blockade regulates the local angiotensin II system in cerebral microvessels from spontaneously hypertensive rats. Stroke 2006, 37, 1271–1276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kozak, A.; Ergul, A.; El-Remessy, A.B.; Johnson, M.H.; Machado, L.S.; Elewa, H.F.; Abdelsaid, M.; Wiley, D.C.; Fagan, S.C. Candesartan augments ischemia-induced proangiogenic state and results in sustained improvement after stroke. Stroke 2009, 40, 1870–1876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faure, S.; Bureau, A.; Oudart, N.; Javellaud, J.; Fournier, A.; Achard, J.M. Protective effect of candesartan in experimental ischemic stroke in the rat mediated by AT2 and AT4 receptors. J. Hypertens. 2008, 26, 2008–2015. [Google Scholar] [CrossRef]
- Stenman, E.; Jamali, R.; Henriksson, M.; Maddahi, A.; Edvinsson, L. Cooperative effect of angiotensin AT(1) and endothelin ET(A) receptor antagonism limits the brain damage after ischemic stroke in rat. Eur. J. Pharmacol. 2007, 570, 142–148. [Google Scholar] [CrossRef]
- Stenman, E.; Edvinsson, L. Cerebral ischemia enhances vascular angiotensin AT1 receptor-mediated contraction in rats. Stroke 2004, 35, 970–974. [Google Scholar] [CrossRef] [Green Version]
- Brdon, J.; Kaiser, S.; Hagemann, F.; Zhao, Y.; Culman, J.; Gohlke, P. Comparison between early and delayed systemic treatment with candesartan of rats after ischaemic stroke. J. Hypertens. 2007, 25, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Engelhorn, T.; Goerike, S.; Doerfler, A.; Okorn, C.; Forsting, M.; Heusch, G.; Schulz, R. The angiotensin II type 1-receptor blocker candesartan increases cerebral blood flow, reduces infarct size, and improves neurologic outcome after transient cerebral ischemia in rats. J. Cereb. Blood Flow Metab. 2004, 24, 467–474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamakawa, H.; Jezova, M.; Ando, H.; Saavedra, J.M. Normalization of endothelial and inducible nitric oxide synthase expression in brain microvessels of spontaneously hypertensive rats by angiotensin II AT1 receptor inhibition. J. Cereb. Blood Flow Metab. 2003, 23, 371–380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakagawa, T.; Hasegawa, Y.; Uekawa, K.; Senju, S.; Nakagata, N.; Matsui, K.; Kim-Mitsuyama, S. Transient Mild Cerebral Ischemia Significantly Deteriorated Cognitive Impairment in a Mouse Model of Alzheimer’s Disease via Angiotensin AT1 Receptor. Am. J. Hypertens. 2017, 30, 141–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faure, S.; Oudart, N.; Javellaud, J.; Fournier, A.; Warnock, D.G.; Achard, J.M. Synergistic protective effects of erythropoietin and olmesartan on ischemic stroke survival and post-stroke memory dysfunctions in the gerbil. J. Hypertens. 2006, 24, 2255–2261. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Fernandez, M.; Fuentes, B.; Rodriguez-Frutos, B.; Ramos-Cejudo, J.; Otero-Ortega, L.; Diez-Tejedor, E. Different protective and reparative effects of olmesartan in stroke according to time of administration and withdrawal. J. Neurosci. Res. 2015, 93, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Oyama, N.; Yagita, Y.; Sasaki, T.; Omura-Matsuoka, E.; Terasaki, Y.; Sugiyama, Y.; Sakoda, S.; Kitagawa, K. An angiotensin II type 1 receptor blocker can preserve endothelial function and attenuate brain ischemic damage in spontaneously hypertensive rats. J. Neurosci. Res. 2010, 88, 2889–2898. [Google Scholar] [CrossRef]
- Hosomi, N.; Nishiyama, A.; Ban, C.R.; Naya, T.; Takahashi, T.; Kohno, M.; Koziol, J.A. Angiotensin type 1 receptor blockage improves ischemic injury following transient focal cerebral ischemia. Neuroscience 2005, 134, 225–231. [Google Scholar] [CrossRef]
- Li, J.M.; Mogi, M.; Iwanami, J.; Min, L.J.; Tsukuda, K.; Sakata, A.; Fujita, T.; Iwai, M.; Horiuchi, M. Temporary pretreatment with the angiotensin II type 1 receptor blocker, valsartan, prevents ischemic brain damage through an increase in capillary density. Stroke 2008, 39, 2029–2036. [Google Scholar] [CrossRef]
- Miyamoto, N.; Zhang, N.; Tanaka, R.; Liu, M.; Hattori, N.; Urabe, T. Neuroprotective role of angiotensin II type 2 receptor after transient focal ischemia in mice brain. Neurosci. Res. 2008, 61, 249–256. [Google Scholar] [CrossRef]
- Iwai, M.; Liu, H.W.; Chen, R.; Ide, A.; Okamoto, S.; Hata, R.; Sakanaka, M.; Shiuchi, T.; Horiuchi, M. Possible inhibition of focal cerebral ischemia by angiotensin II type 2 receptor stimulation. Circulation 2004, 110, 843–848. [Google Scholar] [CrossRef] [Green Version]
- Dong, Y.F.; Kataoka, K.; Tokutomi, Y.; Nako, H.; Nakamura, T.; Toyama, K.; Sueta, D.; Koibuchi, N.; Yamamoto, E.; Ogawa, H.; et al. Beneficial effects of combination of valsartan and amlodipine on salt-induced brain injury in hypertensive rats. J. Pharmacol. Exp. Ther. 2011, 339, 358–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lou, M.; Blume, A.; Zhao, Y.; Gohlke, P.; Deuschl, G.; Herdegen, T.; Culman, J. Sustained blockade of brain AT1 receptors before and after focal cerebral ischemia alleviates neurologic deficits and reduces neuronal injury, apoptosis, and inflammatory responses in the rat. J. Cereb. Blood Flow Metab. 2004, 24, 536–547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, W.J.; Funk, A.; Herdegen, T.; Unger, T.; Culman, J. Blockade of central angiotensin AT(1) receptors improves neurological outcome and reduces expression of AP-1 transcription factors after focal brain ischemia in rats. Stroke 1999, 30, 2391–2398. [Google Scholar] [CrossRef] [Green Version]
- Tsukuda, K.; Mogi, M.; Iwanami, J.; Min, L.J.; Jing, F.; Oshima, K.; Horiuchi, M. Irbesartan attenuates ischemic brain damage by inhibition of MCP-1/CCR2 signaling pathway beyond AT(1) receptor blockade. Biochem. Biophys. Res. Commun. 2011, 409, 275–279. [Google Scholar] [CrossRef]
- Dalmay, F.; Mazouz, H.; Allard, J.; Pesteil, F.; Achard, J.M.; Fournier, A. Non-AT(1)-receptor-mediated protective effect of angiotensin against acute ischaemic stroke in the gerbil. J. Renin Angiotensin Aldosterone Syst. 2001, 2, 103–106. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Li, G.; Zhang, W.; Sigmund, C.D.; Olson, J.E.; Chen, Y. Ischemia-induced brain damage is enhanced in human renin and angiotensinogen double-transgenic mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 297, R1526–R1531. [Google Scholar] [CrossRef] [Green Version]
- Loh, K.P.; Low, L.S.; Wong, W.H.; Zhou, S.; Huang, S.H.; De Silva, R.; Duan, W.; Chou, W.H.; Zhu, Y.Z. A comparison study of cerebral protection using Ginkgo biloba extract and Losartan on stroked rats. Neurosci. Lett. 2006, 398, 28–33. [Google Scholar] [CrossRef]
- Forder, J.P.; Munzenmaier, D.H.; Greene, A.S. Angiogenic protection from focal ischemia with angiotensin II type 1 receptor blockade in the rat. Am. J. Physiol. Heart Circ. Physiol. 2005, 288, H1989–H1996. [Google Scholar] [CrossRef] [Green Version]
- Miyamoto, N.; Tanaka, Y.; Ueno, Y.; Tanaka, R.; Hattori, N.; Urabe, T. Benefits of prestroke use of angiotensin type 1 receptor blockers on ischemic stroke severity. J. Stroke Cerebrovasc. Dis. 2012, 21, 363–368. [Google Scholar] [CrossRef]
- Jusufovic, M.; Sandset, E.C.; Bath, P.M.; Berge, E.; Scandinavian Candesartan Acute Stroke Trial Study Group. Early blood pressure lowering treatment in acute stroke. Ordinal analysis of vascular events in the Scandinavian Candesartan Acute Stroke Trial (SCAST). J. Hypertens. 2016, 34, 1594–1598. [Google Scholar] [CrossRef]
- Hornslien, A.G.; Sandset, E.C.; Wyller, T.B.; Berge, E.; Scandinavian Candesartan Acute Stroke Trial Study Group. Effects of candesartan in acute stroke on activities of daily living and level of care at 6 months. J. Hypertens. 2015, 33, 1487–1491. [Google Scholar] [CrossRef] [PubMed]
- Sandset, E.C.; Jusufovic, M.; Sandset, P.M.; Bath, P.M.; Berge, E.; Group, S.S. Effects of blood pressure-lowering treatment in different subtypes of acute ischemic stroke. Stroke 2015, 46, 877–879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandset, E.C.; Bath, P.M.; Boysen, G.; Jatuzis, D.; Korv, J.; Lüders, S.; Murray, G.D.; Richter, P.S.; Roine, R.O.; Terent, A.; et al. The angiotensin-receptor blocker candesartan for treatment of acute stroke (SCAST): A randomised, placebo-controlled, double-blind trial. Lancet 2011, 377, 741–750. [Google Scholar] [CrossRef]
- Schrader, J.; Luders, S.; Kulschewski, A.; Berger, J.; Zidek, W.; Treib, J.; Einhäupl, K.; Diener, H.C.; Dominiak, P.; Acute Candesartan Cilexetil Therapy in Stroke Survivors Study Group. The ACCESS Study: Evaluation of Acute Candesartan Cilexetil Therapy in Stroke Survivors. Stroke 2003, 34, 1699–1703. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, T.; Tsutsumi, Y.; Shimizu, Y.; Uchiyama, S. Renin-angiotensin system blockade safely reduces blood pressure in patients with minor ischemic stroke during the acute phase. J. Stroke Cerebrovasc. Dis. 2010, 19, 435–440. [Google Scholar] [CrossRef]
- Hallevi, H.; Hazan-Halevy, I.; Paran, E. Modification of neutrophil adhesion to human endothelial cell line in acute ischemic stroke by dipyridamole and candesartan. Eur. J. Neurol. 2007, 14, 1002–1007. [Google Scholar] [CrossRef]
- Jusufovic, M.; Sandset, E.C.; Bath, P.M.; Karlson, B.W.; Berge, E.; Scandinavian Candesartan Acute Stroke Trial Study Group. Effects of blood pressure lowering in patients with acute ischemic stroke and carotid artery stenosis. Int. J. Stroke 2015, 10, 354–359. [Google Scholar] [CrossRef]
- Oh, M.S.; Yu, K.H.; Hong, K.S.; Kang, D.W.; Park, J.M.; Bae, H.J.; Koo, J.; Lee, J.; Lee, B.C.; Valsartan Efficacy oN modesT blood pressUre Reduction in acute ischemic stroke (VENTURE) study group. Modest blood pressure reduction with valsartan in acute ischemic stroke: A prospective, randomized, open-label, blinded-end-point trial. Int. J. Stroke 2015, 10, 745–751. [Google Scholar] [CrossRef]
- Serebruany, V.L.; Malinin, A.I.; Lowry, D.R.; Sane, D.C.; Webb, R.L.; Gottlieb, S.O.; O’Connor, C.M.; Hennekens, C.H. Effects of valsartan and valeryl 4-hydroxy valsartan on human platelets: A possible additional mechanism for clinical benefits. J. Cardiovasc. Pharmacol. 2004, 43, 677–684. [Google Scholar] [CrossRef]
- Ovbiagele, B.; Bath, P.M.; Cotton, D.; Sha, N.; Diener, H.C.; Investigators, P.R. Low glomerular filtration rate, recurrent stroke risk, and effect of renin-angiotensin system modulation. Stroke 2013, 44, 3223–3225. [Google Scholar] [CrossRef] [Green Version]
- Wadiwala, M.F.; Kamal, A.K. What is better antiplatelet agent to prevent recurrent stroke? J. Pak. Med. Assoc. 2012, 62, 976–977. [Google Scholar] [PubMed]
- Weber, R.; Weimar, C.; Blatchford, J.; Hermansson, K.; Wanke, I.; Möller-Hartmann, C.; Gizweksi, E.R.; Forsting, M.; Demchuck, A.M.; Sacco, R.L.; et al. Telmisartan on top of antihypertensive treatment does not prevent progression of cerebral white matter lesions in the prevention regimen for effectively avoiding second strokes (PRoFESS) MRI substudy. Stroke 2012, 43, 2336–2342. [Google Scholar] [CrossRef] [PubMed]
- Bath, P.M.; Martin, R.H.; Palesch, Y.; Cotton, D.; Yusuf, S.; Saccor, R.; Diener, H.C.; Toni, D.; Estol, C.; Roberts, R.; et al. Effect of telmisartan on functional outcome, recurrence, and blood pressure in patients with acute mild ischemic stroke: A PRoFESS subgroup analysis. Stroke 2009, 40, 3541–3546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yusuf, S.; Diener, H.C.; Sacco, R.L.; Cotton, D.; Ounpuu, S.; Lawton, W.A.; Palesch, Y.; Martin, R.H.; Albers, G.W.; Bath, P.; et al. Telmisartan to prevent recurrent stroke and cardiovascular events. N. Engl. J. Med. 2008, 359, 1225–1237. [Google Scholar] [CrossRef]
- Hong, K.S.; Kang, D.W.; Bae, H.J.; Kim, Y.K.; Han, M.K.; Park, J.M.; Rha, J.H.; Lee, Y.S.; Koo, J.S.; Cho, Y.J.; et al. Effect of cilnidipine vs. losartan on cerebral blood flow in hypertensive patients with a history of ischemic stroke: A randomized controlled trial. Acta Neurol. Scand. 2010, 121, 51–57. [Google Scholar] [CrossRef]
- Kjeldsen, S.E.; Lyle, P.A.; Kizer, J.R.; Dahlhöf, B.; Devereux, R.B.; Julius, S.; Beevers, G.; de Faire, U.; Fyhrquist, F.; Ibsen, H.; et al. The effects of losartan compared to atenolol on stroke in patients with isolated systolic hypertension and left ventricular hypertrophy. The LIFE study. J. Clin. Hypertens. 2005, 7, 152–158. [Google Scholar] [CrossRef] [Green Version]
- Nazir, F.S.; Overell, J.R.; Bolster, A.; Hilditch, T.E.; Reid, J.L.; Lees, K.R. The effect of losartan on global and focal cerebral perfusion and on renal function in hypertensives in mild early ischaemic stroke. J. Hypertens. 2004, 22, 989–995. [Google Scholar] [CrossRef]
- Yamada, K.; Hirayama, T.; Hasegawa, Y. Antiplatelet effect of losartan and telmisartan in patients with ischemic stroke. J. Stroke Cerebrovasc. Dis. 2007, 16, 225–231. [Google Scholar] [CrossRef]
- Van Ginneken, V.; Engel, P.; Fiebach, J.B.; Audebert, H.J.; Nolte, C.H.; Rocco, A. Prior antiplatelet therapy is not associated with larger hematoma volume or hematoma growth in intracerebral hemorrhage. Neurol. Sci. 2018, 39, 745–748. [Google Scholar] [CrossRef]
- Vatter, H.; Konczalla, J.; Seifert, V. Endothelin related pathophysiology in cerebral vasospasm: What happens to the cerebral vessels? Acta Neurochir. Suppl. 2011, 110, 177–180. [Google Scholar]
- Provencio, J.J. Inflammation in subarachnoid hemorrhage and delayed deterioration associated with vasospasm: A review. Acta Neurochir. Suppl. 2013, 115, 233–238. [Google Scholar] [PubMed] [Green Version]
- Larysz-Brysz, M.; Lewin-Kowalik, J.; Czuba, Z.; Kotulska, K.; Olakowska, E.; Marcol, W.; Liskiewicz, A.; Jedrzejowska-Szypulka, H. Interleukin-1beta increases release of endothelin-1 and tumor necrosis factor as well as reactive oxygen species by peripheral leukocytes during experimental subarachnoid hemorrhage. Curr. Neurovasc. Res. 2012, 9, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Paczkowska, E.; Golab-Janowska, M.; Bajer-Czajkowska, A.; Machalinska, A.; Ustianowski, P.; Rybicka, M.; Klos, P.; Dziedziejko, V.; Safranow, K.; Nowacki, P. Increased circulating endothelial progenitor cells in patients with haemorrhagic and ischaemic stroke: The role of endothelin-1. J. Neurol. Sci. 2013, 325, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Andereggen, L.; Beck, J.; Z’Graggen, W.J.; Schroth, G.; Andres, R.H.; Murek, M.; Haenggi, M.; Reinert, M.; Raabe, A.; Gralla, J. Feasibility and Safety of Repeat Instant Endovascular Interventions in Patients with Refractory Cerebral Vasospasms. AJNR Am. J. Neuroradiol. 2017, 38, 561–567. [Google Scholar] [CrossRef] [Green Version]
- Hosmann, A.; Rauscher, S.; Wang, W.T.; Dodier, P.; Bavinzski, G.; Knosp, E.; Gruber, A. Intra-Arterial Papaverine-Hydrochloride and Transluminal Balloon Angioplasty for Neurointerventional Management of Delayed-Onset Post-Aneurysmal Subarachnoid Hemorrhage Vasospasm. World Neurosurg. 2018, 119, e301–e312. [Google Scholar] [CrossRef]
- Macdonald, R.L.; Higashida, R.T.; Keller, E.; Mayer, S.A.; Molyneux, A.; Raabe, A.; Vajkoczy, P.; Wanke, I.; Frey, A.; Marr, A.; et al. Preventing vasospasm improves outcome after aneurysmal subarachnoid hemorrhage: Rationale and design of CONSCIOUS-2 and CONSCIOUS-3 trials. Neurocrit. Care 2010, 13, 416–424. [Google Scholar] [CrossRef] [Green Version]
- Raabe, A.; Beck, J.; Keller, M.; Vatter, H.; Zimmermann, M.; Seifert, V. Relative importance of hypertension compared with hypervolemia for increasing cerebral oxygenation in patients with cerebral vasospasm after subarachnoid hemorrhage. J. Neurosurg. 2005, 103, 974–981. [Google Scholar] [CrossRef]
- Macdonald, R.L.; Kassell, N.F.; Mayer, S.; Ruefenacht, D.; Schmiedek, P.; Weidauer, S.; Frey, A.; Roux, S.; Pasqualin, A.; CONSCIOUS-1 Investigators. Clazosentan to overcome neurological ischemia and infarction occurring after subarachnoid hemorrhage (CONSCIOUS-1): Randomized, double-blind, placebo-controlled phase 2 dose-finding trial. Stroke 2008, 39, 3015–3021. [Google Scholar] [CrossRef] [Green Version]
- Macdonald, R.L.; Higashida, R.T.; Keller, E.; Mayer, S.A.; Molyneux, A.; Raabe, A.; Vajkoczy, P.; Wanke, I.; Bach, D.; Frey, A.; et al. Clazosentan, an endothelin receptor antagonist, in patients with aneurysmal subarachnoid haemorrhage undergoing surgical clipping: A randomised, double-blind, placebo-controlled phase 3 trial (CONSCIOUS-2). Lancet Neurol. 2011, 10, 618–625. [Google Scholar] [CrossRef]
- Macdonald, R.L.; Higashida, R.T.; Keller, E.; Mayer, S.A.; Molyneux, A.; Raabe, A.; Vajkoczy, P.; Wanke, I.; Bach, D.; Frey, A.; et al. Randomized trial of clazosentan in patients with aneurysmal subarachnoid hemorrhage undergoing endovascular coiling. Stroke 2012, 43, 1463–1469. [Google Scholar] [CrossRef] [Green Version]
- Loch Macdonald, R. Management of cerebral vasospasm. Neurosurg. Rev. 2006, 29, 179–193. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, S.F.; Granger, D.N. Cerebral microvascular inflammation in DOCA salt-induced hypertension: Role of angiotensin II and mitochondrial superoxide. J. Cereb. Blood Flow Metab. 2012, 32, 368–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, R.; Witkowski, S.; Fu, Q.; Claassen, J.A.; Levine, B.D. Cerebral hemodynamics after short- and long-term reduction in blood pressure in mild and moderate hypertension. Hypertension 2007, 49, 1149–1155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andereggen, L.; Neuschmelting, V.; von Gunten, M.; Widmer, H.R.; Fandino, J.; Marbacher, S. The role of microclot formation in an acute subarachnoid hemorrhage model in the rabbit. Biomed. Res. Int. 2014, 2014, 161702. [Google Scholar] [CrossRef]
- Bar-Klein, G.; Cacheaux, L.P.; Kamintsky, L.; Prager, O.; Weissberg, I.; Schoknecht, K.; Cheng, P.; Kim, S.Y.; Wood, L.; Heinemann, U.; et al. Losartan prevents acquired epilepsy via TGF-beta signaling suppression. Ann. Neurol. 2014, 75, 864–875. [Google Scholar] [CrossRef]
- Wanderer, S.; Mrosek, J.; Gessler, F.; Seifert, V.; Konczalla, J. Vasomodulatory effects of the angiotensin II type 1 receptor antagonist losartan on experimentally induced cerebral vasospasm after subarachnoid haemorrhage. Acta Neurochir. 2018, 160, 277–284. [Google Scholar] [CrossRef]
- Wanderer, S.; Mrosek, J.; Vatter, H.; Seifert, V.; Konczalla, J. Crosstalk between the angiotensin and endothelin system in the cerebrovasculature after experimental induced subarachnoid hemorrhage. Neurosurg. Rev. 2018, 41, 539–548. [Google Scholar] [CrossRef]
- Gomez-Garre, D.; Martin-Ventura, J.L.; Granados, R.; Sancho, T.; Torres, R.; Ruano, M.; Garcia-Puig, J.; Egido, J. Losartan improves resistance artery lesions and prevents CTGF and TGF-beta production in mild hypertensive patients. Kidney Int. 2006, 69, 1237–1244. [Google Scholar] [CrossRef] [Green Version]
- Tada, Y.; Wada, K.; Shimada, K.; Makino, H.; Liang, E.I.; Murakami, S.; Kudo, M.; Kitazato, K.T.; Nagahiro, S.; Hashimoto, T. Roles of hypertension in the rupture of intracranial aneurysms. Stroke 2014, 45, 579–586. [Google Scholar] [CrossRef] [Green Version]
- Asaeda, M.; Sakamoto, M.; Kurosaki, M.; Tabuchi, S.; Kamitani, H.; Yokota, M.; Watanabe, T. A non-enzymatic derived arachidonyl peroxide, 8-iso-prostaglandin F2 alpha, in cerebrospinal fluid of patients with aneurysmal subarachnoid hemorrhage participates in the pathogenesis of delayed cerebral vasospasm. Neurosci. Lett. 2005, 373, 222–225. [Google Scholar] [CrossRef] [Green Version]
- Konczalla, J.; Vatter, H.; Weidauer, S.; Raabe, A.; Seifert, V. Alteration of the cerebrovascular function of endothelin B receptor after subarachnoidal hemorrhage in the rat. Exp. Biol. Med. 2006, 231, 1064–1068. [Google Scholar]
- Ansar, S.; Vikman, P.; Nielsen, M.; Edvinsson, L. Cerebrovascular ETB, 5-HT1B, and AT1 receptor upregulation correlates with reduction in regional CBF after subarachnoid hemorrhage. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H3750–H3758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakamoto, M.; Takaki, E.; Yamashita, K.; Watanabe, K.; Tabuchi, S.; Watanabe, T.; Satoh, K. Nonenzymatic derived lipid peroxide, 8-iso-PGF2 alpha, participates in the pathogenesis of delayed cerebral vasospasm in a canine SAH model. Neurol. Res. 2002, 24, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Smeda, J.S.; Daneshtalab, N. The effects of poststroke captopril and losartan treatment on cerebral blood flow autoregulation in SHRsp with hemorrhagic stroke. J. Cereb. Blood Flow Metab. 2011, 31, 476–485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tchekalarova, J.D.; Ivanova, N.M.; Pechlivanova, D.M.; Atanasovo, D.; Lazarov, N.; Kortenska, L.; Mitreva, R.; Lozanov, V.; Stoynev, A. Antiepileptogenic and neuroprotective effects of losartan in kainate model of temporal lobe epilepsy. Pharmacol. Biochem. Behav. 2014, 127, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Wu, H.; Yu, X.; Zhang, G.; Zhang, R.; Zhan, S.; Wang, H.; Bu, N.; Ma, X.; Li, Y. Angiotensin II and its receptor in activated microglia enhanced neuronal loss and cognitive impairment following pilocarpine-induced status epilepticus. Mol. Cell Neurosci. 2015, 65, 58–67. [Google Scholar] [CrossRef]
- Tchekalarova, J.D.; Ivanova, N.; Atanasova, D.; Pechlivanova, D.M.; Lazarov, N.; Kortenska, L.; Mitreva, R.; Lozanov, V.; Stoynev, A. Long-Term Treatment with Losartan Attenuates Seizure Activity and Neuronal Damage Without Affecting Behavioral Changes in a Model of Co-morbid Hypertension and Epilepsy. Cell Mol. Neurobiol. 2016, 36, 927–941. [Google Scholar] [CrossRef]
- Nozaki, T.; Ura, H.; Takumi, I.; Kobayashi, S.; Maru, E.; Morita, A. The angiotensin II type I receptor antagonist losartan retards amygdala kindling-induced epileptogenesis. Brain Res. 2018, 1694, 121–128. [Google Scholar] [CrossRef]
- Biancardi, V.C.; Stranahan, A.M.; Krause, E.G.; de Kloet, A.D.; Stern, J.E. Cross talk between AT1 receptors and Toll-like receptor 4 in microglia contributes to angiotensin II-derived ROS production in the hypothalamic paraventricular nucleus. Am. J. Physiol. Heart Circ. Physiol. 2016, 310, H404–H415. [Google Scholar] [CrossRef] [Green Version]
- Maeso, R.; Rodrigo, E.; Munoz-Garcia, R.; Navarro-Cid, J.; Ruilope, L.M.; Lahera, V.; Cachofeiro, V. Losartan reduces constrictor responses to endothelin-1 and the thromboxane A2 analogue in aortic rings from spontaneously hypertensive rats: Role of nitric oxide. J. Hypertens. 1997, 15, 1677–1684. [Google Scholar] [CrossRef]
- Guan, W.; Kozak, A.; El-Remessy, A.B.; Johnson, M.H.; Pillai, B.A.; Fagan, S.C. Acute treatment with candesartan reduces early injury after permanent middle cerebral artery occlusion. Transl. Stroke Res. 2011, 2, 179–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oku, N.; Kitagawa, K.; Imaizumi, M.; Takasawa, M.; Piao, R.; Kimura, Y.; Kajimoto, K.; Matsumoto, M.; Hori, M.; Hatazawa, J. Hemodynamic influences of losartan on the brain in hypertensive patients. Hypertens. Res. 2005, 28, 43–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Habashi, J.P.; Judge, D.P.; Holm, T.M.; Cohn, R.D.; Loeys, B.L.; Cooper, T.K.; Myers, L.; Klein, E.C.; Liu, G.; Calvi, C. Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science 2006, 312, 117–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Povlsen, G.K.; Waldsee, R.; Ahnstedt, H.; Kristiansen, K.A.; Johansen, F.F.; Edvinsson, L. In vivo experimental stroke and in vitro organ culture induce similar changes in vasoconstrictor receptors and intracellular calcium handling in rat cerebral arteries. Exp. Brain Res. 2012, 219, 507–520. [Google Scholar] [CrossRef]
- Vikman, P.; Beg, S.; Khurana, T.S.; Hansen-Schwartz, J.; Edvinsson, L. Gene expression and molecular changes in cerebral arteries following subarachnoid hemorrhage in the rat. J. Neurosurg. 2006, 105, 438–444. [Google Scholar] [CrossRef]
- Dimitropoulou, C.; Chatterjee, A.; McCloud, L.; Yetik-Anacak, G.; Catravas, J.D. Angiotensin, bradykinin and the endothelium. In Handbook of Experimental Pharmacology; Springer: New York, NY, USA, 2006; Volume 176, pp. 255–294. [Google Scholar]
- Tirapelli, C.R.; Bonaventura, D.; Tirapelli, L.F.; de Oliveira, A.M. Mechanisms underlying the vascular actions of endothelin 1, angiotensin II and bradykinin in the rat carotid. Pharmacology 2009, 84, 111–126. [Google Scholar] [CrossRef]
- Mehta, P.K.; Griendling, K.K. Angiotensin II cell signaling: Physiological and pathological effects in the cardiovascular system. Am. J. Physiol. Cell Physiol. 2007, 292, C82–C97. [Google Scholar] [CrossRef]
- Arai, H.; Hori, S.; Aramori, I.; Ohkubo, H.; Nakanishi, S. Cloning and expression of a cDNA encoding an endothelin receptor. Nature 1990, 348, 730–732. [Google Scholar] [CrossRef]
- Toda, N.; Miyazaki, M. Angiotensin-induced relaxation in isolated dog renal and cerebral arteries. Am. J. Physiol. 1981, 240, H247–H254. [Google Scholar] [CrossRef]
- Tsutsumi, Y.; Matsubara, H.; Masaki, H.; Kurihara, H.; Murasawa, S.; Takai, S.; Miyazaki, M.; Nozawa, Y.; Ozono, R.; Nakagawa, K.; et al. Angiotensin II type 2 receptor overexpression activates the vascular kinin system and causes vasodilation. J. Clin. Investig. 1999, 104, 925–935. [Google Scholar] [CrossRef] [Green Version]
- Abassi, Z.A.; Klein, H.; Golomb, E.; Keiser, H.R. Regulation of the urinary excretion of endothelin in the rat. Am. J. Hypertens. 1993, 6, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Brunner, F.; Kukovetz, W.R. Postischemic antiarrhythmic effects of angiotensin-converting enzyme inhibitors. Role of suppression of endogenous endothelin secretion. Circulation 1996, 94, 1752–1761. [Google Scholar] [CrossRef] [PubMed]
- Chua, B.H.; Chua, C.C.; Diglio, C.A.; Siu, B.B. Regulation of endothelin-1 mRNA by angiotensin II in rat heart endothelial cells. Biochim. Biophys. Acta 1993, 1178, 201–206. [Google Scholar] [CrossRef]
- Dohi, Y.; Hahn, A.W.; Boulanger, C.M.; Buhler, F.R.; Luscher, T.F. Endothelin stimulated by angiotensin II augments contractility of spontaneously hypertensive rat resistance arteries. Hypertension 1992, 19, 131–137. [Google Scholar] [CrossRef] [Green Version]
- Kohno, M.; Horio, T.; Ikeda, M.; Yokokawa, K.; Fukui, T.; Yasunari, K.; Kurihara, N.; Takeda, T. Angiotensin II stimulates endothelin-1 secretion in cultured rat mesangial cells. Kidney Int. 1992, 42, 860–866. [Google Scholar] [CrossRef] [Green Version]
- Saavedra, J.M.; Benicky, J.; Zhou, J. Mechanisms of the Anti-Ischemic Effect of Angiotensin II AT(1) Receptor Antagonists in the Brain. Cell Mol. Neurobiol. 2006, 26, 1099–1111. [Google Scholar] [CrossRef]

| Drug | Model | Outcome | Beneficial Effect | Special Remarks |
|---|---|---|---|---|
| TMS [25] | Global ischemic mice model | Cerebral perfusion | Restored cerebral blood flow | - |
| TMS [26] | MCAO mice | Neuroscore, infarct size | Improved neuroscore and decreased infarct size, increased cerebral blood flow, reduced superoxide production and inflammatory cytokine expression | - |
| TMS [27] | Murine model of transient and permanent focal ischemia | Infarct size, reperfusion injury | Reduced stroke volume 72 h after transient ischemia, likewise pro-inflammatory adhesion molecules and infiltration of inflammatory cells in the ischemic region | No reduction in stroke volume 72 h after permanent ischemia |
| TMS [28] | MCAO mice | Focal brain ischemia, atherosclerotic lesions | Attenuated ischemic brain damage, neurological deficits and superoxide production in ischemic area; attenuated reduction of cerebral blood flow in the penumbra without significantly changing blood pressure | Anti-atherosclerotic effects |
| TMS [29] | MCAO rat | Cerebral perfusion | Improved cerebral blood flow, enhanced vascular density (CD31 immunofluorescence staining), antiapoptotic effects | - |
| TMS [30] | MCAO rat | Cognitive function, level of matrix metalloproteinases | Improved spatial memory ability, decreased expression levels of MMP-2 and MMP-9 | - |
| TMS [31] | MCAO rat | Behavior alterations, neuroprotective effects on secondary reperfusion phase | Normalized behavioral alterations comparable to pre-ischemic treatment (protected neurons from ischemic reperfusion injury), attenuated excitatory amino acid release in secondary reperfusion phase | In combination with nimodipine. Drug treatments immediately after reperfusion, effects compared with pretreatment |
| TMS [32] | MCAO rat | Effects on neurovascular unit and neuroinflammation | Reduced decrease of NAGO-positive endothelium, similar increase of MMP-9 positive neurons and NLRP3-positive inflammasome in the cerebral cortex | Low dose TMS improved changes without lowering blood pressure, high dose TMS further improved changes with lowering blood pressure |
| TMS [33] | Open skull preparation rat | Cerebral arteriolar pressure, cerebral blood flow, internal vessel diameter | Normalization of arteriolar pressure and lower limit of cerebral autoregulation | Combined with Ramipril |
| TMS [34] | MCAO rats | Metabolic related post-ischemic changes | Ameliorated metabolic related post-ischemic changes | - |
| TMS [35] | MCAO rats | Neurological outcome, infarct volume, inflammation | Improved outcome, reduced infarct volume and inflammation | Subcutaneous TMS application 5 days prior to MCAO with reperfusion |
| TMS [36] | MCAO rats | Infarct volume, immunohistochemical parameters | Significantly reduced infarct volume, reduced neurotoxic cytosolic phospholipase A2, ameliorates ischemic changes of neurons in the peri-infarct area | Pretreatment for 7 days |
| TMS [37] | Collagenase infusion or autologous blood injection to induce intracerebral hemorrhage in rats | Hemorrhage volume, functional recovery | Reduced hemorrhage volume, brain edema, inflammatory/apoptotic cells in perihematomal area; induced endothelial nitric-oxide-synthase, decreased oxidative stress, apoptotic signals, and TNFα | - |
| TMS [38] | Stroke-resistant spontaneously hypertensive rats | Oxidative stress | Reduced advanced glycation end product, 4-hydroxy-2-nonenal- and phosphorylated a-synuclein-positive cells in the cerebral cortex and hippocampus | - |
| CS [39] | MCAO mice | Ischemic brain damage | Reduced ischemic brain area and neurological deficits in non-hypotensive doses; improved reduction of brain surface blood flow and inhibited superoxide production in the cortex and brain arterial wall at non-hypotensive and hypotensive doses; AT2-2-R expression in the ischemic area was increased by prior pretreatment with CS | - |
| CS [40] | MCAO mice | Antioxidant enzyme activity | Restored superoxide dismutase activity and cerebral blood flow | - |
| CS [41] | MCAO rats | Neurobehavioral outcome, infarct size, vascular density | Improved neurobehavioral outcome, reduced infarct size and vascular density | In vitro vascular density was assessed using human brain endothelial cells |
| CS [42] | MCAO rats | Infarct size, neurological outcome | Improved neurobehavioral and motor functions, decreased infarct size | Intravenous CS administration |
| CS [43] | MCAO rats | Neurological outcome | Improved recovery from ischemic stroke | Only 0.3 mg/kg CS with neuroprotective function |
| CS [44] | MCAO rats | Neurological outcome, oxidative enzymes | Improved motor function and reduced endoplasmatic reticulum stress markers | Only early beneficial effect after 24 h |
| CS [45] | MCAO rats | Neurological outcome, vascular density/synaptogenesis | Improved functional outcome, increased vascular density/synaptogenesis only in the control group | Intracerebroventricular injection of short hairpin RNA lentiviral particles to knock down brain-derived neurotrophic factor or nontargeting control vector |
| CS [46] | MCAO rats | Angiogenesis | Induced prolonged proangiogenic effect and upregulation of VEGF-A and VEGF-B; stabilized hypoxia-inducible factor-1a and preserves angiopoetin-1 | - |
| CS [47] | Spontaneously hypertensive rats | Angiogenesis | Exerted proangiogenic effects on brain microvascular endothelial cells | - |
| CS [48] | In vitro monolayer model using rat brain capillary endothelial cells | Stability of blood brain barrier | Improved cell function and viability of brain capillary endothelial cells under OGD | Normoxia versus 6 h OGD |
| CS [49] | MCAO rats | Neurological outcome, infarct size | Improved neurological function, significantly reduced blood brain barrier disruption/edema/infarct volume | - |
| CS [50] | MCAO rats | Infarct size, functional recovery, neuroplasticity | Significantly reduced infarct size, ameliorated functional recovery and increased neuroplasticity markers | - |
| CS [51] | MCAO rats | Infarct size, neurological outcome | Decreased infarct size and improved neurological outcome | - |
| CS [52] | MCAO rats | Mortality, infarct size | Significantly reduced mortality and infarct size | - |
| CS [53] | MCAO rats | Infarct size | Reduced infarct size | Oral administration |
| CS [54] | MCAO rats | Infarct size, edema, neurological outcome | Reduced infarct size, edema formation and improves neurological outcome | - |
| CS [55] | MCAO rats | Infarct size, neurological outcome | Significantly reduced stroke volume and improved neurological outcome | - |
| CS [56] | MCAO rats | Infarct size, edema | Reduced infarct size and edema, improved neurologic function | - |
| CS [57] | MCAO rats | Infarct volume, neurological deficit | Reduced infarct size and improved neurologic outcome | - |
| CS [58] | MCAO rats | Infarct volume, neurological deficits | Reduced infarct size, improved neurological outcome, reduced lipid peroxidation | Subcutaneous infusion for 14 days |
| CS [59] | MCAO rats | Infarct volume, neurological deficits | Reduced infarct size/edema and improved neurological outcome | Long-term blockade (subcutaneous injection twice daily 5 days before ischemia), not short-term administration (intravenous once 4 h prior to ischemia), improves neurological outcome |
| CS [60] | MCAO rats | Infarct volume, brain edema | Significantly reduced cortical infarct volume and brain edema | - |
| CS [61] | Bilateral CCAO rats | Neurological outcome, oxidative damage | Attenuated neurobehavioral alterations, oxidative damage and restored mitochondrial enzyme dysfunction | Occlusion for 30 min, followed by 24 h reperfusion; CS pretreatment for 7 days |
| CS [62] | MCAO rats | Infarct size | Reduced infarct area | - |
| CS [63] | MCAO rats | Infarct size, neurological outcome | Pretreatment reduced infarct area and improved neurological outcome | - |
| CS [64] | MCAO rats | Infarct size, neurological outcome | Reduced infarct size and neurological deficits; significantly reduced mRNA expression of inflammatory markers | - |
| CS [65] | Spontaneously hypertensive rats | AT2-1-R expression | Increased AT2-2-R expression in spontaneously hypertensive rats | CS application via subcutaneous osmotic minipumps for 4 weeks |
| CS [66] | MCAO rats | Neurological outcome, vascular density | Improved neurological outcome and increased vascular density | - |
| CS [67] | Embolic stroke model | Mortality, neurological outcome, infarct size | Significantly decreased mortality, neurological deficits, and infarct size | Injection of calibrated microspheres |
| CS [68] | MCAO rat | Infarct size, neurological outcome | Reduced infarct size and improved neurological outcome | Combined treatment with ETA-R antagonist |
| CS [69] | MCAO rats | Contractile response to angiontensin II | Abolished the enhanced responses to angiotensin II | - |
| CS [70] | MCAO rats | Infarct volume, neurological outcome | Reduced infarct size with low but not high dose of CS, improved neurological outcome | Subcutaneous CS administration |
| CS [71] | MCAO rats | Infarct size, neuroscores, cerebral blood flow | Reduced infarct size and increased cerebral blood flow | Intravenous CS administration |
| CS [72] | Spontaneously hypertensive rats | Vascular remodeling, expression of eNOS/iNOS | Reversed negative vascular remodeling and alterations in eNOS/iNOS expression | - |
| OMS [73] | Bilateral CCAO mice | Cognitive impairment | Ameliorated cognitive impairment | - |
| OMS [74] | Single carotid ligation stroke model gerbil | Survival | Significantly increased survival at day 30 | - |
| OMS [75] | MCAO rats | Neurological outcome, infarct size, cell death | Significantly improved functional scores, reduced infarct size and cell death | Only continuous administration of OMS before and after stroke reduced oxidative stress levels |
| OMS [76] | MCAO rats | Infarct volume | Reduced infarct volume 48 h after transient focal brain ischemia | OMS administration via drinking water |
| OMS [77] | MCAO rats | Stroke index score, infarct volume, quantity of MMPs | Improved stroke index score, infarct volume, reduced cerebral edema and upregulation of MMPs | - |
| VS [78] | MCAO mice | Infarct volume, DNA damage, superoxide production | Significantly reduced infarct size, DNA damage, superoxide production, mRNA levels of monocyte chemoattractant protein-1, increases cerebral blood flow, increased eNOS activation and nitric oxide production | - |
| VS [79] | MCAO mice | Infarct volume, neurological outcome | Significantly reduced infarct volume and improved neurological outcome | - |
| VS [80] | MCAO mice | Infarct volume, neurological outcome | Significantly reduced ischemic area, neurological deficits, reduction of cerebral blood flow and superoxide production | - |
| VS [81] | High salt loaded SR-SHR | Brain injury | Enhanced protective effects against brain injury, white matter lesions and glial activation | Combined with amlodipine |
| IS [82] | MCAO rats | Infarct size, neurological outcome | Reduced infarct size and number of apoptotic cells in the peri-infarct cortex on day 3, attenuated invasion of microglia and macrophages on day 3 and 7 after ischemia | - |
| IS [83] | MCAO rats | Neurological outcome | Significantly improved neurological outcome | Administration of IS intracerebroventricularly over 5 days |
| IS [84] | MCAO rats | Infarct size | Reduced infarct volume | Coadministration of propagermanium (CCR2 antagonist) |
| LS [85] | Single carotid ligation stroke model gerbil | Mortality | Did not increase mortality after unilateral carotid ligation in gerbils | - |
| LS [86] | MCAO mice | OGD-induced cell injury | Abolished OGD-induced exaggeration of cell injury in mice overexpressing renin and angiotensinogen animals | - |
| LS [87] | MCAO rats | Gene expression levels of pro-apoptotic genes | Significant reduced gene expression of pro-apoptotic genes | - |
| LS [88] | Cerebral focal ischemia by cauterization of cortical surface vessels rats | Cessation of blood flow, infarct size | Maintained angiogenesis, vascular delivery, and significantly decreased infarct size | Administration of LS in drinking water 2 weeks before inducing ischemia |
| Drug | Outcome | Beneficial Effect | Special Remarks |
|---|---|---|---|
| CS [90] | Vascular event (vascular death, nonfatal stroke or nonfatal myocardial infarction) over 6 months and mRS | No overall effect on vascular events in ischemic and/or hemorrhagic stroke, adjusted odds ratio for vascular events of patients treated within 6 h reached significance | Administration at least within 30 h of ischemic or hemorrhagic stroke. CS treatment for 7 days, increasing from 4 mg on day 1 to 16 mg on day 3 to 7 |
| CS [91] | Barthel index and level of care assessed after 6 months | No significant effects on Barthel Index or level of care at 6 months | Administration at least within 30 h of ischemic or hemorrhagic stroke. CS treatment for 7 days, increasing from 4 mg on day 1 to 16 mg on day 3 to 7 |
| CS [92] | Vascular death, myocardial infarction, stroke during first 6 months and functional outcome at 6 months | Significant trend towards a better effect of CS in patients with larger infarcts; no differences in treatment effect for composite vascular end point | CS treatment for 7 days, increasing from 4 mg on day 1 to 16 mg on day 3 to 7 |
| CS [93] | Vascular death, myocardial infarction, stroke during first 6 months and functional outcome at 6 months | After 6 months the risk of the composite vascular endpoint did not differ between treatment groups | CS treatment for 7 days, increasing from 4 mg on day 1 to 16 mg on day 3 to 7 |
| CS [94] | Safety of modest blood pressure reduction by CS cilexetil in the early treatment of stroke | The cumulative 12 months mortality and the number of vascular events differed significantly in favor of the CS cilexetil group | CS treatment with 4 mg on day 1; dosage increased to 8 mg on day 2 or 16 mg if blood pressure exceeded 160 mmHg systolic or 100 mmHg diastolic |
| CS [95] | Short-term safety of blood pressure reduction in hypertensive patients with acute ischemic stroke | CS treatment safely reduces blood pressure in hypertensive patients with acute ischemic stroke | 4 mg/day for 14 days |
| CS [96] | Adhesion of neutrophils to human endothelial cells in acute ischemic stroke | CS inhibited the adhesion of neutrophils to vascular endothelium in ischemic stroke patients (not in chronic stroke patients or healthy volunteers) | Incubation with 10−9 mol for 30 min |
| CS [97] | Effect of blood pressure lowering in patients with acute ischemic stroke and carotid artery stenosis (Vascular death, stroke, myocardial infarction, and functional outcome at 6 months) | No evidence that CS effect is qualitatively different in patients with carotid artery stenosis | CS treatment for 7 days, increasing from 4 mg on day 1 to 16 mg on day 3 to 7 |
| VS [98] | Safety of modest blood pressure reduction within 48 h of acute ischemic stroke | After 90 days the mRS as well the rate of major vascular events differed not significantly between both groups | 80 mg/day (dose was modified in the subsequent six-days of treatment if the target systolic blood pressure was not achieved) |
| VS [99] | Effect of vs. on human platelet aggregation | VS exhibited significant inhibition of human platelets and therefore might be able to reduce vascular ischemic events | 10 nmol to 100 μmol |
| TMS [100] | Time to first recurrent stroke | Low glomerular filtration rate (<60 mL/min) is independently associated with a higher risk of recurrent stroke, TMS not able to mitigate this risk | TMS dosage not reported |
| TMS [101] | Recurrent stroke of any type | Similar rates of recurrent strokes comparing aspirin plus extended-release dipyridamole with clopidogrel and TMS | 80 mg/day |
| TMS [102] | Prevention of cerebral white matter lesions | TMS on top of existing antihypertensive medication did not prevent the progression of white matter lesions | 80 mg/day. Analysis limited by the relatively short follow-up |
| TMS [103] | Functional outcome at 30 days (primary outcome), death, recurrence, and hemodynamic measures up to 90 days (secondary outcomes) | TMS treatment appears to be safe with no excess in adverse events and not associated with a significant effect on functional dependency, death, or stroke recurrence | 80 mg/day |
| TMS [104] | Recurrent stroke | TMS initiated soon after ischemic stroke and continued for 2.5 years did not significantly lower the rate of recurrent stroke, major cardiovascular events, or diabetes | 80 mg/day |
| LS [105] | Global change of cerebral blood flow | LS treatment increases the global cerebral blood flow despite blood pressure lowering | 50–100 mg/day for 4 weeks |
| LS [106] | Effect on stroke in patients with isolated systolic hypertension and left ventricular hypertrophy | Incidence of any stroke (40% risk reduction), fatal stroke (70% risk reduction), and atherothrombotic stroke (45% risk reduction) was significantly lower in the LS treated group compared to atenolol treated patients | Mean LS dose of 79 mg |
| LS [107] | Effect on global and focal cerebral blood flow in hypertensive patients 2–7 days after stroke | No neurological deterioration in the LS group | 25–50 mg/day |
| LS [108] | Spontaneous platelet aggregation and P-selectin levels (in patients with hypertension and chronic ischemic stroke) | Spontaneous platelet aggregation was not, P-selectin levels significantly reduced after LS treatment. This suggests that standard doses of LS display antiplatelet effect | 50 mg/day |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wanderer, S.; Grüter, B.E.; Strange, F.; Sivanrupan, S.; Di Santo, S.; Widmer, H.R.; Fandino, J.; Marbacher, S.; Andereggen, L. The Role of Sartans in the Treatment of Stroke and Subarachnoid Hemorrhage: A Narrative Review of Preclinical and Clinical Studies. Brain Sci. 2020, 10, 153. https://doi.org/10.3390/brainsci10030153
Wanderer S, Grüter BE, Strange F, Sivanrupan S, Di Santo S, Widmer HR, Fandino J, Marbacher S, Andereggen L. The Role of Sartans in the Treatment of Stroke and Subarachnoid Hemorrhage: A Narrative Review of Preclinical and Clinical Studies. Brain Sciences. 2020; 10(3):153. https://doi.org/10.3390/brainsci10030153
Chicago/Turabian StyleWanderer, Stefan, Basil E. Grüter, Fabio Strange, Sivani Sivanrupan, Stefano Di Santo, Hans Rudolf Widmer, Javier Fandino, Serge Marbacher, and Lukas Andereggen. 2020. "The Role of Sartans in the Treatment of Stroke and Subarachnoid Hemorrhage: A Narrative Review of Preclinical and Clinical Studies" Brain Sciences 10, no. 3: 153. https://doi.org/10.3390/brainsci10030153
APA StyleWanderer, S., Grüter, B. E., Strange, F., Sivanrupan, S., Di Santo, S., Widmer, H. R., Fandino, J., Marbacher, S., & Andereggen, L. (2020). The Role of Sartans in the Treatment of Stroke and Subarachnoid Hemorrhage: A Narrative Review of Preclinical and Clinical Studies. Brain Sciences, 10(3), 153. https://doi.org/10.3390/brainsci10030153





