Keeping in Touch with Mental Health: The Orienting Reflex and Behavioral Outcomes from Calatonia
Depression and Anxiety: The Significance of Touch in Psychiatry—Clinical and Neuroscientific Approaches
)
Abstract
:1. Introduction
2. History of the Technique
3. The Calatonia Technique
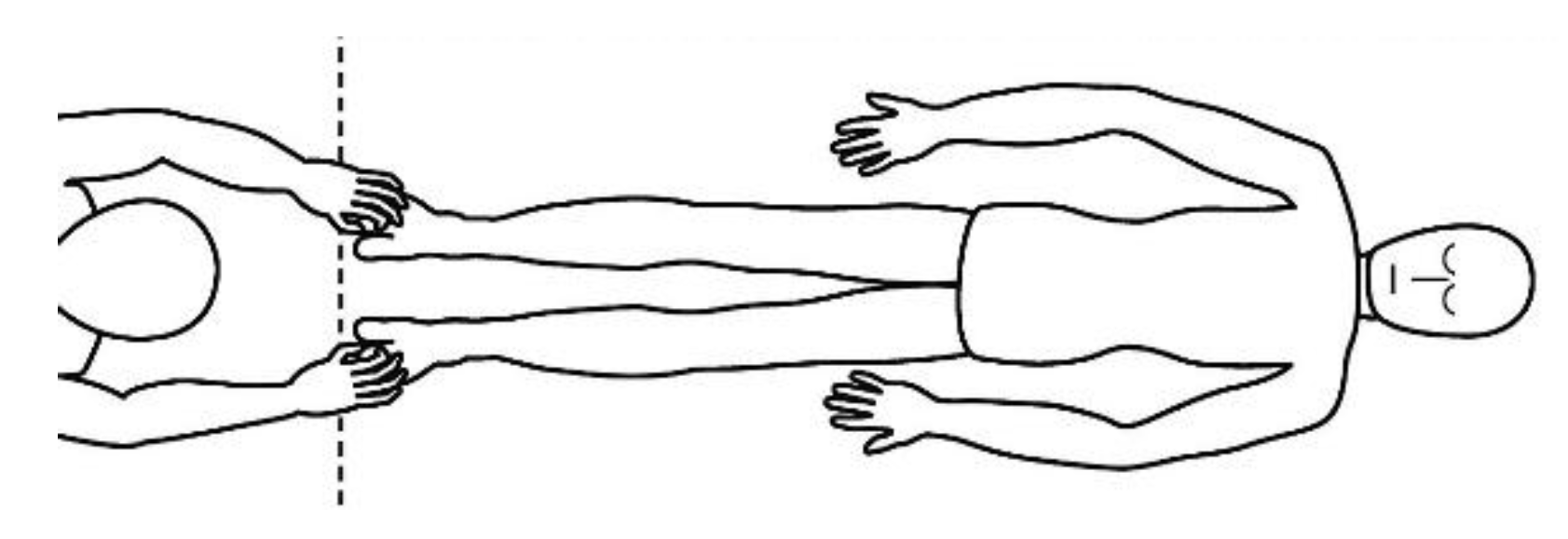
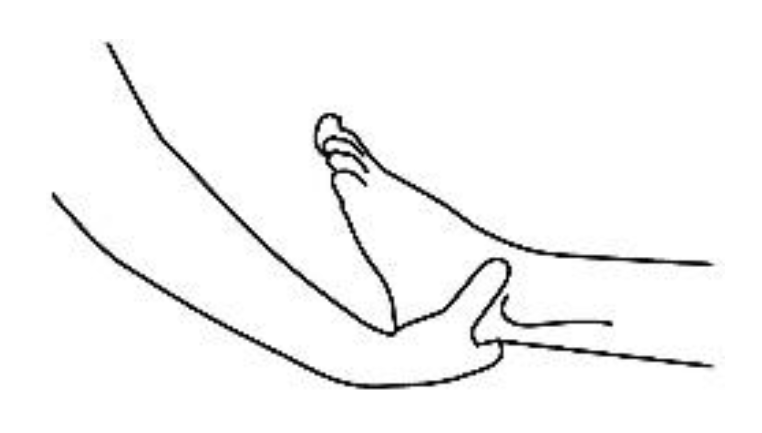
4. Touch to the Feet or Hands
5. Novel Stimuli in Psychotherapy
6. The Orienting Reflex in Calatonia
7. Brain Areas Associated with the Orienting Reflex
8. The Appraisal of New Stimuli
9. ORs in Clinical Practice
10. Habituation: Does Repeated Calatonia Cease to Generate an OR?
11. A Clinical Vignette
12. Conclusions
13. Final Considerations
- (a)
- (b)
- (c)
- The simultaneous engagement of low threshold (sensitive to light touch) skin receptors from the affective-affiliative system in the mammalian nervous system, primarily composed of C-tactile fibers and/or receptors [155,156,157,158,159], polymodal C-receptors, unmyelinated free nerve endings [160] and the low threshold discriminative-spatial system, associated with Merkel’s cell–neurite complex receptors and Ruffini corpuscle proprioceptors [47,137,161,162,163,164,165].
- (d)
Author Contributions
Funding
Conflicts of Interest
References
- Fiskum, C. Psychotherapy Beyond All the Words: Dyadic Expansion, Vagal Regulation, and Biofeedback in Psychotherapy. J. Psychother. Integr. 2019, 29, 412–425. [Google Scholar] [CrossRef]
- Knaster, M. Discovering the Body’s Wisdom: A Comprehensive Guide to more than Fifty Mind-Body Practices That Can Relieve Pain, Reduce Stress, and Foster Health, Spiritual Growth, and Inner Peace; Bantam: New York, NY, USA, 1996. [Google Scholar]
- Van der Kolk, B. The Body Keeps the Score: Brain, Mind, and Body in the Healing of Trauma; Penguin Books: New York, NY, USA, 2015. [Google Scholar]
- Field, T. Complementary and Alternative Therapies Research; American Psychological Association: Washington, DC, USA, 2009. [Google Scholar]
- Anderson, J.G.; Taylor, A.G. Effects of Healing Touch in Clinical Practice: A Systematic Review of Randomized Clinical Trials. J. Holist. Nurs. 2011, 29, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Tantia, J.F. Toward a Somatically-Informed Paradigm in Embodied Research. Int. Body Psychother. J. 2019, 18, 134–145. [Google Scholar]
- Bradley, M.M. Natural selective attention: Orienting and emotion. Psychophysiology 2009, 46, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, W. Motivational Interviewing: III. On the Ethics of Motivational Intervention. Behav. Cogn. Psychother. 1994, 22, 111–123. [Google Scholar] [CrossRef]
- Bradley, M.M.; Codispoti, M.; Cuthbert, B.N.; Lang, P. Emotion and Motivation I: Defensive and Appetitive Reactions in Picture Processing. Emotion 2001, 1, 276–298. [Google Scholar] [CrossRef]
- Harmon-Jones, E.; Harmon-Jones, C.; Summerell, E. On the Importance of Both Dimensional and Discrete Models of Emotion. Behav. Sci. 2017, 7, 66. [Google Scholar] [CrossRef] [Green Version]
- Panksepp, J. Neurologizing the psychology of affects: How appraisal-based constructivism and basic emotion theory can coexist. Perspect. Psychol. Sci. 2007, 2, 281–296. [Google Scholar] [CrossRef]
- Weiqi, Z.; Ye, L.; Hong, L.C.; Yu-Hsin, C.; Qian, C.; Xiaolan, F. The Influence of Event Valence and Emotional States on the Metaphorical Comprehension of Time. Front. Psychol. 2019, 10, 410. [Google Scholar]
- Lang, P.J. Emotion and Motivation: Toward Consensus Definitions and a Common Research Purpose. Emot. Rev. 2010, 2, 229–233. [Google Scholar] [CrossRef]
- Vrtička, P.; Vuilleumier, P. Neuroscience of human social interactions and adult attachment style. Front. Hum. Neurosci. 2012, 6, 212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrós-Loscertales, A.; Ventura-Campos, N.; Sanjuán-Tomás, A.; Belloch, V.; Parcet, M.A.; Avila, C. Behavioral activation system modulation on brain activation during appetitive and aversive stimulus processing. Soc. Cogn. Affect. Neurosci. 2010, 5, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Grossman, S.P. Motivation, Aversive, Biological Bases. In States of Brain and Mind. Readings from the Encyclopedia of Neuroscience Series; Hobson, J.A., Ed.; Birkhäuser: Boston, MA, USA, 1988; pp. 63–65. [Google Scholar]
- Blanchard, A.R. Calatonia: Novel Insights from Neuroscience. In Calatonia: A Therapeutic Approach that Promotes Somatic and Psychological Regulation; Blanchard, A.R., Rios, A.M.G., Seixas, L.P., Eds.; Alma Street Enterprise: Miami, FL, USA, 2019; pp. 286–304. [Google Scholar]
- Farah, R. Calatonia: Subtle Touch in Psychotherapy; Companhia Ilimitada: São Paulo, Brazil, 2017. [Google Scholar]
- Sándor, P. Calatonia. Bol. De Psicol. 1969, XXI, 92–100. [Google Scholar]
- Sándor, P. Calatonia. In Calatonia: A Therapeutic Approach that Promotes Somatic and Psychological Regulation; Blanchard, A.R., Rios, A.M.G., Seixas, L.P., Eds.; Alma Street Enterprise: Miami, FL, USA, 2019; pp. 1–13. [Google Scholar]
- Armando, M.D. Calatonia and Resillience. In Calatonia: A Therapeutic Approach that Promotes Somatic and Psychological Regulation; Blanchard, A.R., Rios, A.M.G., Seixas, L.P., Eds.; Alma Street Enterprise: Miami, FL, USA, 2019; pp. 263–285. [Google Scholar]
- Insel, T.; Cuthbert, B.; Garvey, M.; Heinssen, R.; Pine, D.S.; Quinn, K.; Sanislow, C.; Wang, P. Research domain criteria (RDoC): Toward a new classification framework for research on mental disorders. Am. J. Psychiatry 2010, 167, 167–748. [Google Scholar] [CrossRef] [Green Version]
- Gupta, A.; Love, A.; Kilpatrick, L.A.; Labus, J.S.; Bhatt, R.; Chang, L.; Tillisch, K.; Naliboff, B.; Mayer, E.A. Morphological Brain Measures of Cortico-Limbic Inhibition Related to Resilience. J. Neurosci. Res. 2017, 95, 1760–1775. [Google Scholar] [CrossRef] [Green Version]
- Farah, R. The Academic Teaching of Calatonia. In Calatonia: A Therapeutic Approach that Promotes Somatic and Psychological Regulation; Blanchard, A.R., Rios, A.M.G., Seixas, L.P., Eds.; Alma Street Enterprise: Miami, FL, USA, 2019; pp. 26–45. [Google Scholar]
- Machado Filho, P.T. The Legacy of Sándor. In Calatonia: A Therapeutic Approach that Promotes Somatic and Psychological Regulation; Blanchard, A.R., Rios, A.M.G., Seixas, L.P., Eds.; Alma Street Enterprise: Miami, FL, USA, 2019; pp. 14–25. [Google Scholar]
- Kirsch, T. The Jungians; Routledge: London, UK, 2000. [Google Scholar]
- Delmanto, S. Subtle Touches: Calatonia, A Life Experience with Pethö Sándor’s Work; Summus: São Paulo, Brazil, 2008. [Google Scholar]
- Gonçalves, M.I.C.; Pereira, M.A.; Ribeiro, A.J.; Rios, A.M.G. Subtle touch, calatonia and other somatic interventions with children and adolescents. Int. Body Psychother. J. 2007, 6, 33–47. [Google Scholar]
- Rios, A.M.G.; Seixas, L.P.; Blanchard, A.R. The Body in Psychotherapy: Calatonia and Subtle Touch Techniques. In Body, Mind, and Healing After Jung: A Space of Questions; Jones, R., Ed.; Routledge: London, UK, 2010; pp. 228–250. [Google Scholar]
- Blanchard, A.R.; Rios, A.M.G.; Seixas, L.P. (Eds.) Calatonia: A Therapeutic Approach that Promotes Somatic and Psychological Regulation; Alma Street Enterprise: Miami, FL, USA, 2019. [Google Scholar]
- Morrison, I. Keep Calm and Cuddle on: Social Touch as a Stress Buffer. Adapt. Hum. Behav. Physiol. 2016, 2, 344–362. [Google Scholar] [CrossRef] [Green Version]
- Greger Tavares, S.M.; Vannuchi, B.P.; Machado, F.P.T.; Andrade, A.L.M. Efeitos psicofisiológicos da Calatonia em adultos: Um estudo piloto na abordagem quanti-qualitativa. Jung Corpo 2015, 15, 17–33. [Google Scholar]
- Lasaponari, E.F. A Utilização Da Calatonia No Período Pós-Operatório Imediato. Unpublished. Master’s Thesis, Nursing School, University of São Paulo, São Paulo, Brazil, 2011. Available online: http://www.teses.usp.br/teses/disponiveis/7/7139/tde-21062011-152045/ (accessed on 6 February 2020).
- Nossow, V.; Peniche, A.C.G. Paciente cirurgico ambulatorial: Calatonia e ansiedade. Acta Paul. De Enferm. 2007, 20, 161–167. [Google Scholar] [CrossRef] [Green Version]
- Löken, L.S.; Olausson, H. The skin as a social organ. Exp. Brain Res. 2010, 204, 305–314. [Google Scholar]
- Craig, A.D. How do you feel? Interoception: The sense of the physiological condition of the body. Nat. Rev. Neurosci. 2002, 3, 655–666. [Google Scholar] [CrossRef] [PubMed]
- Craig, A.D. Interoception and emotion: A neuroanatomical perspective. In Handbook of Emotions; Lewis, M., Haviland-Jones, J.M., Feldman Barrett, L., Eds.; The Guildford Press: New York, NY, USA, 2008; pp. 272–288. [Google Scholar]
- Löken, L.S.; Wessberg, J.; Morrison, I.; McGlone, F.; Olausson, H. Coding of pleasant touch by unmyelinated afferents in humans. Nat. Neurosci. 2009, 12, 547–548. [Google Scholar] [CrossRef] [PubMed]
- Moehring, F.; Halder, P.; Seal, R.P.; Stucky, C.L. Uncovering the cells and circuits of touch in normal and pathological settings. Neuron 2018, 100, 349–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abraham, J.; Mathew, S. Merkel Cells: A Collective Review of Current Concepts. Int. J. Appl. Basic Med. Res. 2019, 9, 9–13. [Google Scholar]
- Chang, W.; Kanda, H.; Ikeda, R.; Ling, J.; DeBerry, J.J.; Gu, J.G. Merkel disc is a serotonergic synapse in the epidermis for transmitting tactile signals in mammals. Proc. Natl. Acad. Sci. USA 2016, 113, E5491–E5500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halata, Z.; Grim, M.; Baumann, K.I. Current understanding of Merkel cells, touch reception and the skin. Expert Rev. Dermatol. 2010, 5, 109–116. [Google Scholar] [CrossRef] [Green Version]
- Johansson, R.S.; Vallbo, A.B. Tactile sensibility in the human hand: Relative and absolute densities of four types of mechanoreceptive units in glabrous skin. J. Physiol. 1979, 286, 283–300. [Google Scholar] [CrossRef]
- Johansson, R.S.; Vallbo, Å.B. Tactile sensory coding in the glabrous skin of the human hand. Trends Neurosci. 1983, 6, 27–32. [Google Scholar] [CrossRef]
- Maksimovic, S.; Baba, Y.; Lumpkin, E.A. Neurotransmitters and synaptic components in the Merkel cell-neurite complex, a gentle touch receptor. Ann. N. Y. Acad. Sci. 2013, 1279, 13–21. [Google Scholar] [CrossRef] [Green Version]
- Maksimovic, S.; Nakatani, M.; Baba, Y.; Nelson, A.M.; Marshall, K.L.; Wellnitz, S.A.; Firozi, P.; Woo, S.H.; Ranade, S.; Patapoutian, A.; et al. Epidermal Merkel cells are mechanosensory cells that tune mammalian touch receptors. Nature 2014, 509, 617–621. [Google Scholar] [CrossRef] [Green Version]
- McGlone, F.; Wessberg, J.; Olausson, H. Discriminative and Affective Touch: Sensing and Feeling. Neuron 2014, 82, 737–755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rolls, E.T. The affective and cognitive processing of touch, oral texture, and temperature in the brain. Neurosci. Biobehav. Rev. 2010, 34, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Hayward, V. A Brief Overview of the Human Somatosensory System. In Musical Haptics; Papetti, S., Saitis, C., Eds.; Springer: Cham, Switzerland, 2018; pp. 29–48. [Google Scholar]
- Millar, S. Reading by Touch; Routledge: New York, NY, USA, 1997. [Google Scholar]
- Klooster, N.B.; Cook, S.W.; Uc, E.Y.; Duff, M.C. Gestures make memories, but what kind? Patients with impaired procedural memory display disruptions in gesture production and comprehension. Front. Hum. Neurosci. 2015, 8, 1054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Squire, L.R.; Dede, A.J. Conscious and unconscious memory systems. Cold Spring Harb. Perspect. Biol. 2015, 7, a021667. [Google Scholar] [CrossRef] [Green Version]
- Allum, J.H.J.; Bloem, B.R.; Carpenter, M.G.; Hulliger, M.; Hadders-Algra, M. Proprioceptive control of posture: A review of new concepts. Gait Posture 1998, 8, 214–242. [Google Scholar] [CrossRef]
- Drew, T.; Prentice, S.; Schepens, B. Cortical and brainstem control of locomotion. Prog. Brain Res. 2004, 143, 251–261. [Google Scholar]
- Kennedy, P.M.; Inglis, J.T. Distribution and behaviour of glabrous cutaneous receptors in the human foot sole. J. Physiol. 2002, 538, 995–1002. [Google Scholar] [CrossRef]
- Malcuit, G.; Bastien, C.; Pomerleau, A. Habituation of the orienting response to stimuli of different functional values in 4-month-old infants. J. Exp. Child Psychol. 1996, 62, 272–291. [Google Scholar] [CrossRef]
- Buodo, G.; Sarlo, M.; Palomba, D. Attentional resources measured by reaction times highlight differences within pleasant and unpleasant, high arousing stimuli. Motiv. Emot. 2002, 26, 123–138. [Google Scholar] [CrossRef]
- Fan, J.; McCandliss, B.D.; Fossella, J.; Flombaum, J.I.; Posner, M. The activation of attentional networks. NeuroImage 2005, 26, 471–479. [Google Scholar] [CrossRef]
- Lang, P.J.; Bradley, M.M. Appetitive and Defensive Motivation: Goal-Directed or Goal-Determined? Emot. Rev. J. Int. Soc. Res. Emot. 2013, 5, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Posner, M.I.; Petersen, S.E. The Attention System of the Human Brain. Annu. Rev. Neurosci. 2003, 13, 25–42. [Google Scholar] [CrossRef] [PubMed]
- Ross-Sheehy, S.; Schneegans, S.; Spencer, J.P. The Infant Orienting with Attention task: Assessing the neural basis of spatial attention in infancy. Infancy Off. J. Int. Soc. Infant Stud. 2015, 20, 467–506. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.M.; Brammer, M.J.; Skerrett, D.; Lagopolous, J.; Rennie, C.; Kozek, K.; Olivieri, G.; Peduto, T.; Gordon, E. The neural correlates of orienting: An integration of fMRI and skin conductance orienting. Neuroreport 2000, 11, 3011–3015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geva, R.; Zivan, M.; Warsha, A.; Olchik, D. Alerting, orienting or executive attention networks: Differential patterns of pupil dilations. Front. Behav. Neurosci. 2013, 7, 145. [Google Scholar] [CrossRef] [Green Version]
- Posner, M.I. Attention in the Social World; Oxford University Press: New York, NY, USA, 2012. [Google Scholar]
- Posner, M.I. Attentional networks and consciousness. Front. Psychol. 2012, 3, 64. [Google Scholar] [CrossRef] [Green Version]
- Pavlov, I.P. Conditioned Reflexes; Oxford University Press: Oxford, UK, 1927. [Google Scholar]
- Loewenstein, G. The psychology of curiosity: A review and reinterpretation. Psychol. Bull. 1994, 116, 75–98. [Google Scholar] [CrossRef]
- DeGangi, G.A. The Dysregulated Adult: Integrated Treatment Approaches; Academic Press: Cambridge, MA, USA, 2012. [Google Scholar]
- DeGangi, G.A. Pediatric Disorders of Regulation in Affect and Behavior: A Therapist’s Guide to Assessment and Treatment; Academic Press: Cambridge, MA, USA, 2017. [Google Scholar]
- Sokolov, E.N. Perception and the Conditioned Reflex; Macmillan: New York, NY, USA, 1963. [Google Scholar]
- Bradley, M.M.; Keil, A.; Lang, P.J. Orienting and Emotional Perception: Facilitation, Attenuation, and Interference. Front. Psychol. 2012, 3, 493. [Google Scholar] [CrossRef] [Green Version]
- Graham, F.K.; Clifton, R.K. Heart-rate change as a component of the orienting response. Psychol. Bull. 1966, 65, 305–320. [Google Scholar] [CrossRef]
- Bernstein, A.S. The Orienting Response as Novelty and Significance Detector: Reply to O’Gorman. Psychophysiology 1979, 16, 263–273. [Google Scholar] [CrossRef]
- Sambo, C.F.; Forster, B. Sustained Spatial Attention in Touch: Modality-Specific and Multimodal Mechanisms. Sci. World J. 2011, 11, 199–213. [Google Scholar] [CrossRef] [PubMed]
- Engbert, K.; Wohlschläger, A.; Haggard, P. Who is causing what? The sense of agency is relational and efferent-triggered. Cognition 2008, 107, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Mangalam, M.; Cutts, S.A.; Fragaszy, D.M. Sense of ownership and not the sense of agency is spatially bounded within the space reachable with the unaugmented hand. Exp. Brain Res. 2019, 237, 2911–2924. [Google Scholar] [CrossRef] [PubMed]
- Tsakiris, M.; Schütz-Bosbach, S.; Gallagher, S. On agency and body-ownership: Phenomenological and neurocognitive reflections. Conscious. Cogn. 2007, 16, 645–660. [Google Scholar] [CrossRef]
- Tomkins, S. Affect Imagery Consciousness, Vol I: The Positive Affects; Springer: New York, NY, USA, 1962. [Google Scholar]
- Stevens, C.; Bavelier, D. The role of selective attention on academic foundations: A cognitive neuroscience perspective. Dev. Cogn. Neurosci. 2012, 2, S30–S48. [Google Scholar] [CrossRef] [PubMed]
- Harjunen, V.J.; Spapé, M.; Ahmed, I.; Jacucci, G.; Ravaja, N. Individual differences in affective touch: Behavioral inhibition and gender define how an interpersonal touch is perceived. Personal. Individ. Differ. 2017, 107, 88–95. [Google Scholar] [CrossRef]
- Parvizi, J.; Damasio, A. Consciousness and the brain-stem. Cognition 2001, 79, 135–160. [Google Scholar] [CrossRef]
- Martins, I.; Tavares, I. Reticular Formation and Pain: The Past and the Future. Front. Neuroanat. 2017, 11, 51. [Google Scholar] [CrossRef] [Green Version]
- Neugebauer, V. Chapter 15: Amygdala pain mechanisms. In Handbook of Experimental Pharmacology; Barrett, J.E., Ed.; Springer: New York, NY, USA, 2015; pp. 261–284. [Google Scholar]
- Venkatraman, A.; Edlow, B.L.; Immordino-Yang, M.H. The Brainstem in Emotion: A Review. Front. Neuroanat. 2017, 11, 15. [Google Scholar] [CrossRef]
- Youssef, A.M.; Macefield, V.G.; Henderson, L.A. Cortical influences on brainstem circuitry responsible for conditioned pain modulation in humans. Hum. Brain Mapp. 2016, 37, 2630–2644. [Google Scholar] [CrossRef]
- Bremner, J.D. Traumatic stress: Effects on the brain. Dialogues Clin. Neurosci. 2006, 8, 445–461. [Google Scholar] [PubMed]
- Jeewajee, A.; Lever, C.; Burton, S.; O’Keefe, J.; Burgess, N. Environmental novelty is signaled by reduction of the hippocampal theta frequency. Hippocampus 2008, 18, 340–348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uddin, L.Q.; Nomi, J.S.; Hébert-Seropian, B.; Ghaziri, J.; Boucher, O. Structure and Function of the Human Insula. J. Clin. Neurophysiol. 2017, 34, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Howe, M.L.; Cicchetti, D.; Toth, S.L. Children’s basic memory processes, stress, and maltreatment. Dev. Psychopathol. 2006, 18, 759–769. [Google Scholar] [CrossRef] [Green Version]
- Balderston, N.L.; Schultz, D.H.; Helmstetter, F.J. The human amygdala plays a stimulus specific role in the detection of novelty. NeuroImage 2011, 55, 1889–1898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonnet, L.; Comte, A.; Tatu, L.; Millot, J.-L.; Moulin, T.; Medeiros de Bustos, E. The role of the amygdala in the perception of positive emotions: An “intensity detector”. Front. Behav. Neurosci. 2015, 9, 178. [Google Scholar] [CrossRef]
- Morrison, S.E.; Salzman, C.D. Revaluing the amygdala. Curr. Opin. Neurobiol. 2010, 20, 221–230. [Google Scholar] [CrossRef]
- Murray, E.A. The amygdala, reward and emotion. Trends Cogn. Sci. 2007, 11, 489–497. [Google Scholar] [CrossRef]
- Weymar, M.; Schwabe, L. Amygdala and Emotion: The Bright Side of It. Front. Neurosci. 2016, 10, 224. [Google Scholar] [CrossRef] [Green Version]
- Phelps, E.A.; LeDoux, J.E. Contributions of the amygdala to emotion processing: From animal models to human behavior. Neuron 2005, 48, 175–187. [Google Scholar] [CrossRef] [Green Version]
- Vasa, R.A.; Pine, D.S.; Thorn, J.M.; Nelson, T.E.; Spinelli, S.; Nelson, E.; Maheu, F.S.; Ernst, M.; Bruck, M.; Mostofsky, S.H. Enhanced Right Amygdala Activity in Adolescents during Encoding of Positively-Valenced Pictures. Dev. Cogn. Neurosci. 2011, 1, 88–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scherer, K.R. Emotions are emergent processes: They require a dynamic computational architecture. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 3459–3474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blackford, J.U.; Buckholtz, J.W.; Avery, S.N.; Zald, D.H. A unique role for the human amygdala in novelty detection. NeuroImage 2010, 50, 1188–1193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vuilleumier, P. How brains beware: Neural mechanisms of emotional attention. Trends Cogn. Sci. 2005, 9, 585–594. [Google Scholar] [CrossRef]
- Lang, P.J.; Bradley, M.M. Emotion and the motivational brain. Biol. Psychol. 2010, 84, 437–450. [Google Scholar] [CrossRef] [Green Version]
- Schomaker, J.; Rangel-Gomez, M.; Meeter, M. Happier, faster: Developmental changes in the effects of mood and novelty on responses. Q. J. Exp. Psychol. 2016, 69, 37–47. [Google Scholar] [CrossRef]
- Hammer, N.; Glätzner, J.; Feja, C.; Kühne, C.; Meixensberger, J.; Planitzer, U.; Schleifenbaum, S.; Tillmann, B.N.; Winkler, D. Human vagus nerve branching in the cervical region. PLoS ONE 2015, 10, e0118006. [Google Scholar] [CrossRef]
- Keding, T.J.; Herringa, R.J. Paradoxical Prefrontal—Amygdala Recruitment to Angry and Happy Expressions in Pediatric Posttraumatic Stress Disorder. Neuropsychopharmacology 2016, 41, 2903–2912. [Google Scholar] [CrossRef] [Green Version]
- Marusak, H.A.; Martin, K.R.; Etkin, A.; Thomason, M.E. Childhood Trauma Exposure Disrupts the Automatic Regulation of Emotional Processing. Neuropsychopharmacology 2015, 40, 1250–1258. [Google Scholar] [CrossRef]
- White, S.F.; Costanzo, M.E.; Blair, J.R.; Roy, M.J. PTSD symptom severity is associated with increased recruitment of top-down attentional control in a trauma-exposed sample. Neuroimage Clin. 2015, 7, 19–27. [Google Scholar] [CrossRef] [Green Version]
- Streeck-Fischer, A.; van der Kolk, B.A. Down will come baby, cradle and all: Diagnostic and therapeutic implications of chronic trauma on child development. Aust. N. Z. J. Psychiatry 2000, 34, 903–918. [Google Scholar] [CrossRef] [PubMed]
- Block, S.R.; King, A.P.; Sripada, R.K.; Weissman, D.H.; Welsh, R.; Liberzon, I. Behavioral and neural correlates of disrupted orienting attention in posttraumatic stress disorder. Cogn. Affect. Behav. Neurosci. 2017, 17, 422–436. [Google Scholar] [CrossRef] [PubMed]
- Teicher, M.H.; Alaptagin, K. Childhood maltreatment, cortical and amygdala morphometry, functional connectivity, laterality, and psychopathology. Child Maltreat. 2019, 24, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Butler, O.; Adolf, J.; Gleich, T.; Willmund, G.; Zimmermann, P.; Lindenberger, U.; Gallinat, J.; Kühn, S. Military deployment correlates with smaller prefrontal gray matter volume and psychological symptoms in a subclinical population. Transl. Psychiatry 2017, 7, e1031. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Kim, J.S.; Jin, M.J.; Im, C.-H.; Lee, S.-H. Dysfunctional frontal lobe activity during inhibitory tasks in individuals with childhood trauma: An event-related potential study. Neuroimage Clin. 2018, 17, 935–942. [Google Scholar] [CrossRef]
- Sherin, J.E.; Nemeroff, C.B. Post-traumatic stress disorder: The neurobiological impact of psychological trauma. Dialogues Clin. Neurosci. 2011, 13, 263–278. [Google Scholar]
- Herbert, C. Calatonia and Subtle Touch in the Healing of Trauma. In Calatonia: A Therapeutic Approach that Promotes Somatic and Psychological Regulation; Blanchard, A.R., Rios, A.M.G., Seixas, L.P., Eds.; Alma Street Enterprise: Miami, FL, USA, 2019; pp. 70–86. [Google Scholar]
- Herbert, C. Overcoming Traumatic Stress—A Self-Help Guide Using Cognitive Behavioural Techniques; Robinson, Little Brown Book Group: London, UK, 2017. [Google Scholar]
- Resick, P.A.; Monson, C.M.; Chard, K.M. Cognitive Processing Therapy for PTSD: A Comprehensive Manual; Guilford Press: New York, NY, USA, 2016. [Google Scholar]
- Ogden, P.; Minton, K.; Pain, C. Trauma and the Body: A Sensory Motor Approach to Therapy; W.W. Norton: New York, NY, USA, 2006. [Google Scholar]
- Payne, P.; Levine, P.A.; Crane-Godreau, M.A. Somatic experiencing: Using interoception and proprioception as core elements of trauma therapy. Front. Psychol. 2015, 6, 93. [Google Scholar]
- Shapiro, F.; Forrest, M.S. EMDR: The Break-Through Therapy for Overcoming Anxiety, Stress, and Trauma; Basic Books: New York, NY, USA, 2004. [Google Scholar]
- Seidler, G.H.; Wagner, F.E. Comparing the efficacy of EMDR and trauma-focused cognitive-behavioral therapy in the treatment of PTSD: A meta-analytic study. Psychol. Med. 2006, 36, 1515–1522. [Google Scholar] [CrossRef]
- Patihis, L.; Cruz, C.S.; McNally, R.J. Eye Movement Desensitization and Reprocessing (EMDR). In Encyclopedia of Personality & Individual Differences; Zeigler-Hill, V., Shackelford, T.K., Eds.; Springer: New York, NY, USA, 2017. [Google Scholar]
- Chamberlin, D.E. The Predictive Processing Model of EMDR. Front. Psychol. 2019, 10, 2267. [Google Scholar] [CrossRef]
- Friston, K. The free-energy principle: A rough guide to the brain? Trends Cogn. Sci. 2009, 13, 293–301. [Google Scholar] [CrossRef]
- Sokolov, E.N.; Spinks, J.A.; Naatanen, R.; Lyytinen, H. The Orienting Response in Information Processing; Lawrence Erlbaum: Mahwah, NJ, USA, 2002. [Google Scholar]
- Torta, D.M.; Liang, M.; Valentini, E.; Mouraux, A.; Iannetti, G.D. Dishabituation of laser-evoked EEG responses: Dissecting the effect of certain and uncertain changes in stimulus spatial location. Exp. Brain Res. 2012, 218, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Schomaker, J.; Meeter, M. Short- and long-lasting consequences of novelty, deviance and surprise on brain and cognition. Neurosci. Biobehav. Rev. 2015, 55, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Harshaw, C. Interoceptive dysfunction: Toward an integrated framework for understanding somatic and affective disturbance in depression. Psychol. Bull. 2015, 141, 311–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, L.; Zhang, A.; Sun, N.; Liu, P.; Yang, C.; Li, G.; Liu, Z.; Wang, Y.; Zhang, K. Functional connectivity between the thalamus and the primary somatosensory cortex in major depressive disorder: A resting-state fMRI study. BMC Psychiatry 2018, 18, 339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daffner, K.R.; Scinto, L.F.; Weitzman, A.M.; Faust, R.; Rentz, D.M.; Budson, A.E.; Holcomb, P.J. Frontal and parietal components of a cerebral network mediating voluntary attention to novel events. J. Cogn. Neurosci. 2003, 15, 294–313. [Google Scholar] [CrossRef]
- Cacioppo, S.; Zhou, H.; Monteleone, G.; Majka, E.A.; Quinn, K.A.; Ball, A.B.; Norman, G.J.; Semin, G.R.; Cacioppo, J.T. You are in sync with me: Neural correlates of interpersonal synchrony with a partner. Neuroscience 2014, 277, 842–858. [Google Scholar] [CrossRef]
- Dumas, G.; Nadel, J.; Soussignan, R.; Martinerie, J.; Garnero, L. Inter-brain synchronization during social interaction. PLoS ONE 2010, 5, e12166. [Google Scholar] [CrossRef] [Green Version]
- Hove, M.J.; Risen, J.L. It’s All in the Timing: Interpersonal Synchrony Increases Affiliation. Soc. Cogn. 2009, 27, 949–960. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Hu, Y.; Li, X.; Pan, Y.; Cheng, X. Brain-to-brain synchronization across two persons predicts mutual prosociality. Soc. Cogn. Affect. Neurosci. 2017, 12, 1835–1844. [Google Scholar] [CrossRef]
- Mu, Y.; Guo, C.; Han, S. Oxytocin enhances inter-brain synchrony during social coordination in male adults. Soc. Cogn. Affect. Neurosci. 2016, 11, 1882–1893. [Google Scholar] [CrossRef] [Green Version]
- Koole, S.L.; Tschacher, W. Synchrony in Psychotherapy: A Review and an Integrative Framework for the Therapeutic Alliance. Front. Psychol. 2016, 7, 862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schore, A.N. Relational trauma and the developing right brain: An interface of psychoanalytic self-psychology and neuroscience. Ann. N. Y. Acad. Sci. 2009, 1159, 189–203. [Google Scholar] [CrossRef] [PubMed]
- Siegel, D.J. The Developing Mind; The Guilford Press: New York, NY, USA, 2012. [Google Scholar]
- Jones, S.R. When brain rhythms aren’t ‘rhythmic’: Implication for their mechanisms and meaning. Curr. Opin. Neurobiol. 2016, 40, 72–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciaunica, A.; Fotopoulou, A. The touched self: Psychological and philosophical perspectives on proximal intersubjectivity and the self. In Embodiment, Enaction, and Culture: Investigating the Constitution of the Shared World; Durt, C., Fuchs, T., Tewes, C., Eds.; MIT Press: Cambridge, MA, USA, 2017; pp. 173–192. [Google Scholar]
- Hallam, G.P.; Webb, T.L.; Sheeran, P.; Miles, E.; Niven, K.; Wilkinson, I.D.; Hunter, M.D.; Woodruff, P.W.; Totterdell, P.; Farrow, T.F. The neural correlates of regulating another person’s emotions: An exploratory fMRI study. Front. Hum. Neurosci. 2014, 8, 376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naruse, K.; Hirai, T. Effects of slow tempo exercise on respiration, heart rate, and mood state. Percept. Mot. Ski. 2000, 91, 729–740. [Google Scholar] [CrossRef]
- Szirmai, I. How does the brain create rhythms? Ideggyógy. Szle. 2010, 63, 13–23. [Google Scholar]
- Deco, G.; Kringelbach, M.L.; Jirsa, V.K.; Ritter, P. The dynamics of resting fluctuations in the brain: Metastability and its dynamical cortical core. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Smallwood, J.; Schooler, J.W. The Science of Mind Wandering: Empirically Navigating the Stream of Consciousness. Annu. Rev. Psychol. 2015, 66, 487–518. [Google Scholar] [CrossRef]
- Bell, P.T.; Shine, J.M. Subcortical contributions to large-scale network communication. Neurosci. Biobehav. Rev. 2016, 71, 313–322. [Google Scholar] [CrossRef] [Green Version]
- Goldberg, I.I.; Harel, M.; Malach, R. When the Brain Loses Its Self: Prefrontal Inactivation during Sensorimotor Processing. Neuron 2006, 50, 329–339. [Google Scholar] [CrossRef] [Green Version]
- Gollo, L.L.; Zalesky, A.; Hutchison, R.M.; van den Heuvel, M.; Breakspear, M. Dwelling quietly in the rich club: Brain network determinants of slow cortical fluctuations. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2015, 370, 20140165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hari, R.; Parkkonen, L. The brain timewise: How timing shapes and supports brain function. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2015, 370, 20140170. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, R.H.; Andrews-Hanna, J.R.; Wager, T.D.; Pizzagalli, D.A. Large-Scale Network Dysfunction in Major Depressive Disorder: A Meta-analysis of Resting-State Functional Connectivity. JAMA Psychiatry 2015, 72, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Friston, K. Structural and functional brain networks: From connections to cognition. Science 2013, 342, 1238411. [Google Scholar] [CrossRef] [Green Version]
- Santangelo, V. Large-Scale brain networks supporting divided attention across spatial locations and sensory modalities. Front. Integr. Neurosci. 2018, 12, 8. [Google Scholar] [CrossRef] [PubMed]
- Compton, R.J.; Carp, J.; Chaddock, L.; Fineman, S.L.; Quandt, L.C.; Ratliff, J.B. Trouble Crossing the Bridge: Altered Interhemispheric Communication of Emotional Images in Anxiety. Emotion 2008, 8, 684–692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Compton, R.J.; Feigenson, K.; Widick, P. Take it to the bridge: An interhemispheric processing advantage for emotional faces. Cogn. Brain Res. 2005, 24, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Hearne, L.J.; Dean, R.J.; Robinson, G.A.; Richards, L.J.; Mattingley, J.B.; Cocchi, L. Increased cognitive complexity reveals abnormal brain network activity in individuals with corpus callosum dysgenesis. Neuroimage Clin. 2019, 21, 101595. [Google Scholar] [CrossRef] [PubMed]
- Roland, J.L.; Snyder, A.Z.; Hacker, C.D.; Mitra, A.; Shimony, J.S.; Limbrick, D.D.; Raichle, M.E.; Smyth, M.D.; Leuthardt, E.C. On the role of the corpus callosum in interhemispheric functional connectivity in humans. Proc. Natl. Acad. Sci. USA 2017, 114, 13278–13283. [Google Scholar] [CrossRef] [Green Version]
- Skumlien, M.; Sederevicius, D.; Fjell, A.M.; Walhovd, K.B.; Westerhausen, R. Parallel but independent reduction of emotional awareness and corpus callosum connectivity in older age. PLoS ONE 2018, 13, e0209915. [Google Scholar] [CrossRef] [Green Version]
- Brauer, J.; Xiao, Y.; Poulain, T.; Friederici, A.D.; Schirmer, A. Frequency of Maternal Touch Predicts Resting Activity and Connectivity of the Developing Social Brain. Cereb. Cortex 2016, 26, 3544–3552. [Google Scholar] [CrossRef] [PubMed]
- Iggo, A. Cutaneous mechanoreceptors with afferent C fibres. J. Physiol. 1960, 152, 337–353. [Google Scholar] [CrossRef] [PubMed]
- Iggo, A.; Muir, A.R. The structure and function of a slowly adapting touch corpuscle in hairy skin. J. Physiol. 1969, 200, 763–796. [Google Scholar] [CrossRef] [PubMed]
- Olausson, H.; Lamarre, Y.; Backlund, H.; Morin, C.; Wallin, B.G.; Starck, G.; Ekholm, S.; Strigo, I.; Worsley, K.; Vallbo, Å.B.; et al. Unmyelinated tactile afferents signal touch and project to insular cortex. Nat. Neurosci. 2002, 5, 900–904. [Google Scholar] [CrossRef]
- Olausson, H.; Wessberg, J.; Morrison, I.; McGlone, F.; Vallbo, Å. The neurophysiology of unmyelinated tactile afferents. Neurosci. Biobehav. Rev. 2010, 34, 185–191. [Google Scholar] [CrossRef]
- Monroe, C.M. The effects of therapeutic touch on pain. J. Holist. Nurs. Off. J. Am. Holist. Nurses’ Assoc. 2009, 27, 85–92. [Google Scholar] [CrossRef]
- Birznieks, I.; Macefield, V.G.; Westling, G.; Johansson, R.S. Slowly Adapting Mechanoreceptors in the Borders of the Human Fingernail Encode Fingertip Forces. J. Neurosci. 2009, 29, 9370–9379. [Google Scholar] [CrossRef] [Green Version]
- Ebisch, S.J.; Salone, A.; Martinotti, G.; Carlucci, L.; Mantini, D.; Perrucci, M.G.; Saggino, A.; Romani, G.L.; Di Giannantonio, M.; Northoff, G.; et al. Integrative Processing of Touch and Affect in Social Perception: An fMRI Study. Front. Hum. Neurosci. 2016, 10, 209. [Google Scholar] [CrossRef] [Green Version]
- Grion, N.; Akrami, A.; Zuo, Y.; Stella, F.; Diamond, M.E. Coherence between Rat sensorimotor system and hippocampus is enhanced during tactile discrimination. PLoS Biol. 2016, 14, e1002384. [Google Scholar] [CrossRef] [Green Version]
- Macefield, V.G. Physiological characteristics of lowthreshold mechanoreceptors in joints, muscle and skin in human subjects. Clin. Exp. Pharmacol. Physiol. 2005, 32, 135–144. [Google Scholar] [CrossRef]
- Mountcastle, V.C. The Sensory Hand: Neural Mechanisms of Somatic Sensation; Harvard University Press: Harvard, MA, USA, 2005. [Google Scholar]
- Rushworth, M.F.S.; Paus, T.; Sipila, P.K. Attention systems and the organization of the human parietal cortex. NeuroImage 2001, 13, 353. [Google Scholar] [CrossRef]
- O’Brien, K.M.; Afzal, K.; Tronick, E. Relational psychophysiology and mutual regulation during dyadic therapeutic and developmental relating. In Interdisciplinary Handbook of the Person-Centered Approach; Cornelius-White, J., Motschnig-Pitrik, R., Lux, M., Eds.; Springer: New York, NY, USA, 2013; pp. 183–197. [Google Scholar]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blanchard, A.R.; Comfort, W.E. Keeping in Touch with Mental Health: The Orienting Reflex and Behavioral Outcomes from Calatonia. Brain Sci. 2020, 10, 182. https://doi.org/10.3390/brainsci10030182
Blanchard AR, Comfort WE. Keeping in Touch with Mental Health: The Orienting Reflex and Behavioral Outcomes from Calatonia. Brain Sciences. 2020; 10(3):182. https://doi.org/10.3390/brainsci10030182
Chicago/Turabian StyleBlanchard, Anita Ribeiro, and William Edgar Comfort. 2020. "Keeping in Touch with Mental Health: The Orienting Reflex and Behavioral Outcomes from Calatonia" Brain Sciences 10, no. 3: 182. https://doi.org/10.3390/brainsci10030182
APA StyleBlanchard, A. R., & Comfort, W. E. (2020). Keeping in Touch with Mental Health: The Orienting Reflex and Behavioral Outcomes from Calatonia. Brain Sciences, 10(3), 182. https://doi.org/10.3390/brainsci10030182



