Transcranial Direct Current Stimulation at 4 mA Induces Greater Leg Muscle Fatigability in Women Compared to Men
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Protocol
2.3. Isokinetic Strength Testing
2.4. Isokinetic Fatigue Testing
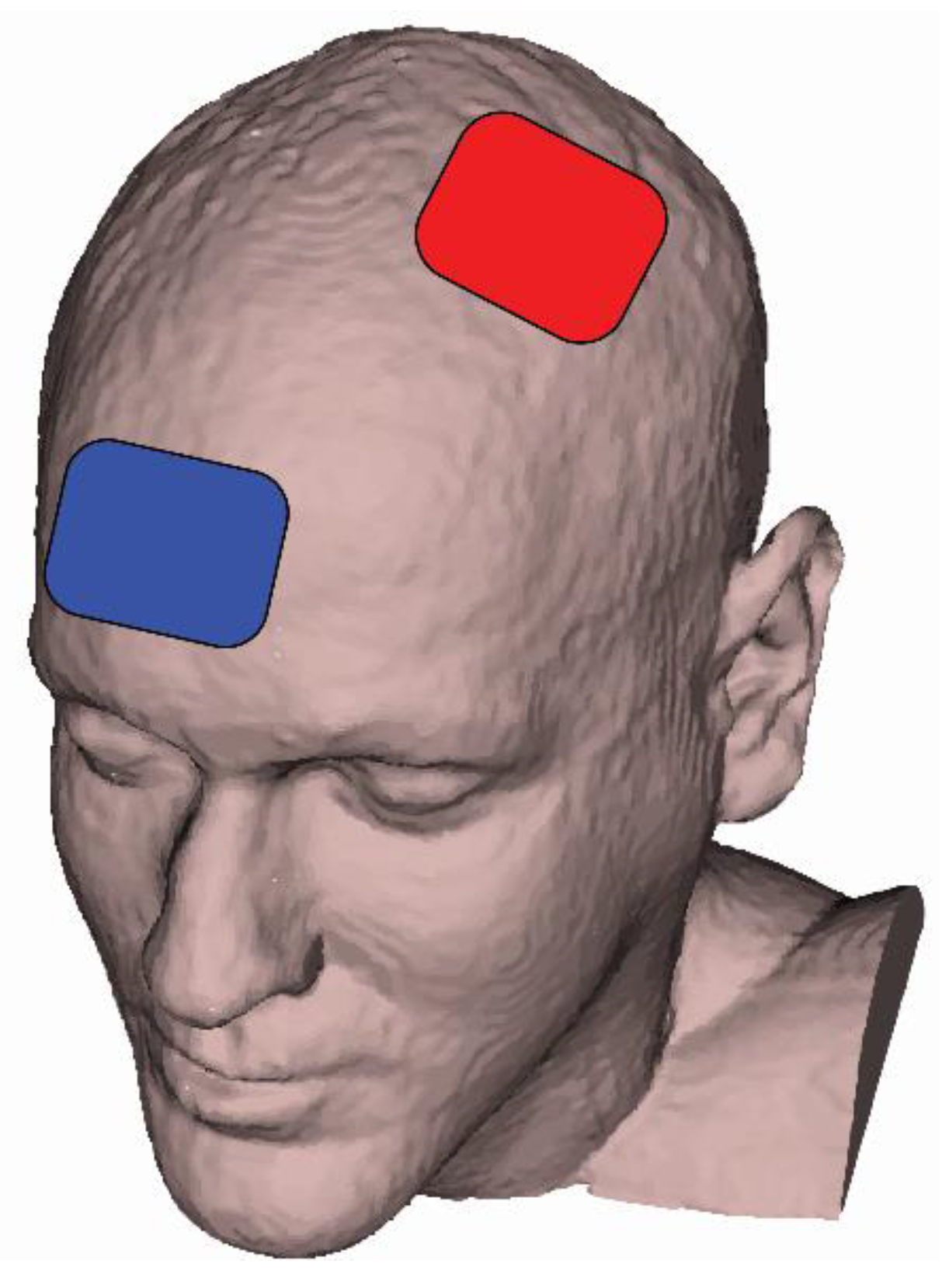
2.5. tDCS Sessions
2.6. Data Analysis
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nitsche, M.A.; Paulus, W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J. Physiol. 2000, 527 Pt 3, 633–639. [Google Scholar] [CrossRef]
- Lefaucheur, J.P.; Antal, A.; Ayache, S.S.; Benninger, D.H.; Brunelin, J.; Cogiamanian, F.; Cotelli, M.; De Ridder, D.; Ferrucci, R.; Langguth, B.; et al. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clin. Neurophysiol. 2017, 128, 56–92. [Google Scholar] [CrossRef] [PubMed]
- Horvath, J.C.; Forte, J.D.; Carter, O. Evidence that transcranial direct current stimulation (tDCS) generates little-to-no reliable neurophysiologic effect beyond MEP amplitude modulation in healthy human subjects: A systematic review. Neuropsychologia 2015, 66, 213–236. [Google Scholar] [CrossRef]
- Voroslakos, M.; Takeuchi, Y.; Brinyiczki, K.; Zombori, T.; Oliva, A.; Fernandez-Ruiz, A.; Kozak, G.; Kincses, Z.T.; Ivanyi, B.; Buzsaki, G.; et al. Direct effects of transcranial electric stimulation on brain circuits in rats and humans. Nat. Commun. 2018, 9, 483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuo, M.F.; Paulus, W.; Nitsche, M.A. Sex differences in cortical neuroplasticity in humans. Neuroreport 2006, 17, 1703–1707. [Google Scholar] [CrossRef] [PubMed]
- Russell, M.; Goodman, T.; Wang, Q.; Groshong, B.; Lyeth, B.G. Gender Differences in Current Received during Transcranial Electrical Stimulation. Front. Psychiatry 2014, 5, 104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaieb, L.; Antal, A.; Paulus, W. Gender-specific modulation of short-term neuroplasticity in the visual cortex induced by transcranial direct current stimulation. Vis. Neurosci. 2008, 25, 77–81. [Google Scholar] [CrossRef] [Green Version]
- Krause, B.; Cohen Kadosh, R. Not all brains are created equal: The relevance of individual differences in responsiveness to transcranial electrical stimulation. Front. Syst. Neurosci. 2014, 8, 25. [Google Scholar] [CrossRef]
- Meiron, O.; Lavidor, M. Unilateral prefrontal direct current stimulation effects are modulated by working memory load and gender. Brain Stimul. 2013, 6, 440–447. [Google Scholar] [CrossRef]
- de Tommaso, M.; Invitto, S.; Ricci, K.; Lucchese, V.; Delussi, M.; Quattromini, P.; Bettocchi, S.; Pinto, V.; Lancioni, G.; Livrea, P.; et al. Effects of anodal TDCS stimulation of left parietal cortex on visual spatial attention tasks in men and women across menstrual cycle. Neurosci. Lett. 2014, 574, 21–25. [Google Scholar] [CrossRef]
- Lapenta, O.M.; Fregni, F.; Oberman, L.M.; Boggio, P.S. Bilateral temporal cortex transcranial direct current stimulation worsens male performance in a multisensory integration task. Neurosci. Lett. 2012, 527, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Boggio, P.S.; Rocha, R.R.; da Silva, M.T.; Fregni, F. Differential modulatory effects of transcranial direct current stimulation on a facial expression go-no-go task in males and females. Neurosci. Lett. 2008, 447, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Rudroff, T.; Kindred, J.H.; Ketelhut, N.B. Fatigue in Multiple Sclerosis: Misconceptions and Future Research Directions. Front. Neurol. 2016, 7, 122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Workman, C.D.; Kamholz, J.; Rudroff, T. The Tolerability and Efficacy of 4 mA Transcranial Direct Current Stimulation on Leg Muscle Fatigability. Brain Sci. 2019, 10, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Workman, C.D.; Kamholz, J.; Rudroff, T. Increased leg muscle fatigability during 2 mA and 4 mA transcranial direct current stimulation over the left motor cortex. Exp. Brain Res. 2020, 238, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Angius, L.; Pascual-Leone, A.; Santarnecchi, E. Brain stimulation and physical performance. Prog. Brain Res. 2018, 240, 317–339. [Google Scholar] [CrossRef]
- Cancelli, A.; Cottone, C.; Giordani, A.; Migliore, S.; Lupoi, D.; Porcaro, C.; Mirabella, M.; Rossini, P.M.; Filippi, M.M.; Tecchio, F. Personalized, bilateral whole-body somatosensory cortex stimulation to relieve fatigue in multiple sclerosis. Mult. Scler. 2018, 24, 1366–1374. [Google Scholar] [CrossRef]
- Ferrucci, R.; Priori, A. Transcranial cerebellar direct current stimulation (tcDCS): Motor control, cognition, learning and emotions. Neuroimage 2014, 85 Pt 3, 918–923. [Google Scholar] [CrossRef] [Green Version]
- Lefaucheur, J.P.; Chalah, M.A.; Mhalla, A.; Palm, U.; Ayache, S.S.; Mylius, V. The treatment of fatigue by non-invasive brain stimulation. Neurophysiol. Clin. 2017, 47, 173–184. [Google Scholar] [CrossRef]
- Proessl, F.; Poston, B.; Rudroff, T. Does a single application of anodal tDCS improve knee extensor fatigability in people with multiple sclerosis? Brain Stimul. 2018, 11, 1388–1390. [Google Scholar] [CrossRef]
- Tecchio, F.; Cancelli, A.; Cottone, C.; Zito, G.; Pasqualetti, P.; Ghazaryan, A.; Rossini, P.M.; Filippi, M.M. Multiple sclerosis fatigue relief by bilateral somatosensory cortex neuromodulation. J. Neurol. 2014, 261, 1552–1558. [Google Scholar] [CrossRef] [PubMed]
- Workman, C.D.; Kamholz, J.; Rudroff, T. Transcranial direct current stimulation (tDCS) for the treatment of a Multiple Sclerosis symptom cluster. Brain Stimul. 2020, 13, 263–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gleeson, N.P.; Mercer, T.H. Reproducibility of isokinetic leg strength and endurance characteristics of adult men and women. Eur. J. Appl. Physiol. Occup. Physiol. 1992, 65, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.; Lee, J.Y.; Lee, K.I.; Park, K.M. Are there differences in brain morphology according to handedness? Brain Behav. 2017, 7, e00730. [Google Scholar] [CrossRef] [PubMed]
- Saenz, A.; Avellanet, M.; Hijos, E.; Chaler, J.; Garreta, R.; Pujol, E.; Sandoval, B.; Buen, C.; Farreny, A. Knee isokinetic test-retest: A multicentre knee isokinetic test-retest study of a fatigue protocol. Eur. J. Phys. Rehabil. Med. 2010, 46, 81–88. [Google Scholar]
- Ciccone, A.B.; Deckert, J.A.; Schlabs, C.R.; Tilden, M.J.; Herda, T.J.; Gallagher, P.M.; Weir, J.P. Transcranial Direct Current Stimulation of the Temporal Lobe Does Not Affect High-Intensity Work Capacity. J. Strength Cond. Res. 2019, 33, 2074–2086. [Google Scholar] [CrossRef]
- Hameau, S.; Bensmail, D.; Roche, N.; Zory, R. Adaptations of fatigue and fatigability after a short intensive, combined rehabilitation program in patients with multiple sclerosis. J. Rehabil. Med. 2018, 50, 59–66. [Google Scholar] [CrossRef] [Green Version]
- Lambert, C.P.; Archer, R.L.; Evans, W.J. Muscle strength and fatigue during isokinetic exercise in individuals with multiple sclerosis. Med. Sci. Sports Exerc. 2001, 33, 1613–1619. [Google Scholar] [CrossRef]
- Mackey, C.S.; Thiele, R.M.; Conchola, E.C.; DeFreitas, J.M. Comparison of fatigue responses and rapid force characteristics between explosive- and traditional-resistance-trained males. Eur. J. Appl. Physiol. 2018, 118, 1539–1546. [Google Scholar] [CrossRef]
- Thorstensson, A.; Karlsson, J. Fatiguability and fibre composition of human skeletal muscle. Acta Physiol. Scand. 1976, 98, 318–322. [Google Scholar] [CrossRef]
- Klem, G.H.; Luders, H.O.; Jasper, H.H.; Elger, C. The ten-twenty electrode system of the International Federation. The International Federation of Clinical Neurophysiology. Electroencephalogr. Clin. Neurophysiol. Suppl. 1999, 52, 3–6. [Google Scholar] [PubMed]
- Schambra, H.M.; Abe, M.; Luckenbaugh, D.A.; Reis, J.; Krakauer, J.W.; Cohen, L.G. Probing for hemispheric specialization for motor skill learning: A transcranial direct current stimulation study. J. Neurophysiol. 2011, 106, 652–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jayaram, G.; Stinear, J.W. The effects of transcranial stimulation on paretic lower limb motor excitability during walking. J. Clin. Neurophysiol. 2009, 26, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Foerster, A.S.; Rezaee, Z.; Paulus, W.; Nitsche, M.A.; Dutta, A. Effects of Cathode Location and the Size of Anode on Anodal Transcranial Direct Current Stimulation Over the Leg Motor Area in Healthy Humans. Front. Neurosci. 2018, 12, 443. [Google Scholar] [CrossRef]
- DaSilva, A.F.; Volz, M.S.; Bikson, M.; Fregni, F. Electrode positioning and montage in transcranial direct current stimulation. J. Vis. Exp. 2011, e2744. [Google Scholar] [CrossRef] [Green Version]
- Nitsche, M.A.; Cohen, L.G.; Wassermann, E.M.; Priori, A.; Lang, N.; Antal, A.; Paulus, W.; Hummel, F.; Boggio, P.S.; Fregni, F.; et al. Transcranial direct current stimulation: State of the art 2008. Brain Stimul. 2008, 1, 206–223. [Google Scholar] [CrossRef]
- Alix-Fages, C.; Romero-Arenas, S.; Castro-Alonso, M.; Colomer-Poveda, D.; Rio-Rodriguez, D.; Jerez-Martinez, A.; Fernandez-Del-Olmo, M.; Marquez, G. Short-Term Effects of Anodal Transcranial Direct Current Stimulation on Endurance and Maximal Force Production. A Systematic Review and Meta-Analysis. J. Clin. Med. 2019, 8, 536. [Google Scholar] [CrossRef] [Green Version]
- Stagg, C.J.; Jayaram, G.; Pastor, D.; Kincses, Z.T.; Matthews, P.M.; Johansen-Berg, H. Polarity and timing-dependent effects of transcranial direct current stimulation in explicit motor learning. Neuropsychologia 2011, 49, 800–804. [Google Scholar] [CrossRef] [Green Version]
- Ammann, C.; Spampinato, D.; Marquez-Ruiz, J. Modulating Motor Learning through Transcranial Direct-Current Stimulation: An Integrative View. Front. Psychol. 2016, 7, 1981. [Google Scholar] [CrossRef] [Green Version]
- Martin, D.M.; Liu, R.; Alonzo, A.; Green, M.; Loo, C.K. Use of transcranial direct current stimulation (tDCS) to enhance cognitive training: Effect of timing of stimulation. Exp. Brain Res. 2014, 232, 3345–3351. [Google Scholar] [CrossRef]
- Stagg, C.J.; Lin, R.L.; Mezue, M.; Segerdahl, A.; Kong, Y.; Xie, J.; Tracey, I. Widespread modulation of cerebral perfusion induced during and after transcranial direct current stimulation applied to the left dorsolateral prefrontal cortex. J. Neurosci. 2013, 33, 11425–11431. [Google Scholar] [CrossRef] [PubMed]
- Aparicio, L.V.M.; Guarienti, F.; Razza, L.B.; Carvalho, A.F.; Fregni, F.; Brunoni, A.R. A Systematic Review on the Acceptability and Tolerability of Transcranial Direct Current Stimulation Treatment in Neuropsychiatry Trials. Brain Stimul. 2016, 9, 671–681. [Google Scholar] [CrossRef] [PubMed]
- Gur, H.; Akova, B.; Punduk, Z.; Kucukoglu, S. Effects of age on the reciprocal peak torque ratios during knee muscle contractions in elite soccer players. Scand. J. Med. Sci. Sports 1999, 9, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Schulte-Geers, C.; Obert, M.; Schilling, R.L.; Harth, S.; Traupe, H.; Gizewski, E.R.; Verhoff, M.A. Age and gender-dependent bone density changes of the human skull disclosed by high-resolution flat-panel computed tomography. Int. J. Legal Med. 2011, 125, 417–425. [Google Scholar] [CrossRef]
- Workman, C.D.; Fietsam, A.C.; Uc, E.Y.; Rudroff, T. Cerebellar Transcranial Direct Current Stimulation in People with Parkinson’s Disease: A Pilot Study. Brain Sci. 2020, 10, 96. [Google Scholar] [CrossRef] [Green Version]
- Chhatbar, P.Y.; Kautz, S.A.; Takacs, I.; Rowland, N.C.; Revuelta, G.J.; George, M.S.; Bikson, M.; Feng, W. Evidence of transcranial direct current stimulation-generated electric fields at subthalamic level in human brain in vivo. Brain Stimul. 2018, 11, 727–733. [Google Scholar] [CrossRef]
- Hunter, S.K. The Relevance of Sex Differences in Performance Fatigability. Med. Sci. Sports Exerc. 2016, 48, 2247–2256. [Google Scholar] [CrossRef] [Green Version]
- Ditor, D.S.; Hicks, A.L. The effect of age and gender on the relative fatigability of the human adductor pollicis muscle. Can. J. Physiol. Pharmacol. 2000, 78, 781–790. [Google Scholar] [CrossRef]
- Russ, D.W.; Lanza, I.R.; Rothman, D.; Kent-Braun, J.A. Sex differences in glycolysis during brief, intense isometric contractions. Muscle Nerve 2005, 32, 647–655. [Google Scholar] [CrossRef]
- Enoka, R.M.; Stuart, D.G. Neurobiology of muscle fatigue. J. Appl. Physiol. 1992, 72, 1631–1648. [Google Scholar] [CrossRef]
- Hunter, S.K. Sex differences and mechanisms of task-specific muscle fatigue. Exerc. Sport Sci. Rev. 2009, 37, 113–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, T.; Doyel, R.; Widule, C.; Hunter, S.K. Sex differences with aging in the fatigability of dynamic contractions. Exp. Gerontol. 2015, 70, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Senefeld, J.; Yoon, T.; Bement, M.H.; Hunter, S.K. Fatigue and recovery from dynamic contractions in men and women differ for arm and leg muscles. Muscle Nerve 2013, 48, 436–439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barclay, C.J.; Constable, J.K.; Gibbs, C.L. Energetics of fast- and slow-twitch muscles of the mouse. J. Physiol. 1993, 472, 61–80. [Google Scholar] [CrossRef]
- Fertonani, A.; Ferrari, C.; Miniussi, C. What do you feel if I apply transcranial electric stimulation? Safety, sensations and secondary induced effects. Clin. Neurophysiol. 2015, 126, 2181–2188. [Google Scholar] [CrossRef] [PubMed]
- Kessler, S.K.; Turkeltaub, P.E.; Benson, J.G.; Hamilton, R.H. Differences in the experience of active and sham transcranial direct current stimulation. Brain Stimul. 2012, 5, 155–162. [Google Scholar] [CrossRef] [Green Version]
- Weightman, M.; Brittain, J.-S.; Punt, D.; Miall, R.C.; Jenkinson, N. Targeted tDCS selectively improves motor adaptation with the proximal and distal upper limb. Brain Stimul. 2020, 13, 707–716. [Google Scholar] [CrossRef] [Green Version]
- Russo, R.; Wallace, D.; Fitzgerald, P.B.; Cooper, N.R. Perception of comfort during active and sham transcranial direct current stimulation: A double blind study. Brain Stimul. 2013, 6, 946–951. [Google Scholar] [CrossRef]
- Kawata, M. Roles of steroid hormones and their receptors in structural organization in the nervous system. Neurosci. Res. 1995, 24, 1–46. [Google Scholar] [CrossRef]
- Lambert, J.J.; Belelli, D.; Hill-Venning, C.; Peters, J.A. Neurosteroids and GABAA receptor function. Trends Pharmacol. Sci. 1995, 16, 295–303. [Google Scholar] [CrossRef]
- Mellon, S.H. Neurosteroids: Biochemistry, modes of action, and clinical relevance. J. Clin. Endocrinol. Metab. 1994, 78, 1003–1008. [Google Scholar] [CrossRef] [PubMed]
- Rupprecht, R.; Holsboer, F. Neuroactive steroids: Mechanisms of action and neuropsychopharmacological perspectives. Trends Neurosci. 1999, 22, 410–416. [Google Scholar] [CrossRef]
- Inghilleri, M.; Conte, A.; Curra, A.; Frasca, V.; Lorenzano, C.; Berardelli, A. Ovarian hormones and cortical excitability. An rTMS study in humans. Clin. Neurophysiol. 2004, 115, 1063–1068. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, N.E.; Cossar, J.; Marston, L.; Wand, B.M.; Bunce, D.; Moseley, G.L.; De Souza, L.H. Rethinking clinical trials of transcranial direct current stimulation: Participant and assessor blinding is inadequate at intensities of 2 mA. PLoS ONE 2012, 7, e47514. [Google Scholar] [CrossRef] [Green Version]


| Sham | 2 mA | 4 mA | ||||
|---|---|---|---|---|---|---|
| Women | Men | Women | Men | Women | Men | |
| Sensation | ||||||
| Tingling | 2.0 ± 0.0 (n = 1) | 1.8 ± 0.8 (n = 5) | 2.8 ± 1.0 (n = 4) | 1.8 ± 1.1 (n = 5) | 5.0 ± 0.0 (n = 1) | 2.7 ± 1.5 (n = 6) |
| Itching | 2.7 ± 1.5 (n = 3) | 1.7 ± 0.6 (n = 3) | 4.3 ± 1.2 (n = 8) | 3.5+ 0 0.7 (n = 2) | 4.0 ± 1.8 (n = 6) | 2.0 ± 1.0 (n = 3) |
| Burning | 4.0 ± 1.4 (n = 2) | 2.7 ± 0.6 (n = 3) | 3.7 ± 3.1 (n = 3) | 1.6 ± 0.9 (n = 5) | 6.0 ± 1.4 (n = 5) | 4.0 ± 1.4 (n = 5) |
| Prickling | 3.0 ± 1.2 (n = 4) | NR | 5.5 + 0.7 (n = 2) | 3.0 ± 0.0 (n = 1) | 4.0 ± 0.0 (n = 1) | NR |
| Poking | 6.0 ± 0.0 (n = 1) | NR | NR | NR | 2.0 ± 0.0 (n = 1) | 4.0 ± 0.0 (n = 1) |
| Pins/Needles | NR | NR | 3.0 ± 0.0 (n = 1) | NR | 6.0 ± 0.0 (n = 1) | 2.0 ± 1.4 (n = 2) |
| Stinging | NR | 1.5 ± 0.7 (n = 2) | NR | 2.0 ± 0.0 (n = 2) | NR | NR |
| Pinching | NR | NR | 4.0 ± 0.0 (n = 1) | NR | NR | NR |
| Blinding | ||||||
| Guessed sham | 60% | 70% | 20% | 20% | 20% | 10% |
| Guessed 2 mA | 40% | 30% | 50% | 60% | 40% | 40% |
| Guessed 4 mA | 0% | 0% | 30% | 20% | 40% | 50% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Workman, C.D.; Fietsam, A.C.; Rudroff, T. Transcranial Direct Current Stimulation at 4 mA Induces Greater Leg Muscle Fatigability in Women Compared to Men. Brain Sci. 2020, 10, 244. https://doi.org/10.3390/brainsci10040244
Workman CD, Fietsam AC, Rudroff T. Transcranial Direct Current Stimulation at 4 mA Induces Greater Leg Muscle Fatigability in Women Compared to Men. Brain Sciences. 2020; 10(4):244. https://doi.org/10.3390/brainsci10040244
Chicago/Turabian StyleWorkman, Craig D., Alexandra C. Fietsam, and Thorsten Rudroff. 2020. "Transcranial Direct Current Stimulation at 4 mA Induces Greater Leg Muscle Fatigability in Women Compared to Men" Brain Sciences 10, no. 4: 244. https://doi.org/10.3390/brainsci10040244
APA StyleWorkman, C. D., Fietsam, A. C., & Rudroff, T. (2020). Transcranial Direct Current Stimulation at 4 mA Induces Greater Leg Muscle Fatigability in Women Compared to Men. Brain Sciences, 10(4), 244. https://doi.org/10.3390/brainsci10040244






