Epileptiform Neuronal Discharges Impair Astrocyte Syncytial Isopotentiality in Acute Hippocampal Slices
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Preparation of Acute Hippocampal Slices and Freshly Dissociated Astrocytes
2.3. Electrophysiology
2.4. Imaging of Aldh1l1-eGFP Astrocytes and Analysis
2.5. Chemical Reagents
2.6. Data Analyses
3. Results
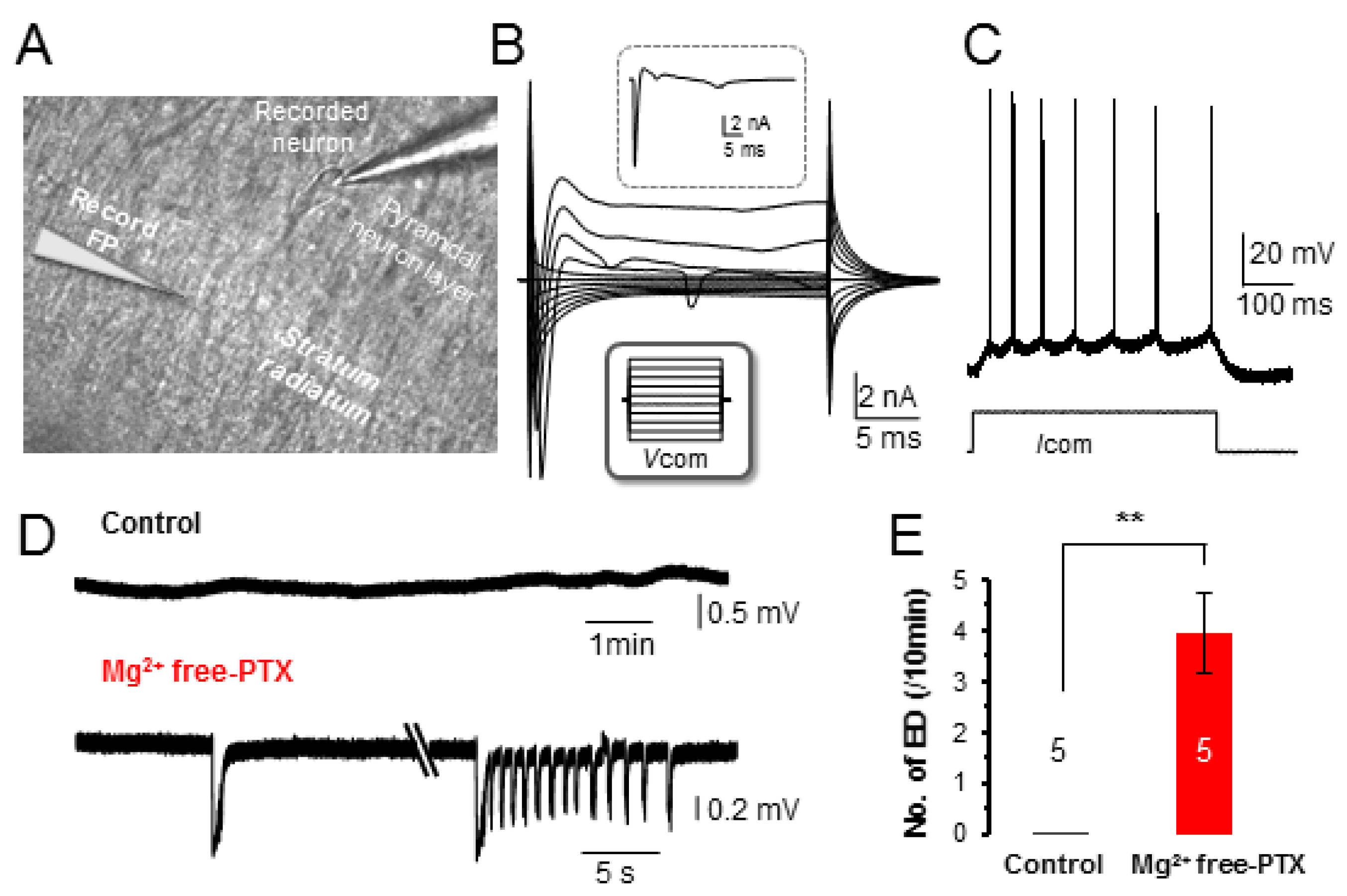
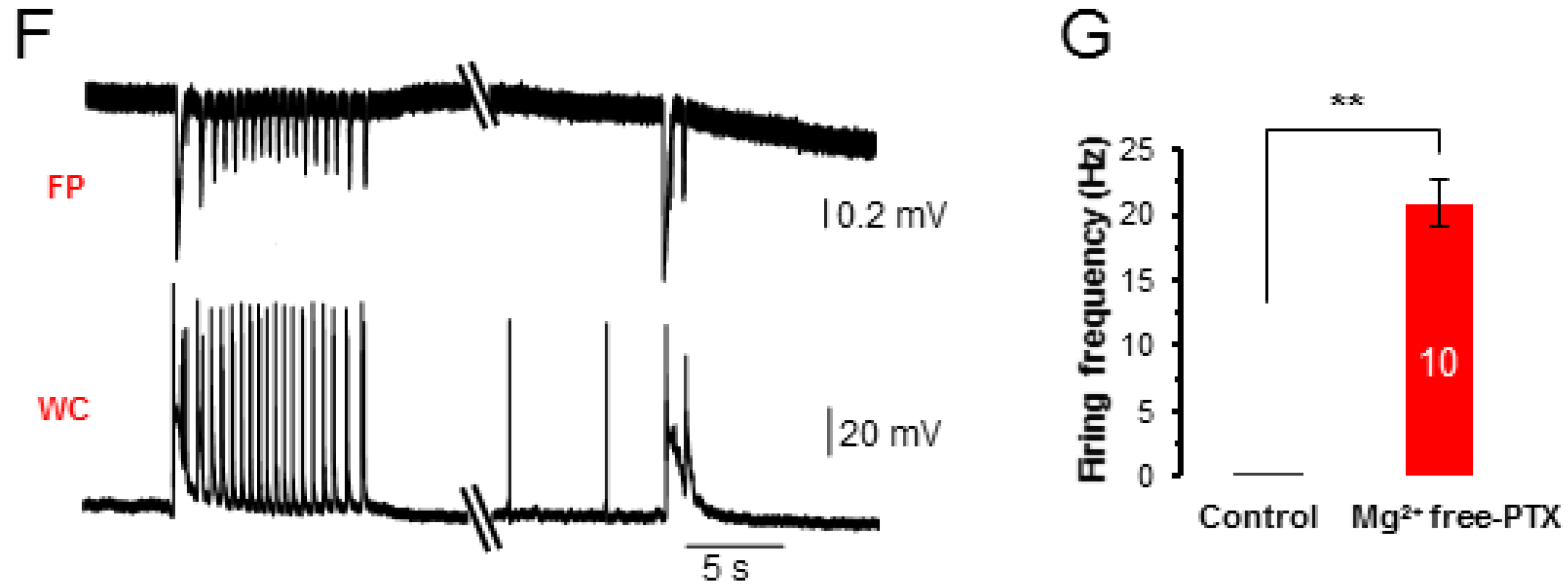
3.1. Induction of Neuronal Epileptiform Discharges in Hippocampal Slices
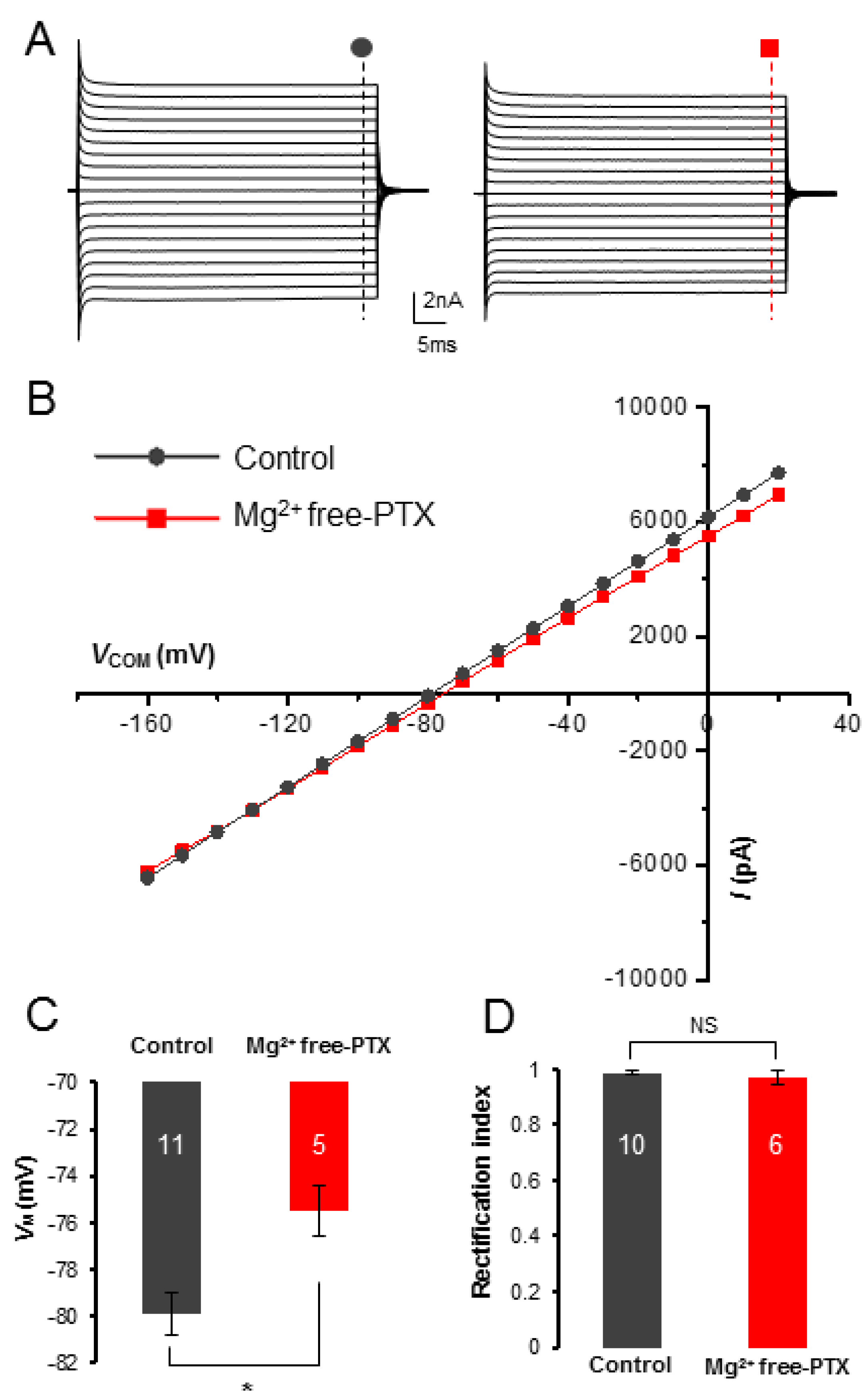
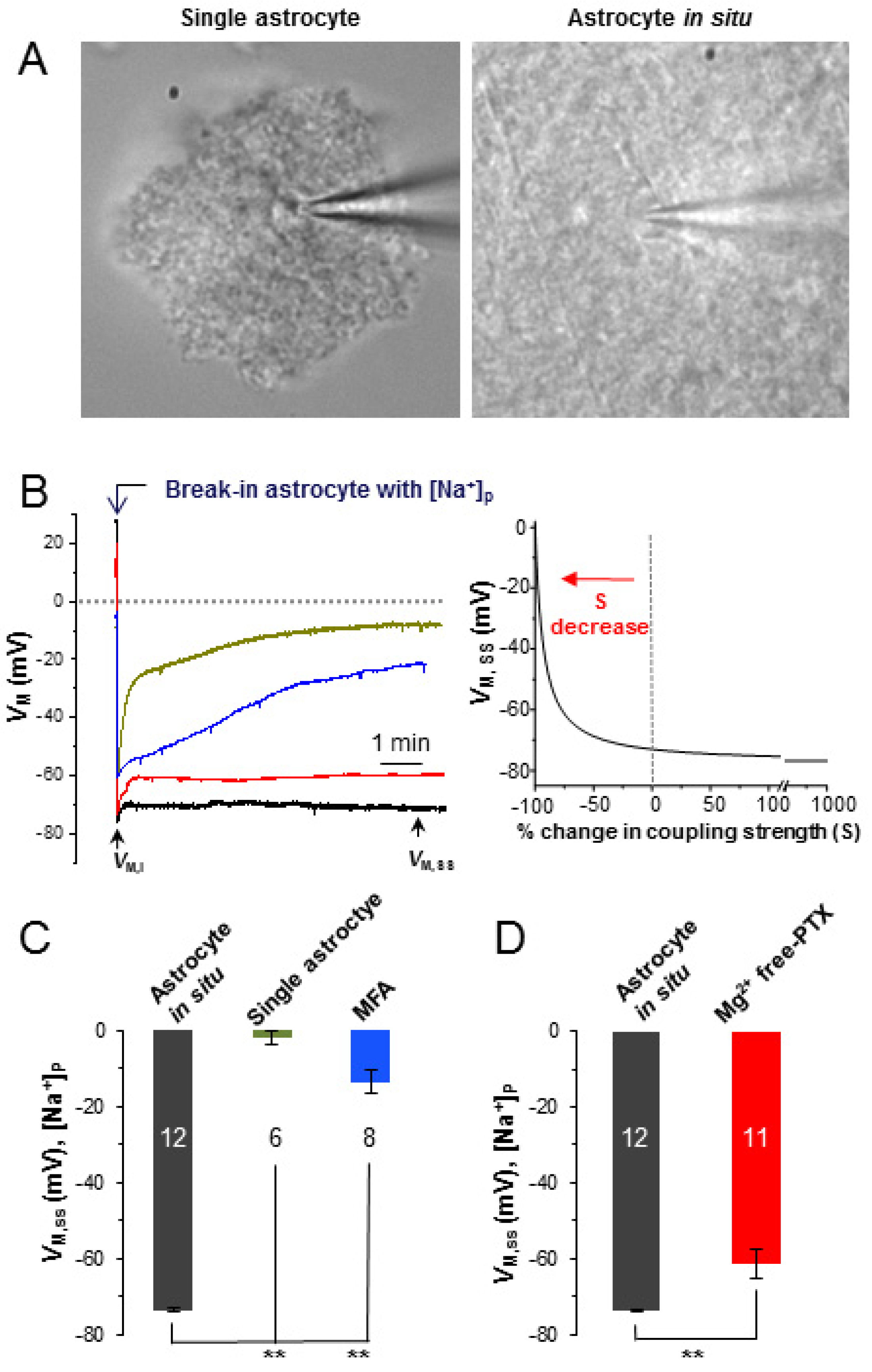
3.2. Mg2+ Free-PTX Condition Depolarizes Astrocyte VM without Altering of Passive Conductance
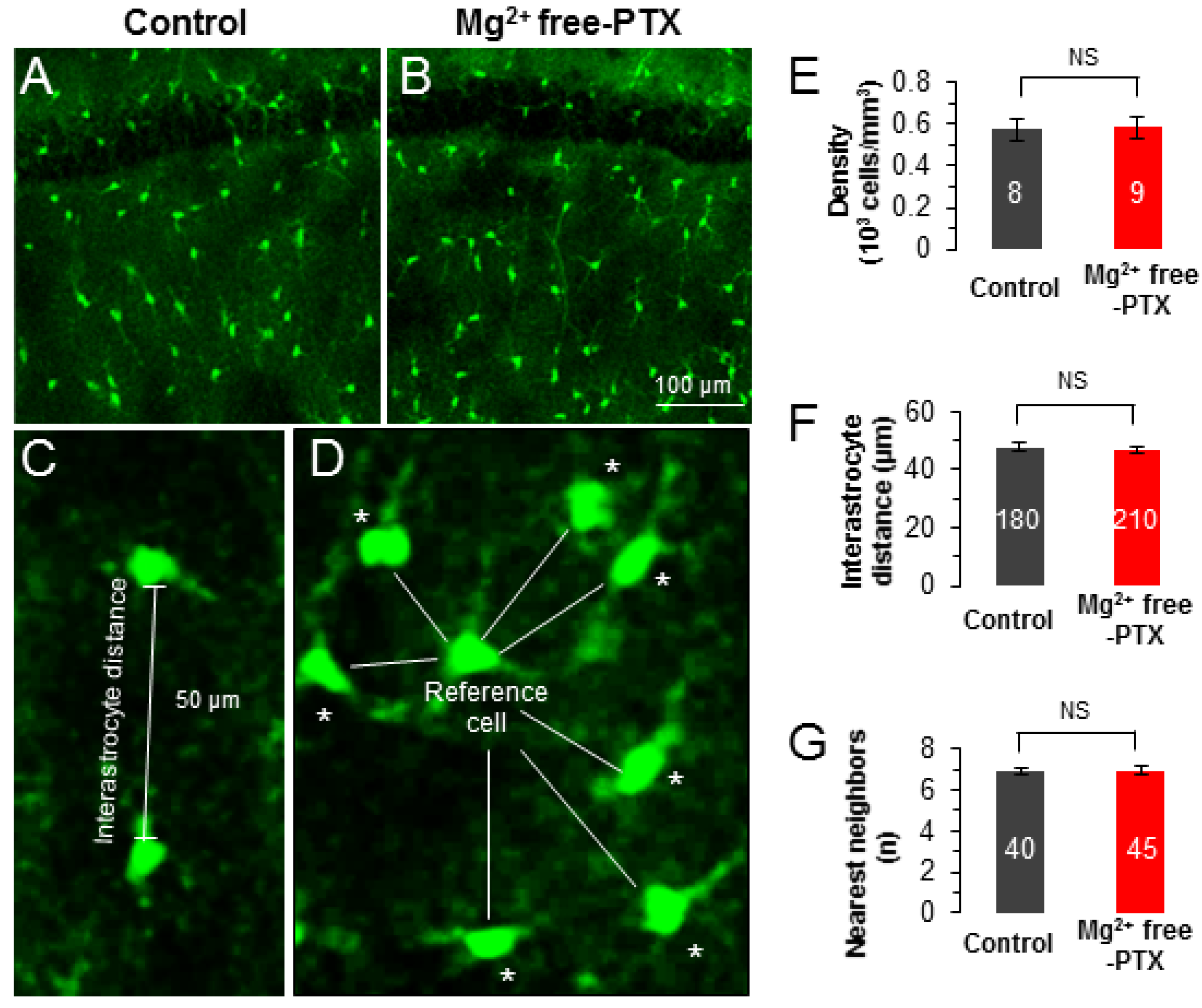
3.3. Epileptiform Neuronal Discharges Impair the Strength of Syncytial Isopotentiality
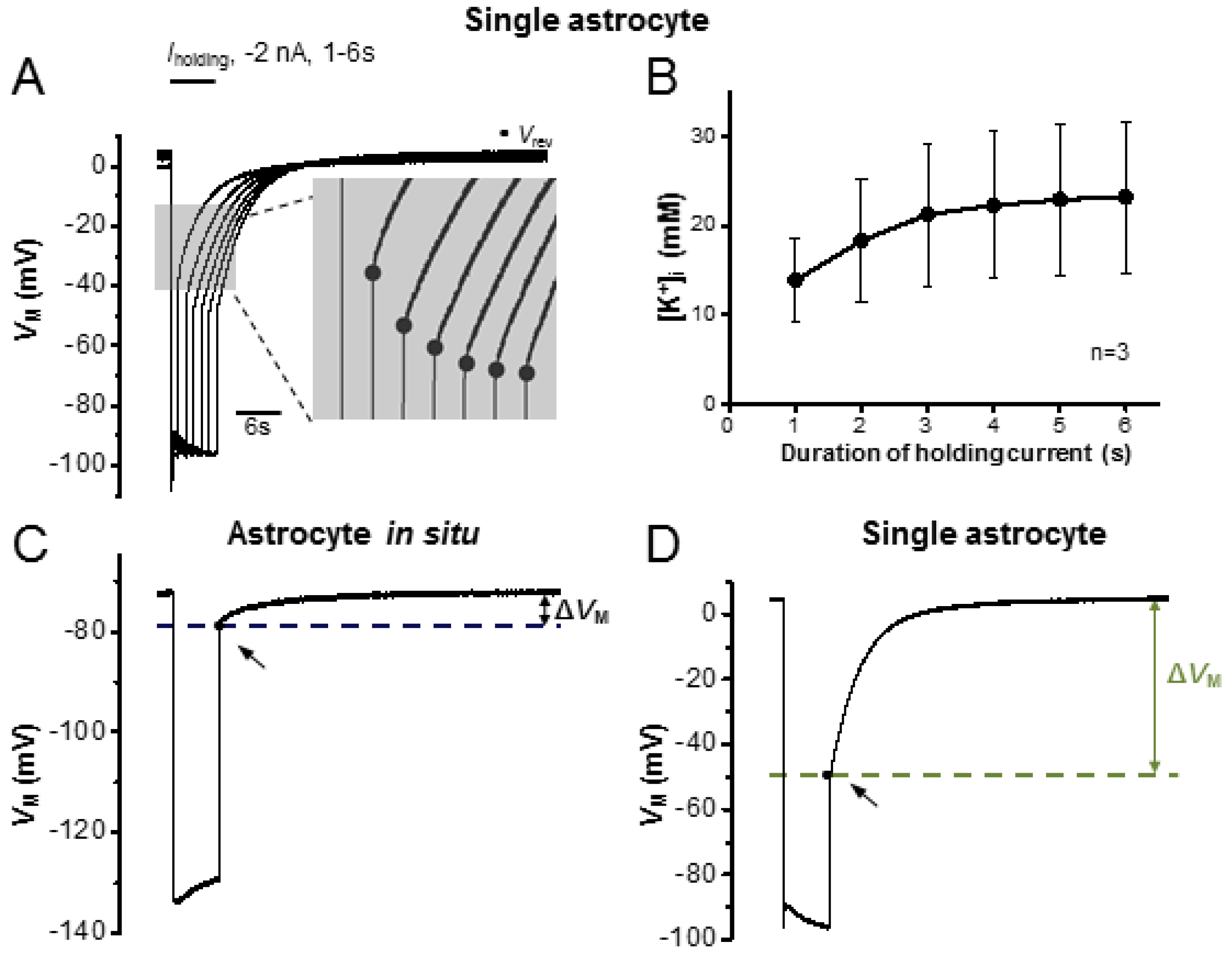
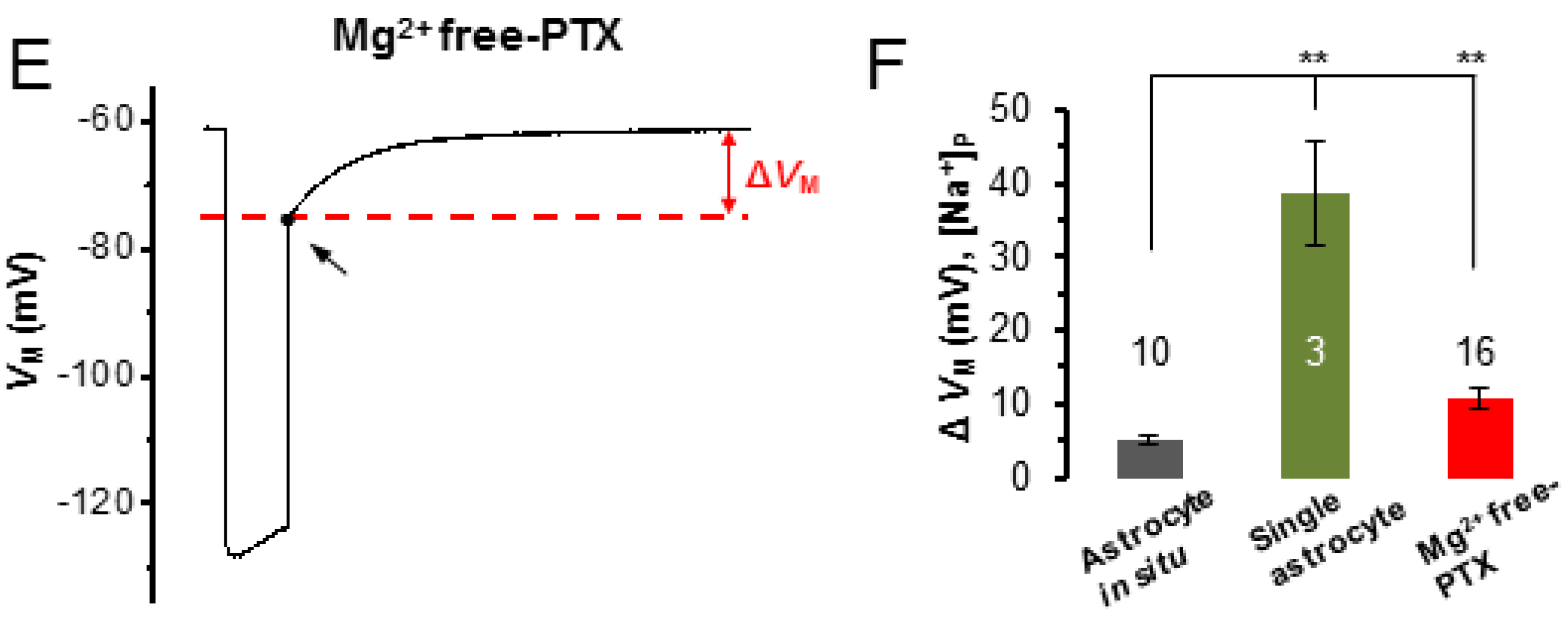
3.4. Epileptiform Discharges in Slices Impair the K+ Redistribution Capacity of an Astrocyte Syncytium
4. Discussion
4.1. Epileptiform Neuronal Discharges Impair Astrocyte Gap Junction Coupling
4.2. Epileptiform Discharges Impair the Redistribution Capacity of a Syncytium for K+ Ions
4.3. Acute vs Chronic Impact of Epileptiform Discharges on Astrocyte Syncytium
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Binmoller, F.J.; Muller, C.M. Postnatal development of dye-coupling among astrocytes in rat visual cortex. Glia 1992, 6, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Gutnick, M.J.; Connors, B.W.; Ransom, B.R. Dye-coupling between glial cells in the guinea pig neocortical slice. Brain Res. 1981, 213, 486–492. [Google Scholar] [CrossRef]
- Xu, G.; Wang, W.; Kimelberg, H.K.; Zhou, M. Electrical coupling of astrocytes in rat hippocampal slices under physiological and simulated ischemic conditions. Glia 2010, 58, 481–493. [Google Scholar] [CrossRef] [PubMed]
- Kuga, N.; Sasaki, T.; Takahara, Y.; Matsuki, N.; Ikegaya, Y. Large-scale calcium waves traveling through astrocytic networks in vivo. J. Neurosci. 2011, 31, 2607–2614. [Google Scholar] [CrossRef] [PubMed]
- Langer, J.; Stephan, J.; Theis, M.; Rose, C.R. Gap junctions mediate intercellular spread of sodium between hippocampal astrocytes in situ. Glia 2012, 60, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Rouach, N.; Koulakoff, A.; Abudara, V.; Willecke, K.; Giaume, C. Astroglial metabolic networks sustain hippocampal synaptic transmission. Science 2008, 322, 1551–1555. [Google Scholar] [CrossRef]
- Newman, E.A. Propagation of intercellular calcium waves in retinal astrocytes and Muller cells. J. Neurosci. 2001, 21, 2215–2223. [Google Scholar] [CrossRef]
- Bellot-Saez, A.; Cohen, G.; van Schaik, A.; Ooi, L.; Morley, J.W.; Buskila, Y. Astrocytic modulation of cortical oscillations. Sci. Rep. 2018, 8, 11565. [Google Scholar] [CrossRef]
- Lutz, S.E.; Zhao, Y.; Gulinello, M.; Lee, S.C.; Raine, C.S.; Brosnan, C.F. Deletion of astrocyte connexins 43 and 30 leads to a dysmyelinating phenotype and hippocampal CA1 vacuolation. J. Neurosci. 2009, 29, 7743–7752. [Google Scholar] [CrossRef]
- Pannasch, U.; Vargova, L.; Reingruber, J.; Ezan, P.; Holcman, D.; Giaume, C.; Sykova, E.; Rouach, N. Astroglial networks scale synaptic activity and plasticity. Proc. Natl. Acad. Sci. USA 2011, 108, 8467–8472. [Google Scholar] [CrossRef]
- Theis, M.; Jauch, R.; Zhuo, L.; Speidel, D.; Wallraff, A.; Doring, B.; Frisch, C.; Sohl, G.; Teubner, B.; Euwens, C.; et al. Accelerated hippocampal spreading depression and enhanced locomotory activity in mice with astrocyte-directed inactivation of connexin43. J. Neurosci. 2003, 23, 766–776. [Google Scholar] [CrossRef] [PubMed]
- Wallraff, A.; Kohling, R.; Heinemann, U.; Theis, M.; Willecke, K.; Steinhauser, C. The impact of astrocytic gap junctional coupling on potassium buffering in the hippocampus. J. Neurosci. 2006, 26, 5438–5447. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Buckalew, R.; Du, Y.; Kiyoshi, C.M.; Alford, C.C.; Wang, W.; McTigue, D.M.; Enyeart, J.J.; Terman, D.; Zhou, M. Gap junction coupling confers isopotentiality on astrocyte syncytium. Glia 2016, 64, 214–226. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Du, Y.; Kiyoshi, C.M.; Wu, X.; Askwith, C.C.; McTigue, D.M.; Zhou, M. Syncytial Isopotentiality: An Electrical Feature of Spinal Cord Astrocyte Networks. Neuroglia 2018, 1, 271–279. [Google Scholar] [CrossRef]
- Kiyoshi, C.M.; Du, Y.; Zhong, S.; Wang, W.; Taylor, A.T.; Xiong, B.; Ma, B.; Terman, D.; Zhou, M. Syncytial isopotentiality: A system-wide electrical feature of astrocytic networks in the brain. Glia 2018, 66, 2756–2769. [Google Scholar] [CrossRef]
- Kiyoshi, C.M.; Zhou, M. Astrocyte syncytium: A functional reticular system in the brain. Neural Regen. Res. 2019, 14, 595–596. [Google Scholar] [CrossRef]
- Samoilova, M.; Wentlandt, K.; Adamchik, Y.; Velumian, A.A.; Carlen, P.L. Connexin 43 mimetic peptides inhibit spontaneous epileptiform activity in organotypic hippocampal slice cultures. Exp. Neurol. 2008, 210, 762–775. [Google Scholar] [CrossRef]
- Steinhauser, C.; Seifert, G. Glial membrane channels and receptors in epilepsy: Impact for generation and spread of seizure activity. Eur. J. Pharm. 2002, 447, 227–237. [Google Scholar] [CrossRef]
- Fiacco, T.A.; Agulhon, C.; McCarthy, K.D. Sorting out astrocyte physiology from pharmacology. Annu. Rev. Pharmacol. Toxicol. 2009, 49, 151–174. [Google Scholar] [CrossRef]
- Halassa, M.M.; Fellin, T.; Haydon, P.G. Tripartite synapses: Roles for astrocytic purines in the control of synaptic physiology and behavior. Neuropharmacology 2009, 57, 343–346. [Google Scholar] [CrossRef]
- Bennett, M.V.; Barrio, L.C.; Bargiello, T.A.; Spray, D.C.; Hertzberg, E.; Saez, J.C. Gap junctions: New tools, new answers, new questions. Neuron 1991, 6, 305–320. [Google Scholar] [CrossRef]
- Christ, G.J.; Moreno, A.P.; Melman, A.; Spray, D.C. Gap junction-mediated intercellular diffusion of Ca2+ in cultured human corporal smooth muscle cells. Am. J. Physiol. 1992, 263, C373–C383. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Kiyoshi, C.M.; Terman, D.; Zhou, M. Analysis of the functional states of an astrocyte syncytium. In Basic Neurobiology Techniques; Wright, N., Ed.; Humana: New York, NY, USA, 2020; Volume 152, pp. 285–313. [Google Scholar]
- Yang, Y.; Vidensky, S.; Jin, L.; Jie, C.; Lorenzini, I.; Frankl, M.; Rothstein, J.D. Molecular comparison of GLT1+ and ALDH1L1+ astrocytes in vivo in astroglial reporter mice. Glia 2011, 59, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.; Du, Y.; Kiyoshi, C.M.; Ma, B.; Alford, C.C.; Wang, Q.; Yang, Y.; Liu, X.; Zhou, M. Electrophysiological behavior of neonatal astrocytes in hippocampal stratum radiatum. Mol. Brain 2016, 9, 34. [Google Scholar] [CrossRef]
- Ma, B.F.; Xie, M.J.; Zhou, M. Bicarbonate efflux via GABA(A) receptors depolarizes membrane potential and inhibits two-pore domain potassium channels of astrocytes in rat hippocampal slices. Glia 2012, 60, 1761–1772. [Google Scholar] [CrossRef]
- Nimmerjahn, A.; Kirchhoff, F.; Kerr, J.N.; Helmchen, F. Sulforhodamine 101 as a specific marker of astroglia in the neocortex in vivo. Nat. Methods 2004, 1, 31–37. [Google Scholar] [CrossRef]
- Du, Y.; Ma, B.; Kiyoshi, C.M.; Alford, C.C.; Wang, W.; Zhou, M. Freshly dissociated mature hippocampal astrocytes exhibit passive membrane conductance and low membrane resistance similarly to syncytial coupled astrocytes. J. Neurophysiol. 2015, 113, 3744–3750. [Google Scholar] [CrossRef]
- Du, Y.; Wang, W.; Lutton, A.D.; Kiyoshi, C.M.; Ma, B.; Taylor, A.T.; Olesik, J.W.; McTigue, D.M.; Askwith, C.C.; Zhou, M. Dissipation of transmembrane potassium gradient is the main cause of cerebral ischemia-induced depolarization in astrocytes and neurons. Exp. Neurol. 2018, 303, 1–11. [Google Scholar] [CrossRef]
- Ma, B.; Xu, G.; Wang, W.; Enyeart, J.J.; Zhou, M. Dual patch voltage clamp study of low membrane resistance astrocytes in situ. Mol. Brain 2014, 7, 18. [Google Scholar] [CrossRef]
- Zhang, H.; Xie, M.; Schools, G.P.; Feustel, P.F.; Wang, W.; Lei, T.; Kimelberg, H.K.; Zhou, M. Tamoxifen mediated estrogen receptor activation protects against early impairment of hippocampal neuron excitability in an oxygen/glucose deprivation brain slice ischemia model. Brain Res. 2009, 1247, 196–211. [Google Scholar] [CrossRef]
- Wang, W.; Kiyoshi, C.M.; Du, Y.; Taylor, A.T.; Sheehan, E.R.; Wu, X.; Zhou, M. TREK-1 Null Impairs Neuronal Excitability, Synaptic Plasticity, and Cognitive Function. Mol. Neurobiol. 2020, 57, 1332–1346. [Google Scholar] [CrossRef] [PubMed]
- Chever, O.; Dossi, E.; Pannasch, U.; Derangeon, M.; Rouach, N. Astroglial networks promote neuronal coordination. Sci. Signal. 2016, 9, ra6. [Google Scholar] [CrossRef] [PubMed]
- Baylor, D.A.; Nicholls, J.G. Changes in extracellular potassium concentration produced by neuronal activity in the central nervous system of the leech. J. Physiol. 1969, 203, 555–569. [Google Scholar] [CrossRef] [PubMed]
- Heinemann, U.; Lux, H.D. Ceiling of stimulus induced rises in extracellular potassium concentration in the cerebral cortex of cat. Brain Res. 1977, 120, 231–249. [Google Scholar] [CrossRef]
- Frankenhaeuser, B.; Hodgkin, A.L. The after-effects of impulses in the giant nerve fibres of Loligo. J. Physiol. 1956, 131, 341–376. [Google Scholar] [CrossRef]
- Zhou, M.; Kimelberg, H.K. Freshly isolated astrocytes from rat hippocampus show two distinct current patterns and different [K(+)](o) uptake capabilities. J. Neurophysiol. 2000, 84, 2746–2757. [Google Scholar] [CrossRef][Green Version]
- Du, Y.; Kiyoshi, C.M.; Wang, Q.; Wang, W.; Ma, B.; Alford, C.C.; Zhong, S.; Wan, Q.; Chen, H.; Lloyd, E.E.; et al. Genetic Deletion of TREK-1 or TWIK-1/TREK-1 Potassium Channels does not Alter the Basic Electrophysiological Properties of Mature Hippocampal Astrocytes In Situ. Front. Cell. Neurosci. 2016, 10, 13. [Google Scholar] [CrossRef]
- Wang, W.; Kiyoshi, C.M.; Du, Y.; Ma, B.; Alford, C.C.; Chen, H.; Zhou, M. mGluR3 Activation Recruits Cytoplasmic TWIK-1 Channels to Membrane that Enhances Ammonium Uptake in Hippocampal Astrocytes. Mol. Neurobiol. 2016, 53, 6169–6182. [Google Scholar] [CrossRef]
- Xu, G.; Wang, W.; Zhou, M. Spatial organization of NG2 glial cells and astrocytes in rat hippocampal CA1 region. Hippocampus 2014, 24, 383–395. [Google Scholar] [CrossRef]
- Orkand, R.K.; Nicholls, J.G.; Kuffler, S.W. Effect of nerve impulses on the membrane potential of glial cells in the central nervous system of amphibia. J. Neurophysiol. 1966, 29, 788–806. [Google Scholar] [CrossRef]
- Kofuji, P.; Newman, E.A. Potassium buffering in the central nervous system. Neuroscience 2004, 129, 1045–1056. [Google Scholar] [CrossRef] [PubMed]
- Giaume, C.; Koulakoff, A.; Roux, L.; Holcman, D.; Rouach, N. Astroglial networks: A step further in neuroglial and gliovascular interactions. Nat. Rev. Neurosci. 2010, 11, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Steinhauser, C.; Seifert, G.; Bedner, P. Astrocyte dysfunction in temporal lobe epilepsy: K+ channels and gap junction coupling. Glia 2012, 60, 1192–1202. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zeng, L.H.; Wong, M. Impaired astrocytic gap junction coupling and potassium buffering in a mouse model of tuberous sclerosis complex. Neurobiol. Dis. 2009, 34, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, D.K.; Vargas, J.R.; Wilcox, K.S. Increased coupling and altered glutamate transport currents in astrocytes following kainic-acid-induced status epilepticus. Neurobiol. Dis. 2010, 40, 573–585. [Google Scholar] [CrossRef] [PubMed]
- Samoilova, M.; Li, J.; Pelletier, M.R.; Wentlandt, K.; Adamchik, Y.; Naus, C.C.; Carlen, P.L. Epileptiform activity in hippocampal slice cultures exposed chronically to bicuculline: Increased gap junctional function and expression. J. Neurochem. 2003, 86, 687–699. [Google Scholar] [CrossRef]
- Mishima, T.; Hirase, H. In vivo intracellular recording suggests that gray matter astrocytes in mature cerebral cortex and hippocampus are electrophysiologically homogeneous. J. Neurosci. 2010, 30, 3093–3100. [Google Scholar] [CrossRef]
- Amzica, F.; Massimini, M. Glial and neuronal interactions during slow wave and paroxysmal activities in the neocortex. Cereb. Cortex 2002, 12, 1101–1113. [Google Scholar] [CrossRef]
- Amzica, F.; Massimini, M.; Manfridi, A. Spatial buffering during slow and paroxysmal sleep oscillations in cortical networks of glial cells in vivo. J. Neurosci. 2002, 22, 1042–1053. [Google Scholar] [CrossRef]
- McKhann, G.M., 2nd; Schoenfeld-McNeill, J.; Born, D.E.; Haglund, M.M.; Ojemann, G.A. Intraoperative hippocampal electrocorticography to predict the extent of hippocampal resection in temporal lobe epilepsy surgery. J. Neurosurg. 2000, 93, 44–52. [Google Scholar] [CrossRef]
- Oberheim, N.A.; Tian, G.F.; Han, X.; Peng, W.; Takano, T.; Ransom, B.; Nedergaard, M. Loss of astrocytic domain organization in the epileptic brain. J. Neurosci. 2008, 28, 3264–3276. [Google Scholar] [CrossRef] [PubMed]
- Bordey, A.; Lyons, S.A.; Hablitz, J.J.; Sontheimer, H. Electrophysiological characteristics of reactive astrocytes in experimental cortical dysplasia. J. Neurophysiol. 2001, 85, 1719–1731. [Google Scholar] [CrossRef] [PubMed]
- Hille, B. Ion Channels of Excitable Cells; Sinauer: Sunderland, MA, USA, 2001. [Google Scholar]
- Nimmerjahn, A.; Mukamel, E.A.; Schnitzer, M.J. Motor behavior activates Bergmann glial networks. Neuron 2009, 62, 400–412. [Google Scholar] [CrossRef] [PubMed]
- Panatier, A.; Vallee, J.; Haber, M.; Murai, K.K.; Lacaille, J.C.; Robitaille, R. Astrocytes are endogenous regulators of basal transmission at central synapses. Cell 2011, 146, 785–798. [Google Scholar] [CrossRef] [PubMed]
- Schummers, J.; Yu, H.; Sur, M. Tuned responses of astrocytes and their influence on hemodynamic signals in the visual cortex. Science 2008, 320, 1638–1643. [Google Scholar] [CrossRef]
- Wang, N.; De Bock, M.; Antoons, G.; Gadicherla, A.K.; Bol, M.; Decrock, E.; Evans, W.H.; Sipido, K.R.; Bukauskas, F.F.; Leybaert, L. Connexin mimetic peptides inhibit Cx43 hemichannel opening triggered by voltage and intracellular Ca2+ elevation. Basic Res. Cardiol. 2012, 107, 304. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Wang, W.; Aten, S.; Kiyoshi, C.M.; Du, Y.; Zhou, M. Epileptiform Neuronal Discharges Impair Astrocyte Syncytial Isopotentiality in Acute Hippocampal Slices. Brain Sci. 2020, 10, 208. https://doi.org/10.3390/brainsci10040208
Wang Q, Wang W, Aten S, Kiyoshi CM, Du Y, Zhou M. Epileptiform Neuronal Discharges Impair Astrocyte Syncytial Isopotentiality in Acute Hippocampal Slices. Brain Sciences. 2020; 10(4):208. https://doi.org/10.3390/brainsci10040208
Chicago/Turabian StyleWang, Qi, Wei Wang, Sydney Aten, Conrad M. Kiyoshi, Yixing Du, and Min Zhou. 2020. "Epileptiform Neuronal Discharges Impair Astrocyte Syncytial Isopotentiality in Acute Hippocampal Slices" Brain Sciences 10, no. 4: 208. https://doi.org/10.3390/brainsci10040208
APA StyleWang, Q., Wang, W., Aten, S., Kiyoshi, C. M., Du, Y., & Zhou, M. (2020). Epileptiform Neuronal Discharges Impair Astrocyte Syncytial Isopotentiality in Acute Hippocampal Slices. Brain Sciences, 10(4), 208. https://doi.org/10.3390/brainsci10040208





