Ex Vivo MRI Analytical Methods and Brain Pathology in Preterm Lambs Treated with Postnatal Dexamethasone †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Studies
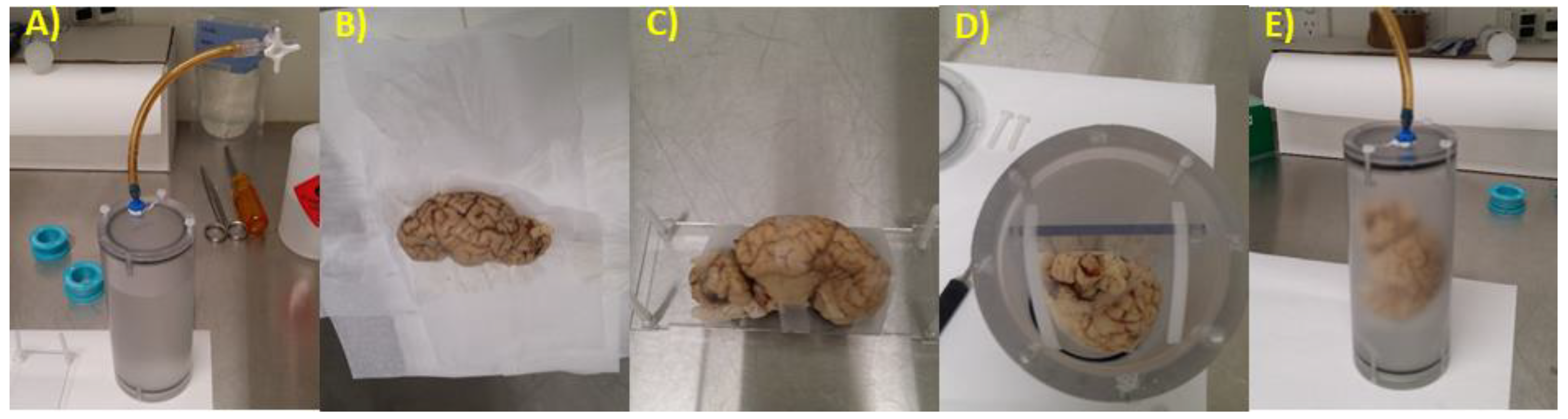
2.2. Brain Preparation for MRI
2.3. Image Sequence Development
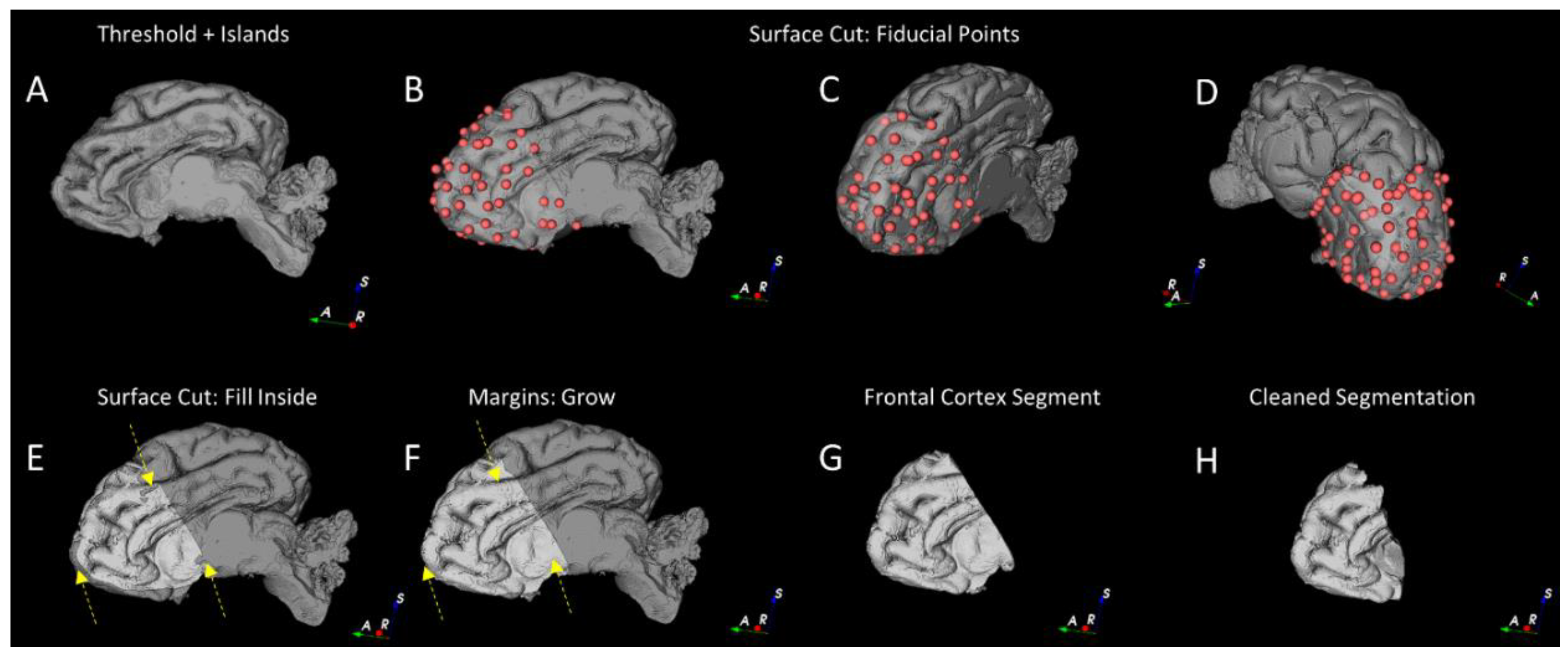
2.4. Grey and White Matter Tissue Segmentation
2.4.1. Pre-Processing
2.4.2. Segmentation of the Cortical Structures and Hippocampus
2.4.3. Segmentation of the White and Grey Matter
2.4.4. Gross Anatomical Measurements
2.5. Histology
2.6. Immunohistochemistry
2.7. Data Analysis
3. Results
3.1. Clinical Variables
3.2. Magnetic Resonance Imaging
3.2.1. Volumetric measures
3.2.2. Anatomical Measures
3.3. Neuropathology
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Barton, S.K.; Tolcos, M.; Miller, S.L.; Christoph-Roehr, C.; Schmolzer, G.M.; Moss, T.J.; Hooper, S.B.; Wallace, E.M.; Polglase, G.R. Ventilation-Induced Brain Injury in Preterm Neonates: A Review of Potential Therapies. Neonatology 2016, 110, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Onland, W.; Offringa, M.; De Jaegere, A.P.; van Kaam, A.H. Finding the optimal postnatal dexamethasone regimen for preterm infants at risk of bronchopulmonary dysplasia: A systematic review of placebo-controlled trials. Pediatrics 2009, 123, 367–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doyle, L.W.; Cheong, J.L.; Ehrenkranz, R.A.; Halliday, H.L. Early (<8 days) systemic postnatal corticosteroids for prevention of bronchopulmonary dysplasia in preterm infants. Cochrane Database Syst. Rev. 2017, 10, CD001146. [Google Scholar] [CrossRef] [PubMed]
- Parikh, N.A.; Lasky, R.E.; Kennedy, K.A.; Moya, F.R.; Hochhauser, L.; Romo, S.; Tyson, J.E. Postnatal dexamethasone therapy and cerebral tissue volumes in extremely low birth weight infants. Pediatrics 2007, 119, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Murphy, B.P.; Inder, T.E.; Huppi, P.S.; Warfield, S.; Zientara, G.P.; Kikinis, R.; Jolesz, F.A.; Volpe, J.J. Impaired cerebral cortical gray matter growth after treatment with dexamethasone for neonatal chronic lung disease. Pediatrics 2001, 107, 217–221. [Google Scholar] [CrossRef]
- Thompson, D.K.; Wood, S.J.; Doyle, L.W.; Warfield, S.K.; Lodygensky, G.A.; Anderson, P.J.; Egan, G.F.; Inder, T.E. Neonate hippocampal volumes: Prematurity, perinatal predictors, and 2-year outcome. Ann. Neurol. 2008, 63, 642–651. [Google Scholar] [CrossRef]
- Cheong, J.L.; Burnett, A.C.; Lee, K.J.; Roberts, G.; Thompson, D.K.; Wood, S.J.; Connelly, A.; Anderson, P.J.; Doyle, L.W.; Victorian Infant Collaborative Study Group. Association between postnatal dexamethasone for treatment of bronchopulmonary dysplasia and brain volumes at adolescence in infants born very preterm. J. Pediatr. 2014, 164, 737–743.e731. [Google Scholar] [CrossRef]
- Baud, O.; Foix-L’Helias, L.; Kaminski, M.; Audibert, F.; Jarreau, P.H.; Papiernik, E.; Huon, C.; Lepercq, J.; Dehan, M.; Lacaze-Masmonteil, T. Antenatal glucocorticoid treatment and cystic periventricular leukomalacia in very premature infants. N. Engl. J. Med. 1999, 341, 1190–1196. [Google Scholar] [CrossRef]
- Shinwell, E.S.; Karplus, M.; Reich, D.; Weintraub, Z.; Blazer, S.; Bader, D.; Yurman, S.; Dolfin, T.; Kogan, A.; Dollberg, S.; et al. Early postnatal dexamethasone treatment and increased incidence of cerebral palsy. Arch. Dis. Child. Fetal Neonatal Ed. 2000, 83, F177–F181. [Google Scholar] [CrossRef] [Green Version]
- Tam, E.W.; Chau, V.; Ferriero, D.M.; Barkovich, A.J.; Poskitt, K.J.; Studholme, C.; Fok, E.D.; Grunau, R.E.; Glidden, D.V.; Miller, S.P. Preterm cerebellar growth impairment after postnatal exposure to glucocorticoids. Sci. Transl. Med. 2011, 3, 105ra105. [Google Scholar] [CrossRef] [Green Version]
- Doyle, L.W.; Halliday, H.L.; Ehrenkranz, R.A.; Davis, P.G.; Sinclair, J.C. Impact of postnatal systemic corticosteroids on mortality and cerebral palsy in preterm infants: Effect modification by risk for chronic lung disease. Pediatrics 2005, 115, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Doyle, L.W.; Halliday, H.L.; Ehrenkranz, R.A.; Davis, P.G.; Sinclair, J.C. An update on the impact of postnatal systemic corticosteroids on mortality and cerebral palsy in preterm infants: Effect modification by risk of bronchopulmonary dysplasia. J. Pediatr. 2014, 165, 1258–1260. [Google Scholar] [CrossRef] [PubMed]
- Doyle, L.W.; Davis, P.G.; Morley, C.J.; McPhee, A.; Carlin, J.B.; Investigators, D.S. Low-dose dexamethasone facilitates extubation among chronically ventilator-dependent infants: A multicenter, international, randomized, controlled trial. Pediatrics 2006, 117, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Doyle, L.W.; Davis, P.G.; Morley, C.J.; McPhee, A.; Carlin, J.B.; Investigators, D.S. Outcome at 2 years of age of infants from the DART study: A multicenter, international, randomized, controlled trial of low-dose dexamethasone. Pediatrics 2007, 119, 716–721. [Google Scholar] [CrossRef] [Green Version]
- De Guzman, A.E.; Wong, M.D.; Gleave, J.A.; Nieman, B.J. Variations in post-perfusion immersion fixation and storage alter MRI measurements of mouse brain morphometry. NeuroImage 2016, 142, 687–695. [Google Scholar] [CrossRef]
- Yushkevich, P.A.; Piven, J.; Hazlett, H.C.; Smith, R.G.; Ho, S.; Gee, J.C.; Gerig, G. User-guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. Neuroimage 2006, 31, 1116–1128. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Brady, M.; Smith, S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. Ieee Trans. Med. Imaging 2001, 20, 45–57. [Google Scholar] [CrossRef]
- Smith, S.M.; Jenkinson, M.; Woolrich, M.W.; Beckmann, C.F.; Behrens, T.E.; Johansen-Berg, H.; Bannister, P.R.; De Luca, M.; Drobnjak, I.; Flitney, D.E.; et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 2004, 23 (Suppl. 1), S208–S219. [Google Scholar] [CrossRef] [Green Version]
- Tustison, N.J.; Avants, B.B.; Cook, P.A.; Zheng, Y.; Egan, A.; Yushkevich, P.A.; Gee, J.C. N4ITK: Improved N3 bias correction. IEEE Trans. Med. Imaging 2010, 29, 1310–1320. [Google Scholar] [CrossRef] [Green Version]
- Fedorov, A.; Beichel, R.; Kalpathy-Cramer, J.; Finet, J.; Fillion-Robin, J.-C.; Pujol, S.; Bauer, C.; Jennings, D.; Fennessy, F.; Sonka, M. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn. Reson. Imaging 2012, 30, 1323–1341. [Google Scholar] [CrossRef] [Green Version]
- Coupé, P.; Manjon, J.V. MRI Denoising Software. Available online: https://sites.google.com/site/pierrickcoupe/softwares/denoising-for-medical-imaging/mri-denoising/mri-denoising-software (accessed on 28 October 2019).
- Manjón, J.V.; Coupé, P.; Buades, A.; Collins, D.L.; Robles, M. New methods for MRI denoising based on sparseness and self-similarity. Med. Image Anal. 2012, 16, 18–27. [Google Scholar]
- Fischl, B. FreeSurfer. Neuroimage 2012, 62, 774–781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ella, A.; Delgadillo, J.A.; Chemineau, P.; Keller, M. Computation of a high-resolution MRI 3D stereotaxic atlas of the sheep brain. J. Comp. Neurol. 2017, 525, 676–692. [Google Scholar] [CrossRef] [PubMed]
- Ella, A.; Keller, M. Construction of an MRI 3D high resolution sheep brain template. Magn. Reson. Imaging 2015, 33, 1329–1337. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.I.; Sudheimer, K.D.; Davis, K.K.; Kerndt, G.M.; Winn, B.M. The Sheep Brain Atlas. Available online: https://msu.edu/~brains/brains/sheep/index.html (accessed on 13 March 2017).
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Berg, S.; Kutra, D.; Kroeger, T.; Straehle, C.N.; Kausler, B.X.; Haubold, C.; Schiegg, M.; Ales, J.; Beier, T.; Rudy, M.; et al. ilastik: interactive machine learning for (bio)image analysis. Nat. Methods 2019, 16, 1226–1232. [Google Scholar] [CrossRef]
- Tournier, J.-D.; Smith, R.; Raffelt, D.; Tabbara, R.; Dhollander, T.; Pietsch, M.; Christiaens, D.; Jeurissen, B.; Yeh, C.-H.; Connelly, A. MRtrix3: A fast, flexible and open software framework for medical image processing and visualisation. Neuroimage 2019, 202, 116137. [Google Scholar] [CrossRef]
- Skiold, B.; Wu, Q.; Hooper, S.B.; Davis, P.G.; McIntyre, R.; Tolcos, M.; Pearson, J.; Vreys, R.; Egan, G.F.; Barton, S.K.; et al. Early detection of ventilation-induced brain injury using magnetic resonance spectroscopy and diffusion tensor imaging: An in vivo study in preterm lambs. PLoS ONE 2014, 9, e95804. [Google Scholar] [CrossRef]
- Devi, C.N.; Chandrasekharan, A.; Sundararaman, V.K.; Alex, Z.C. Neonatal brain MRI segmentation: A review. Comput. Biol. Med. 2015, 64, 163–178. [Google Scholar] [CrossRef]
- Dawe, R.J.; Bennett, D.A.; Schneider, J.A.; Vasireddi, S.K.; Arfanakis, K. Postmortem MRI of human brain hemispheres: T2 relaxation times during formaldehyde fixation. Magn. Reson. Med. 2009, 61, 810–818. [Google Scholar] [CrossRef] [Green Version]
- Sun, S.W.; Neil, J.J.; Liang, H.F.; He, Y.Y.; Schmidt, R.E.; Hsu, C.Y.; Song, S.K. Formalin fixation alters water diffusion coefficient magnitude but not anisotropy in infarcted brain. Magn. Reson. Med. 2005, 53, 1447–1451. [Google Scholar] [CrossRef] [PubMed]
- Jefferies, A.L. Postnatal corticosteroids to treat or prevent chronic lung disease in preterm infants. Paediatr. Child Health 2012, 17, 573–574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doyle, L.W.; Cheong, J.L.; Ehrenkranz, R.A.; Halliday, H.L. Late (>7 days) systemic postnatal corticosteroids for prevention of bronchopulmonary dysplasia in preterm infants. Cochrane Database Syst. Rev. 2017, 10, CD001145. [Google Scholar] [CrossRef] [PubMed]
- Riddle, A.; Dean, J.; Buser, J.R.; Gong, X.; Maire, J.; Chen, K.; Ahmad, T.; Cai, V.; Nguyen, T.; Kroenke, C.D.; et al. Histopathological correlates of magnetic resonance imaging-defined chronic perinatal white matter injury. Ann. Neurol. 2011, 70, 493–507. [Google Scholar] [CrossRef] [PubMed]
- Yager, J.Y.; Ashwal, S. Animal models of perinatal hypoxic-ischemic brain damage. Pediatr. Neurol. 2009, 40, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Back, S.A.; Riddle, A.; Dean, J.; Hohimer, A.R. The Instrumented Fetal Sheep as a Model of Cerebral White Matter Injury in the Premature Infant. Neurotherapeutics 2012, 9, 359–370. [Google Scholar] [CrossRef] [Green Version]
- Polglase, G.R.; Miller, S.L.; Barton, S.K.; Baburamani, A.A.; Wong, F.Y.; Aridas, J.D.; Gill, A.W.; Moss, T.J.; Tolcos, M.; Kluckow, M.; et al. Initiation of resuscitation with high tidal volumes causes cerebral hemodynamic disturbance, brain inflammation and injury in preterm lambs. PLoS ONE 2012, 7, e39535. [Google Scholar] [CrossRef] [Green Version]
- Polglase, G.R.; Nitsos, I.; Baburamani, A.A.; Crossley, K.J.; Slater, M.K.; Gill, A.W.; Allison, B.J.; Moss, T.J.; Pillow, J.J.; Hooper, S.B.; et al. Inflammation in utero exacerbates ventilation-induced brain injury in preterm lambs. J. Appl. Physiol. (1985) 2012, 112, 481–489. [Google Scholar] [CrossRef] [Green Version]






| Fetal Group | Postnatal Groups | |||
|---|---|---|---|---|
| Naïve Control (n = 7) | Saline (n = 8) | Low-Dex (n = 9) | High-Dex (n = 8) | |
| Sex (female/male) | 3/4 | 5/3 | 2/7 | 3/5 |
| Birth weight (kg) | 3.99 ± 0.44 *** | 2.94 ± 0.41 | 3.23 ± 0.40 | 2.99 ± 0.34 |
| Post-mortem weight (kg) | 3.99 ± 0.44 **** | 2.84 ± 0.37 | 2.75 ± 0.29 | 2.69 ± 0.44 |
| Post-mortem brain weight (g) | 54.2 ± 6.85 * | 47.4 ± 4.51 | 48.3 ± 4.04 | 47.5 ± 4.94 |
| Proportion of time on mechanical ventilation (%) | NA | 15.5 ± 12.53 | 16.6 ± 16.66 | 31.8 ± 29.60 |
| MRI Region | Effect of Prematurity | Effect of Postnatal Dexamethasone | |
|---|---|---|---|
| Frontal Cortex | Total | a t(13) = 1.071, p = 0.304 | d H(2) = 0.924, p = 0.630 |
| white matter | a t(13) = 1.198, p = 0.252 | d H(2) = 1.366, p = 0.505 | |
| grey matter | a t(13) = 0.098, p = 0.923 | c F(2,22) = 0.829, p = 0.450 | |
| Ratio white:grey | a t(13) = 0.279, p = 0.785 | c F(2,22) = 1.527, p = 0.239 | |
| Hippocampus | Total | b t(8.16) = 0.33, p = 0.750 | c F(2,22) = 0.003, p = 0.997 |
| Fetal Group | Postnatal Groups | |||
|---|---|---|---|---|
| Naïve Control (n = 7) | Saline (n = 8) | Low-Dex (n = 9) | High-Dex (n = 8) | |
| Anterior horn width (mm) | 1.4 ± 0.1 | 1.8 ± 0.3 | 1.9 ± 0.3 | 1.6 ± 0.1 |
| Hemisphere width (mm) | 23.4 ± 0.4 | 22.6 ± 0.4 | 22.8 ± 0.7 | 23.0 ± 0.4 |
| Rostro-caudal length (mm) | 49.7 ± 1.1 | 47.9 ± 0.9 | 48.2 ± 0.7 | 46.4 ± 0.9 |
| Hemi-cerebellar width (mm) | 14.7 ± 1.1 | 12.4 ± 0.3 | 12.3 ± 0.6 | 13.4 ± 0.4 |
| Frontal cortex thickness (mm) | 1.3 ± 0.1 | 1.4 ± 0.0 | 1.4 ± 0.1 | 1.6 ± 0.1 |
| Lateral cortex thickness (mm) | 1.4 ± 0.1 | 1.4 ± 0.1 | 1.4 ± 0.1 | 1.4 ± 0.1 |
| Fetal Group | Postnatal Groups | |||||||
|---|---|---|---|---|---|---|---|---|
| Naïve Control (n = 7) | Saline (n = 8) | Low-Dex (n = 9) | High-Dex (n = 8) | |||||
| Any Pathology | 3 | (42.9%) | 1 | (12.50%) | 1 | (11.1%) | 0 | (0%) |
| frontal | 0 | (0%) | 1 | (12.50%) | 1 | (11.1%) | 0 | (0%) |
| temporoparietal | 1 | (14.3%) | 0 | (0%) | 0 | (0%) | 0 | (0%) |
| occipital | 3 | (42.9%) | 0 | (0%) | 0 | (0%) | 0 | (0%) |
| Cystic dissolution | 2 | (28.6%) | 0 | (0%) | 0 | (0%) | 0 | (0%) |
| Immaturity (occipital) | 1 | (14.3%) | 5 | (62.5%) | 2 | (22.2%) | 2 | (25%) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yates, N.J.; Feindel, K.W.; Mehnert, A.; Beare, R.; Quick, S.; Blache, D.; Pillow, J.J.; Hunt, R.W.
Ex Vivo MRI Analytical Methods and Brain Pathology in Preterm Lambs Treated with Postnatal Dexamethasone
Yates NJ, Feindel KW, Mehnert A, Beare R, Quick S, Blache D, Pillow JJ, Hunt RW.
Ex Vivo MRI Analytical Methods and Brain Pathology in Preterm Lambs Treated with Postnatal Dexamethasone
Yates, Nathanael J., Kirk W. Feindel, Andrew Mehnert, Richard Beare, Sophia Quick, Dominique Blache, J. Jane Pillow, and Rod W. Hunt.
2020. "Ex Vivo MRI Analytical Methods and Brain Pathology in Preterm Lambs Treated with Postnatal Dexamethasone
Yates, N. J., Feindel, K. W., Mehnert, A., Beare, R., Quick, S., Blache, D., Pillow, J. J., & Hunt, R. W.
(2020). Ex Vivo MRI Analytical Methods and Brain Pathology in Preterm Lambs Treated with Postnatal Dexamethasone





