Abstract
Social isolation (SI) stress has been recognized as a major risk factor of morbidity in humans and animals, exerting damaging effects at the physical and mental health levels. Posttraumatic stress disorder (PTSD), on the other hand, occurs as a result of experiencing serious, life-threatening, traumatic events and involves involuntary re-experiencing trauma (intrusion), avoidance symptoms, and distortions of cognition and emotional arousal. The literature shows that PTSD is affected by genetic predisposition and triggers a large neurocircuitry involving the amygdala, insula, hippocampus, anterior cingulate- and prefrontal-cortex, and affects the function of the neuroendocrine and immune systems. Social isolation seems to influence the predisposition, onset and outcome of PTSD in humans, whereas it constitutes a valid model of the disorder in animals. According to the PRISMA (preferred reporting items for systematic reviews and meta-analyses) protocol, we systematically reviewed all original studies involving the neurobiological trajectories between SI and PTSD published till July 2019 (database: PubMed/Medline). Out of 274 studies, 10 met the inclusion criteria. We present the results of the retrieved studies in terms of hypothalamic-pituitary-adrenal (HPA)-axis and endocannabinoid system function, immune reactions, neuroplasticity, novel pharmacological targets, and shortening of telomere length, which confirm a synergistic effect on a neurobiological level between the two entities.
1. Introduction
Posttraumatic stress disorder (PTSD) is a chronic psychiatric condition resulting from direct or indirect exposure to serious trauma (death, threatened death, actual or threatened injury, or sexual violence) and involving intrusive reexperiencing of the traumatic event in the form of unwanted memories, nightmares, flashbacks, emotional and physical distress after exposure to traumatic reminders, persistent avoidance symptoms to trauma-related stimuli, negative alterations of cognitions and mood (dissociative amnesia, negative beliefs about oneself, negative emotional state like fear, horror, guilt and inability of experiencing positive emotions), as well as marked alterations of arousal and reactivity related to the traumatic event (irritability, anger, aggression, self-destructive behavior) [1]. The lifetime prevalence of PTSD in the general population according to the Diagnostic Statistical Manual (DSM)-5 criteria is around 8% with women scoring higher than men [2], whereas the prevalence of the disorder among high-risk groups, like war-veterans, may be four times higher [3]. The risk for PTSD increases with the number of traumatic experiences [4], whereas more than 80% of PTSD patients present one or more comorbidities like depression, substance use, and physical health issues which lead to debilitating outcomes [5,6] as well as increased mortality rates [7], rendering, hence, the understanding and treatment of the disease a complex crossroads for the clinician. Multiple studies have shown that trauma-related disorders are associated with the dysfunctioning of numerous biological systems, and that PTSD symptom severity exerts a cumulative effect on premature aging of the immune system and telomere length [8].
Human functional imaging studies have identified an increased connectivity between the ventromedial prefrontal cortex (vmPFC), locus coeruleus (LC), hypothalamus, hippocampus, basolateral amygdala (BLA), and bed nucleus of the stria terminalis (BNST) in PTSD patients, while more subcortical areas like the periaqueductal gray area (PAG) and central amygdala (CeA) gradually become involved when the severity of a threatening stimulus becomes aggravated [9,10].
Before the last decade, the predominant neurobiological model of PTSD concentrated on the pathway of fear conditioning involving mainly the amygdala system [11]. The preparations of the new DSM-5 broadened the criteria to include further emotionally dysregulated states like anger, guilt, shame, and symptoms of derealization and depersonalization, as well as altered self- and other-related conditions and deficits in social cognitions. Such changes also gave rise to new neurobiological research challenges [12]. The emotional dysregulation in PTSD tends to be viewed as a vulnerability factor relating to genetic and developmental parameters and a response to (a) traumatic event(s) during childhood, adolescence or adulthood [11]. Neuroendocrine and neuroimmune findings in PTSD include lower basal cortisol output, increased glucocorticoid receptor (GR) function, and a proinflammatory response pre-, peri-, and post-trauma [13], whereas early trauma [14] and ongoing threat [15] are associated to similar observations. The endocannabinoid system (e-CB) has also been related to the pathophysiology of stress-related psychiatric disorders as PTSD. It includes the main e-CBs: anandamide (AEA) and 2-arachidonoylglycerol (2-AG) that act on receptors type 1 (CB1) and (CB2). The CB1 receptor is widely expressed in the prefrontal-limbic system [16] and, along with anandamide, it positively modulates fear extinction acting mainly in the hippocampus and basolateral amygdala [17]. N-palmitoylethanolamine (PEA), an endogenous lipid modulator, is considered part of the extended e-CB next to AEA and 2-AG is considered an analgesic, neuroprotective and antioxidant agent, exerting its pharmacological function by stimulating the peroxisome proliferator-activated receptor -α (PPAR-α), a ligand-activated nuclear receptor [18]. Research has shown that PEA levels in blood show strong negative association with PTSD symptom severity in humans [19] and become increased by antidepressants in corticolimbic regions of rats [20]. Furthermore, PEA binding at PPAR-α interacts with the gamma-aminobutyric acidergic (GABAergic) neurosteroid system in the biosynthesis of allopregnanolone (Allo) [18]. Allo is a positive allosteric modulator of GABA action at GABAA receptors and regulates emotional behavior by exerting anxiolytic, antidepressant, sedative effects. It is produced from progesterone in glutamatergic neurons of the cortex, hippocampus and basolateral amygdala through the double enzymatic action of 5α reductase type I (5 α-RI) and 3 α-hydroxysteroid dehydrogenase(3α-HSD) [18,21]. The decrease of Allo in the cerebrospinal fluid of PTSD patients correlates to the severity of reexperiencing and depressive symptoms of both men and women. Interestingly enough, the block in the biosynthesis of Allo is sex-specific relating to 5 α-RI deficiency in men and to 3 α-HSD deficiency in premenopausal women [22,23]. In animal models of PTSD, including the isolated mouse, corticolimbic downregulation of Allo levels correlates to enhanced contextual fear and impaired fear extinction, implying that this neurosteroid may serve as a useful biomarker and treatment option for PTSD across humans and animals [21].
Different models have tried to explain the psychoneurobiological etiology of stress-related mental disorders and their shaping interaction with trauma(s): The three-hit concept of vulnerability and resilience [24] developed by Daskalakis et al. focuses on the interplay between genetic predisposition, early-life adverse events and later-life environment on the occurrence of psychopathology: According to this testable hypothesis, early mild to moderate stress in animals may contribute to stress inoculation and hypothalamic-pituitary-adrenal (HPA) axis adaptability to later adverse events, whereas more severe early stress may affect, beyond HPA-axis functioning, amygdala activation and processing, fear retention, as well as vulnerability to later-life stressors and stress-related disorders (like PTSD) in adulthood. This model does not follow the linearity of the “classic” cumulative stress model (or stress diathesis model), where additive accumulation of different stressors, that exceed a certain threshold, leads to the development of psychopathology to vulnerable individuals, but offers instead an evolutionary counterpart of the dynamic interchange between genetics (hit-1) and early-life events (hit-2) affecting endocrine function and epigenetic modifications, shaping in turn an evolving phenotype which may adapt and resist (resilience) or succumb to mental disorders (vulnerability) when faced with later life stressors (hit-3).
Social bonds play an important role in mediating psychological and physical well-being [25]. Social isolation (SI) stress constitutes a major risk factor of morbidity and mortality for humans as well as nonhuman social species [26]. Research in animals has shown that SI exerts a burdening effect on animal physiology [27], increasing oxidative stress, proinflammatory cytokines, basal cortisol levels, as well as the occurrence of obesity and type II diabetes, urinary catecholamine levels, thus influencing immunity, inflammation control, and genes regulating glucocorticoid responses in a negative way. If post-weaning SI rearing follows maternal separation (reminding us of the three-hit concept of vulnerability), the metabolic risk is multiplied, and rats exhibit up to a 120% increase of fasting glucose compared to group-housed animals [28]. Other study has shown that prior SI in prairie voles alters concentrations of monoamine neurotransmitters following acute restraint, with isolated animals exhibiting elevated serotonin and dopamine levels in the hypothalamus and potentially decreased levels of serotonin in the frontal cortex [29]. Long-term isolated rats also showed a stronger activation of the sympatho-adrenomedullary system (SAS) when faced with new stressors compared to rats chronically exposed to long-term crowded conditions [30]. Neonatal isolation may also increase social dominance and aggression in rats compared to non-isolated controls, resembling the aggressiveness of people who have experienced early neglect. Research has found that neonatal isolation stress increased the stable fraction of actin, which is glucocorticoid dependent and these altered actin dynamics at the spines in the juvenile mPFC may explain neocortical dysfunction leading to altered social behavior later in life [31]. Moreover, in another study, the SI of adolescent mice rendered them unable to forget aversive memories when tested one month after the original event and fear memory retention was explained by the increase of brain-derived neurotrophic factor (BDNF) in the hippocampus [32].
Last but not least, SI is considered a valid animal model of PTSD in the sense that socially isolated animals present symptoms that resemble the human form (face validity) of the disorder like anxiety behavior, contextual fear responses, impaired fear extinction, cognitive alterations, aggressiveness, and neuroendocrine changes [33]. These disturbances are attributed to the corticolimbic downregulation of allopregnanolone (Allo), which normally exerts fast allosteric modulation of the action of GABA at the GABAA receptors, as mentioned before [21].
In humans, the stress of SI refers to the subjectively perceived feeling of isolation or “loneliness” which does not always coincide with objective social isolation or social support. Neurobiological studies are fewer for obvious reasons, involving more peripheral biological variables: Loneliness has been correlated to a variety of medical conditions, like elevated blood pressure, increased HPA activity, metabolic syndrome, as well as Alzheimer’s disease progression [34]. The upregulation of proinflammatory cytokines during acute stress has, moreover, been observed among the lonelier participants of a study involving healthy adults and breast cancer survivors, implying immune system dysregulation [35]. Loneliness may also predict subsequent depressive symptomatology that cannot be attributed to other parameters [34]. Solitary confinement or disciplinary isolation is a penal tool used in the prison system of the U.S. and other countries against the most violent of the inmates. Segregated individuals display higher levels of mental distress compared to the rest of the general prisoner population and manifest a wide range of psychiatric symptoms ranging from anxiety, panic attacks, and depression, to psychotic symptoms, self-mutilation, or even suicide. Another part of isolated inmates become even more violent after this punitive measure. These detrimental effects of disciplinary segregation have led in the U.S. to calls for the reform of solitary confinement, especially for juvenile offenders, given the irreversible damage that can be caused on their still developing brains [36]. A lack of social support is considered a major risk factor for PTSD following traumatic events [37], while the presence of social support seems to influence symptom severity and recovery [38]. PTSD patients may not make use of social support in order to protect others from distress, thinking that others will not understand or for fear that they will undermine their own self-image [39]. Withdrawal and real or expected negative responses from others relating to early experiences of bullying victimization may cultivate a vicious circle of shame and loneliness in these patients [40]. In addition, the neurobiology of social support is still poorly understood [11].
Given the referred interchange between SI stress and PTSD and in the absence of a systematic review, we wanted to illuminate in this paper the neurobiological trajectories between the two entities and the degree to which the interaction with SI aggravates the (neuro)biological progression of PTSD.
2. Methods
2.1. Search Strategy
The literature search was conducted in the electronic databases PubMed/Medline. The database search was carried out between May and July 2019. There was no limit set regarding the publication year. The search strategy is exemplified for PubMed/Medline. The search terms were combined with the Boolean operator as follows: “social isolation” AND “PTSD”.
2.2. Eligibility Criteria
Articles were included that met the following inclusion criteria: (a) animal and human original studies investigating the neurobiological and biological associations between SI and PTSD and with mentioning of both terms in their title, key words or text. In terms of publication status, articles in print or published ahead of print were accepted. The exclusion criteria were: (a) studies dealing with behavioral aspects of PTSD (description of symptomatology among different patient groups, development and use of diagnostic tools, psychotherapy, rehabilitation programs, (b) studies relating PTSD to organicity (head injury, cancer, myocardial infarction, chronic pain syndromes), (c) literature reviews, or (d) no available full text.
2.3. Study Selection
Each study was screened for eligibility by the first author after reading the title and abstract. Any uncertainties concerning eligibility were discussed and resolved among all authors. The decisions for inclusion or exclusion are summarized in a flow chart according to PRISMA (preferred reporting items for systematic reviews and meta-analyses) recommendations. PRISMA is an evidence-based manual for reporting in systematic reviews and meta-analyses and focuses on the reporting of reviews evaluating randomized trials, but can also be used as a basis for reporting systematic reviews of other types of research [41].
3. Results
3.1. Process of Study Selection
A flow diagram outlines the study selection process (Figure 1).
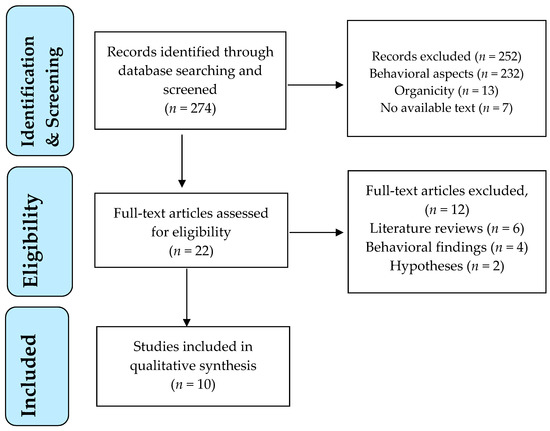
Figure 1.
PRISMA flowchart of study selection process.
The electronic database search retrieved 274 publications (Figure 1). All titles and abstracts were screened for their relevance to the topic. Twenty-two articles were identified as potentially relevant after screening of titles and abstracts. These studies were assessed for eligibility in full text. Of these, 10 studies met the eligibility criteria and were included in the review, dating between 2008 and 2019. The main reasons for study exclusion were: inclusion of behavioral findings with no neurobiological variables and literature reviews or hypotheses without research confirmation.
3.2. Characteristics of Included Studies
Key characteristics of included studies are summarized in Table 1.

Table 1.
Key Characteristics of Included Studies.
All retrieved studies were published between 2008 and 2019. Eight studies concerned animal research and two investigated human subjects. Four out of the eight animal studies [42,43,44,45] used mice as experimental subjects and four studies [46,47,48,49] used rats, divided in socially isolated (experimental) and group-housed animals (controls). Face-validity [33,50] was established in all animal studies, since they phenomenologically resembled the human setting of PTSD with inescapable electric foot shock serving as the most common traumatic experience (male intruder, predator odor, restraint, and forced swimming were, moreover, used in parallel or independently). Construct validity [33,50] was served in terms of the effort to identify common underlying mechanisms with the human disorder, whereas predictive validity [33,50] was present in three of the animal studies [42,44,47] in terms of providing predictions concerning therapeutic responses and novel pharmacological targets. Hippocampal and amygdala involvement was investigated in seven of the studies [42,43,45,46,47,48,49], the role of the HPA-axis and its products in four studies [45,46,48,49], while the endocannabinoid system in three [44,47,48]. The immune profile of stressed animals and upregulation of proinflammatory cytokines was examined in one of the studies [45]. In the two human longitudinal studies conducted by the same research group [51,52], the telomere length of prisoners of war with PTSD was examined 42 years (T2) after war captivity (T0) with an intermediate survey of perceived social isolation (T1) 18 years after trauma occurrence.
In the following section, we will approach the neurobiological trajectories between social isolation and PTSD, presented as main findings in the reviewed studies in terms of HPA-axis and endocannabinoid system function, immune reactions, neuroplasticity, novel pharmacological targets, and shortening of telomere length.
3.2.1. Hypothalamic-Pituitary-Adrenal (HPA)-Axis Function
Total plasma [45,48,49] as well as adrenal CORT levels [46] were decreased in animals exposed to both SI and trauma compared to controls. In Cheng et al. [49] corticosterone levels were higher if compared to neonatally isolated (NI) animals with no stress exposure, but lower compared to the levels of animals exposed only to SPS and not neonatal isolation (NI). Increased pituitary ACTH release was observed as an immediate reaction to stress after social isolation, possibly associated with enhanced c-Fos expression in the amygdala [46,48]. However, a decrease in ACTH levels was observed six months after the incidence repeated unpredictable stress in previously isolated animals [46]. Upregulation of glucocorticoid receptors (GR) immunoreactivity was observed in the hypothalamus and the hippocampus [48,49] of SI animals and it was associated with stress-induced hippocampal neuronal loss and volume reduction. Mineralocorticoid receptors (MR), on the other hand, were downregulated and this observation was associated to a reduced resilience of isolated animals to new stressors [48].
3.2.2. Endocannabinoid System (e-CB)
SI rats that were never exposed to extinction procedures after the electric shock experience were found to have decreased hippocampal anandamide levels (AEA) compared to rats that had followed extinction procedures after the traumatic experience in Morena et al. study [47]. Levels of endogenous AEA can be elevated by URB597, an inhibitor of AEA’s main degrading enzyme, FAAH. In a study by Boero et al. [48], SI increased hypothalamic AEA and CB1R concentrations, while reducing hypothalamic 2-AG levels that may be involved in HPA-axis negative feedback dysregulation.
3.2.3. Proinflammatory Reactions
Lower plasma corticosterone levels six months after RUS was associated with a two-fold increase of proinflammatory cytokines IL-1β and IFN-γ in socially isolated animals of the Algamal et al. study [45].
3.2.4. Neuroplasticity
Brain plasticity also becomes affected by the combined influence of SI and traumatic exposure as relevant studies show. The dorsolateral bed nucleus of the stria terminalis (dlBNST), a subregion of the extended amygdala, plays a critical role in stress-induced plasticity by regulating HPA axis activity. It mediates between the corticolimbic system by receiving stressful stimuli and sending GABAergic projections to the paraventricular nucleus of the hypothalamus (PVN), where corticotrophin releasing hormone (CRH) is released and peripheral stress response gets started under pituitary activation [37]. Acute and chronic social isolation seem to provoke blunting of long-term potentiation in the dlBNST in the Conrad et al. study [43], compared to group-housed animals. Moreover, hippocampal BDNF and pro-BDNF in the amygdala of isolated animals were downregulated six months after RUS in the Algamal study [45]. Also, the expression of synaptic proteins was altered in conditions of combined NI and SPS with Synapsin1 being reduced in the basolateral amygdala and the hippocampal dentate gyrus, while PSDN95 was increased in both the hippocampus and amygdala, whereas the ratio between NLG1/NLG2 was significantly increased in the hippocampus and decreased in the amygdala of NI+SPS animals in the Cheng et al. [49] study.
3.2.5. (Novel) Pharmacological Targets
SI mice present decreased corticolimbic levels of allopregnanolone (Allo) (an endogenous positive allosteric modulator of GABA action at GABAA receptors) associated with the downregulation of 5α-reductase type I, the step enzyme in Allo biosynthesis [42,47]. The decrease of allopregnanolone is associated with altered emotional responses like aggression, increased contextual fear, and delayed contextual fear extinction. It has been shown that selective brain steroidogenic stimulants (SBSS) like fluoxetine and S-norfluoxetine may normalize Allo levels in the mPFC, hippocampus, and BLA and attenuate contextual fear responses related to social isolation [42]. Moreover, in the Locci et al. study [44], S-fluoxetine, along with the Allo analogs GNX, BR351, and BR297 that directly act at GABAA receptors, as well as the endocannabinoid PEA that stimulates brain Allo biosynthesis were used to alleviate symptoms of aggression of isolated mice, as observed in the resident–intruder situation. PEA and BR297 proved to have a stronger anti-aggressive effect than S-fluoxetine [44]. Last but not least, URB597 can increase the levels of the endogenous AEA and is effective in enhancing fear extinction and promote social interaction [47].
3.2.6. Shortening of Telomere Length
Telomeres are nucleoproteins located at the end of chromosomes protecting them from oxidative stress. Although not a neurobiological marker, telomere length (TL) is considered a substantial biomarker of cellular senescence, of immune system integrity and predictor of mortality [51], as well as a reliable peripheral biological measure of stress and hostility of PTSD patients [53,54]. In two retrieved studies, Stein et al. [51,52] showed that, in ex-prisoners of war (POW), solitary confinement during captivity as well as feelings of loneliness, loss of role in the family, lack of social support, and being accused at homecoming were associated with a shortening of telomere length in later life, compared to ex-POWs who did not share these experiences and feelings. A limitation of the study, as stated by the authors, is the small number of participants (N = 83, N = 99 respectively) and the long intervals between the examined periods.
Last but not least, the neurobiological findings of the above mentioned studies were mirrored in behavioral and cognitive symptoms of the stressed humans and animals including: fear responses [42,46], impaired fear extinction [42,45,47], increased anxiety behavior [33,45,49], aggression [44], as well as depression and feelings of loneliness for humans [51,52].
4. Discussion
Neurobiological research on PTSD and social isolation relies heavily on animals and deals mainly with peripheral biological variables in human subjects. Most of the retrieved studies showed a synergistic effect between PTSD and SI resembling to an aggravated version of neurobiological findings that comprise the broader PTSD literature as for example the key feature in many of the retrieved papers [45,48,49] concerning the blunting or hypoactivation of HPA-axis, already known from PTSD research [55]. Nevertheless, qualitatively different is the work of Daskalakis et al. [46] where a novel, isolating environment early in the life of maternally separated rats primes amygdala activation and an enduring fearful phenotype throughout life. In accordance with his three-hit concept of vulnerability [24], the author outlines a profile of fear perpetuation that is the result of social isolation and trauma, acquiring more permanent than transient characteristics.
The upregulation of proinflammatory cytokines in the Algamal study [45] is a more predictable and not pathognomonic finding, in the sense that general inflammatory responses across different psychiatric disorders can affect mood, behavior, and cognition [56]. In terms of neuroplasticity, the decrease of hippocampal BDNF levels and the retention of fear memory six months after the RUS paradigm in isolated rats [45] contradicts another study stating that social isolation of rats during adolescence led to hippocampal BDNF increase and retention of fear memories during adulthood [32] and further research is needed to clarify these opposing findings.
Concerning pharmacotherapy, the recent American Psychiatric Association (APA) and National Institute of Clinical Excellence (NICE) guidelines include antidepressants like the SSRIs fluoxetine, paroxetine, sertraline, and the SNRI venlafaxine, as well as the antipsychotics prazosin, olanzapine, and risperidone as first- and second-line agents for the treatment of PTSD [57]. Studies on the effectiveness of cannabinoids in the treatment of PTSD are small and disputed and better randomized clinical trials are needed [58] in order to corroborate the findings of the retrieved studies. The same holds true for the role of Allo in the treatment of PTSD combined with SI, that also needs further research and clinical support [59].
5. Limitations
The limitations of this review paper include the nature of most of the studies that focused on animal research, thus restricting translational limitations to the human condition. Even the two studies with the ex-POWs suffer from methodological limitations due to the small sample size, the long time-intervals between examination periods, and the retrograde distortion of memory concerning self-reports from the period of captivity [51,52].
6. Conclusions
SI exerts an aggravating influence on neurobiological consequences before, in parallel, or after trauma occurrence in PTSD, as the studies in this paper suggest. Despite the limitations of this review, it is important to retain that addressing social isolation, when trauma occurs, constitutes an essential secondary prevention measure. It would also be of interest for future research to further substantiate the pharmaceutical pathways of neurosteroids and endocannabinoids for the treatment of non-responders to usual agents by organizing well-designated clinical trials. Given the ubiquitous role of genetic predisposition, the three-hit concept of vulnerability [24] is justified in sketching the interplay between PTSD and social isolation. Further research is needed to illuminate this relation by exploring perhaps common biomarkers of allostatic load in human subjects across the two conditions [60,61]. PTSD patients constitute a group of vulnerable individuals whose trauma and the deriving biological and psychosocial repercussions have yet to be better understood and alleviated.
Author Contributions
I.I.V., C.P. and M.M conceived the original idea. I.I.V. did the PubMed search. All three authors decided on the included studies. I.I.V. wrote the first draft of the paper. M.M. corrected the first draft and enriched it with suggestions. All authors have read and agreed to the published version of the manuscript.
Funding
There was no funding for the systematic review.
Conflicts of Interest
The authors declare no conflict of interest.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Arlington, VA, USA, 2013; ISBN 9780890425558. [Google Scholar]
- Kilpatrick, D.G.; Resnick, H.S.; Milanak, M.E.; Miller, M.W.; Keyes, K.M.; Friedman, M.J. National Estimates of Exposure to Traumatic Events and PTSD Prevalence Using DSM-IV and DSM-5 Criteria. J. Trauma. Stress 2013, 26, 537–547. [Google Scholar] [CrossRef]
- Flanagan, J.C.; Mitchell, J.M. Augmenting Treatment for Posttraumatic Stress Disorder and Co-Occurring Conditions with Oxytocin. Curr. Treat. Opt. Psychiatry 2019, 6, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Kolassa, I.T.; Kolassa, S.; Ertl, V.; Papassotiropoulos, A.; De Quervain, D.J. The risk of posttraumatic stress disorder after trauma depends on traumatic load and the catechol-o-methyltransferase Val (158) Met polymorphism. Biol. Psychiatry 2010, 67, 304–308. [Google Scholar] [CrossRef] [PubMed]
- Miao, X.R.; Chen, Q.B.; Wei, K.; Tao, K.M.; Lu, Z.J. Posttraumatic Stress Disorder: From Diagnosis to Prevention. Mil. Med. Res. 2018, 5, 32. [Google Scholar] [CrossRef] [PubMed]
- Nazarian, D.; Kimerling, R.; Frayne, S.M. Posttraumatic stress disorder, substance use disorders, and medical comorbidity among returning U.S. veterans. J. Trauma. Stress 2012, 25, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Schlenger, W.E.; Corry, N.H.; Williams, C.S.; Kulka, R.A.; Mulvaney-Day, N.; DeBakey, S.; Murphy, C.M.; Marmar, C.R. A prospective study of mortality and trauma-related risk factors among a nationally representative sample of Vietnam veterans. Am. J. Epidemiol. 2015, 182, 980–990. [Google Scholar] [CrossRef]
- Thomaes, K.; de Cloet, C.; Wilker, S.; El-Hage, W.; Schäfer, I.; Kleim, B.; Schmahl, C.; van Zuiden, M. Investigating biological traces of traumatic stress in changing societies: Challenges and directions from the ESTSS Task Force on Neurobiology. Eur. J. Psychotraumatol. 2016, 7, 29453. [Google Scholar] [CrossRef]
- Daviua, N.; Bruchas, M.S.; Moghaddamc, B.; Sandid, C.; Beyelere, A. Neurobiological links between stress and anxiety. Neurobiol. Stress 2019, 11, 100191. [Google Scholar] [CrossRef]
- Fenster, R.J.; Lebois, L.A.M.; Ressler, K.J.; Suh, J. Brain circuit dysfunction in post-traumatic stress disorder: From mouse to man. Nat. Rev. Neurosci. 2018, 19, 535–551. [Google Scholar] [CrossRef]
- Olff, M.; Amstadter, A.; Armour, C.; Birkeland, M.S.; Bui, E.; Cloitre, M.; Ehlers, A.; Ford, J.D.; Greene, T.; Hansen, M.; et al. decennial review of psychotraumatology: What did we learn and where are we going? Eur. J. Psychotraumatol. 2019, 10, 1672948. [Google Scholar] [CrossRef]
- Marinova, Z.; Maercker, A. Biological correlates of complex posttraumatic stress disorder-state of research and future directions. Eur. J. Psychotraumatol. 2015, 6, 25913. [Google Scholar] [CrossRef] [PubMed]
- Olff, M.; van Zuiden, M. Neuroendocrine and neuroimmune markers in PTSD: Pre-, peri- and post-trauma glucocorticoid and inflammatory dysregulations. Curr. Opin. Psychol. 2017, 14, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Pervanidou, P.; Agorastos, A.; Kolaitis, G.; Chrousos, G.P. Neuroendocrine responses to early life stress and trauma and susceptibility to disease. Eur. J. Psychotraumatol. 2017, 8, 1351218. [Google Scholar] [CrossRef]
- Fragkaki, I.; Thomaes, K.; Sijbrandij, E.M. Posttraumatic stress disorder under ongoing threat: A review of neurobiological and neuroendocrine findings. Eur. J. Psychotraumatol. 2016, 7, 30915. [Google Scholar] [CrossRef]
- Berardi, A.; Schelling, G.; Campolongo, P. The endocannabinoid system and Post Traumatic Stress Disorder (PTSD): From preclinical findings to innovative therapeutic approaches in clinical settings. Pharm. Res. 2016, 111, 668–678. [Google Scholar] [CrossRef]
- Furini, C.; Myskiw, J.; Izquierdo, I. The learning of fear extinction. Neurosci. Biobehav. Rev. 2014, 47, 670–683. [Google Scholar] [CrossRef]
- Locci, A.; Pinna, G. Stimulation of Peroxisome Proliferator-Activated Receptor-α by N-Palmytoylethanolamine Engages Allopregnanolone Biosynthesis to Modulate Emotional Behavior. Biol. Psychiatry 2019, 85, 1036–1045. [Google Scholar] [CrossRef]
- Wilker, S.; Pfeiffer, A.; Elbert, T.; Ovuga, E.; Karabatsiakis, A.; Krumbholz, A.; Thieme, D.; Schelling, G.; Kolassa, I.T. Endocannabinoid concentrations in hair are associated with PTSD symprom severity. Psychoneuroendocrinology 2016, 67, 198–206. [Google Scholar] [CrossRef]
- Smaga, I.; Bystrowska, B.; Gawlinski, D.; Pomierny, B.; Stankowiz, P.; Philip, M. Antidepressants and changes in concentrations of endocannabinoids and N-acetylolethalomines in rat brain structures. Neurotox. Res. 2014, 26, 190–206. [Google Scholar]
- Pinna, G. Animal Model of PTSD: The Socially Isolated Mouse and the Biomarker Role of Allopregnanolone. Front. Behav. Neurosci. 2019, 13, 114. [Google Scholar] [CrossRef]
- Rasmusson, A.M.; King, M.W.; Valovski, I.; Gregor, K.; Scioli-Salter, E.; Pineles, S.L.; Hamouda, M.; Nillni, Y.I.; Anderson, G.M.; Pinna, G. Relationships Between Cerebrospinal Fluid GABAergic Neurosteroid Levels and Symptom Severity in Men with PTSD. Psychoneuroendocrinology 2019, 102, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Rasmusson, A.M.; Pinna, G.; Paliwal, P.; Weisman, D.; Gottschalk, C.; Charney, D.; Krystal, J.; Guidotti, A. Decreased Cerebrospinal Fluid Allopregnanolone Levels in Women with Posttraumatic Stress Disorder. Biol. Psychiatry 2006, 60, 704–713. [Google Scholar] [CrossRef] [PubMed]
- Daskalakis, N.P.; Bagot, R.C.; Parker, K.J.; Vinkers, C.H.; de Kloet, E.R. The three-hit concept of vulnerability and resilience: Towards understanding adaptation to early-life adversity outcome. Psychoneuroendocrinology 2013, 38, 1858–1873. [Google Scholar] [CrossRef] [PubMed]
- Uchino, B.N. Social Support and health: A review of physiological processes potentially underlying links to disease outcomes. J. Behav. Med. 2006, 29, 377–387. [Google Scholar] [CrossRef]
- Cacioppo, J.T.; Cacioppo, S.; Capitanio, J.P.; Cole, S.W. The Neuroendocrinology of Social Isolation. Annu. Rev. Psychol. 2015, 66, 733–767. [Google Scholar] [CrossRef]
- Cacioppo, J.T.; Hawkley, L.C.; Norman, G.J.; Berntson, G.G. Social Isolation. Ann. N. Y. Acad. Sci. 2011, 1231, 17–22. [Google Scholar] [CrossRef]
- Vargas, J.; Junco, M.; Gomez, C.; Lajud, N. Early Life Stress Increases Metabolic Risk, HPA Axis Reactivity, and Depressive-Like Behavior When Combined with Postweaning Social Isolation in Rats. PLoS ONE 2016, 11, e0162665. [Google Scholar] [CrossRef]
- Mc Neal, N.; Anderson, E.M.; Moenk, D.; Trahanas, D.; Matuszewich, L.; Grippo, A.J. Social isolation alters central nervous system monoamine content in prairie voles following acute restraint. Soc. Neurosci. 2018, 13, 173–183. [Google Scholar] [CrossRef]
- Dronjak, S.; Gavrilovic, L.; Filipovic, D.; Radojcic, M.B. Immobilization and cold stress affect sympatho–adrenomedullary system and pituitary–adrenocortical axis of rats exposed to long-term isolation and crowding. Physiol. Behav. 2004, 81, 409–415. [Google Scholar] [CrossRef]
- Tada, H.; Miyazaku, T.; Takemoto, K.; Takase, K.; Jitsuki, S.; Nakajima, W.; Koide, N.; Yamamoto, N.; Komiya, K.; Sano, A.; et al. Neonatal isolation augments social dominance by altering actin dynamics in the medial prefrontal cortex. Proc. Natl. Acad. Sci. USA 2016, 113, E7097–E7105. [Google Scholar] [CrossRef]
- Liu, J.H.; You, Q.L.; Wei, M.D.; Wang, Q.; Luo, Z.Y.; Lin, S.; Huang, L.; Li, S.J.; Li, X.W.; Gao, T.M. Social Isolation During Adolescence Strengthens Retention of Fear Memories and Facilitates Induction of Late-Phase Long-Term Potentiation. Mol. Neurobiol. 2015, 52, 1421–1429. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Hu, X.Z.; Li, H.; Li, X.; Yu, T.; Dohl, J.; Ursano, R.J. Updates in Animal Models PTSD Characterization. Methods Mol. Biol. 2019, 2011, 331–344. [Google Scholar] [PubMed]
- Cacioppo, J.T.; Hawkley, L.C.; Thisted, R.A. Perceived Social Isolation Makes Me Sad: Five year Cross-Lagged Analyses of Loneliness and Depressive Symptomatology in the Chicago Health, Aging, and Social Relations Study. Psychol. Aging 2010, 25, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Jaremka, L.M.; Fagundes, C.P.; Peng, J.; Bennett, J.M.; Glaser, R.; Malarkey, W.B.; Kiecolt-Glaser, J.K. Loneliness Promotes inflammation During Acute Stress. Psychol. Sci. 2013, 24, 1089–1097. [Google Scholar] [CrossRef]
- Lee, J. Lonely Too Long: Redefining and Reforming Juvenile Solitary Confinement. Law Rev. 2016, 85, 845–875. [Google Scholar] [CrossRef][Green Version]
- Ozer, E.J.; Best, S.R.; Lipsey, T.L.; Weiss, D.S. Predictors of posttraumatic stress disorder and symptoms in adults: A meta-analysis. Psychol. Bull. 2003, 129, 52–73. [Google Scholar] [CrossRef]
- Charuvastra, A.; Cloitre, M. Social bonds and posttraumatic stress disorder. Annu. Rev. Psychol. 2008, 59, 301–328. [Google Scholar] [CrossRef]
- Thoresen, S.; Jensen, T.K.; Wentzel-Larsen, T.; Dyb, G. Social support barriers and mental health in terrorist attack survivors. J. Affect. Disord. 2014, 156, 187–193. [Google Scholar] [CrossRef][Green Version]
- Strøm, I.F.; Aakvaag, H.F.; Birkeland, M.S.; Felix, E.; Thoresen, S. The mediating role of shame in the relationship between childhood bullying victimization and adult psychosocial adjustment. Eur. J. Psychotraumatol. 2018, 9, 1418570. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. The PRISMA Group Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Pibiri, F.; Nelson, M.; Guidotti, A.; Costa, E.; Pinna, G. Decreased corticolimbic allopregnanolone expression during social isolation enhances contextual fear: A model relevant for posttraumatic stress disorder. Proc. Natl. Acad. Sci. USA 2008, 105, 5567–5572. [Google Scholar] [CrossRef]
- Conrad, K.L.; Louderback, K.M.; Gessner, C.P.; Winder, D.G. Stress-induced Alterations in Anxiety-like Behavior and Adaptations in Plasticity in the Bed Nucleus of the Stria Terminalis. Physiol. Behav. 2011, 104, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Locci, A.; Geoffroy, P.; Miesch, M.; Mensah-Nyagan, A.-G.; Pinna, G. Social Isolation in Early versus Late Adolescent Mice Is Associated with Persistent Behavioral Deficits That Can Be Improved by Neurosteroid-Based Treatment. Front. Cell. Neurosci. 2017, 11, 208. [Google Scholar] [CrossRef] [PubMed]
- Algamal, M.; Ojo, J.O.; Lungmus, C.P.; Muza, P.; Cammarata, C.P.; Owens, M.J.; Mouzon, B.C.; Diamond, D.M.; Mullan, M.; Crawford, F. Chronic Hippocampal Abnormalities and Blunted HPA Axis in an Animal Model of Repeated Unpredictable Stress. Front. Behav. Neurosci. 2018, 12, 150. [Google Scholar] [CrossRef] [PubMed]
- Daskalakis, N.P.; Diamantopoulou, A.; Claessens, S.A.F.; Remmers, E.; Tjälve, M.; Oitzl, M.S.; Champagne, D.L.; de Kloet, E.R. Early experience of a novel-environment in isolation primes a fearful phenotype characterized by persistent amygdala activation. Psychoneuroendocrinology 2014, 39, 39–57. [Google Scholar] [CrossRef]
- Morena, M.; Berardi, A.; Colucci, P.; Palmeri, M.; Trezza, V.; Hill, M.N.; Campologno, P. Enhancing Endocannabinoid Neurotransmission augments the Efficacy of Extinction Training and Ameliorates Traumatic Stress-Induced Behavioral Alterations in Rats. Neuropsychopharmacology 2018, 43, 1284–1296. [Google Scholar] [CrossRef]
- Boero, G.; Pisu, M.G.; Biggio, F.; Muredda, L.; Carta, G.; Banni, S.; Paci, E.; Follesa, P.; Concas, A.; Porcu, P.; et al. Impaired glucocorticoid-mediated HPA axis negative feedback induced by juvenile social isolation in male rats. Neuropharmacology 2018, 133, 242–253. [Google Scholar] [CrossRef]
- Cheng, W.; Han, F.; Shi, Y. Neonatal isolation modulates glucocorticoid-receptor function and synaptic plasticity of hippocampal and amygdala neurons in a rat model of single prolonged stress. J. Affect. Dis. 2019, 246, 682–694. [Google Scholar] [CrossRef]
- Aspesi, D.; Pinna, G. Animal Models of post-traumatic stress disorder and novel treatment targets. Behav. Pharmacol. 2019, 30, 130–150. [Google Scholar] [CrossRef]
- Stein, J.Y.; Levin, Y.; Lahav, Y.; Uziel, O.; Abumock, H.; Solomon, Z. Perceived Social Support, Loneliness, and Later Life Telomere Length Following Wartime Captivity. Health Psychol. 2018, 37, 1067–1076. [Google Scholar] [CrossRef]
- Stein, J.Y.; Levin, Y.; Uziel, O.; Abumock, H.; Solomon, Z. Traumatic Stress and Cellular Senescence: The Role of War-Captivity and Homecoming Stressors in Later Life Telomere Length. J. Affect. Dis. 2018, 238, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Hu, X.Z.; Russell, D.W.; Benedek, D.M.; Fullerton, C.S.; Naifeh, J.A.; Li, X.; Chen, Z.; Wu, H.; Ng, T.H.H.; et al. Association between leucocyte telomere length and hostility in U.S. service members. Neurosci. Lett. 2019, 706, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Wolf, E.J.; Morrison, F.G.; Sullivan, D.R.; Logue, M.W.; Guetta, R.E.; Stone, A.; Schichmann, S.A.; Mc Glinchey, R.E.; Milberg, W.P.; Miller, M.W. The goddess who spins the thread of life: Klotho, psychiatric stress and accelerated aging. BrainBehav. Immun. 2019, 80, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Agorastos, A.; Pervanidou, P.; Chrousos, G.P.; Bake, D.G. Developmental Trajectories of Early Life Stress and Trauma: A Narrative Review on Neurobiological Aspects Beyond Stress System Dysregulation. Front. Psychiatry 2019, 10, 118. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M.E.; Teixera, A.L. Inflammation in Psychiatric Disorders. What comes first? Ann. N. Y. Acad. Sci. 2019, 1437, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Ehret, M. Treatment of posttraumatic stress disorder: Focus on pharmacotherapy. Ment. Health Clin. 2019, 9, 373–382. [Google Scholar] [CrossRef]
- Steenkamp, M.M.; Blessing, E.M.; Galatzer-Levy, I.R.; Hollahan, L.C.; Anderson, W.T. Marijuana and other cannabinoids as a treatment for posttraumatic stress disorder: A literature review. Depress Anxiety 2017, 34, 207–216. [Google Scholar] [CrossRef]
- Boero, G.; Porcu, P.; Morrow, L.A. Pleiotropic actions of allopregnanolone underlie therapeutic benefits in stress-related disease. Neurobiol. Stress 2020, 12, 100203. [Google Scholar] [CrossRef]
- Lohr, J.B.; Chang, H.; Sexton, M.; Palmer, B.W. Allostatic load and the endocannabinoid system: Implications for the treatment of physiological abnormalitie sin post traumatic stress disorder (PTSD). Cns Spectr. 2019. [Google Scholar] [CrossRef]
- McEwen, B.S. Allostasis and allostatic load: Implications for neuropsychopharmacology. Neuropsychopharmacology 2000, 22, 108–124. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).