Site-Specific Effects of Online rTMS during a Working Memory Task in Healthy Older Adults
Abstract
1. Introduction
2. Materials and Methods
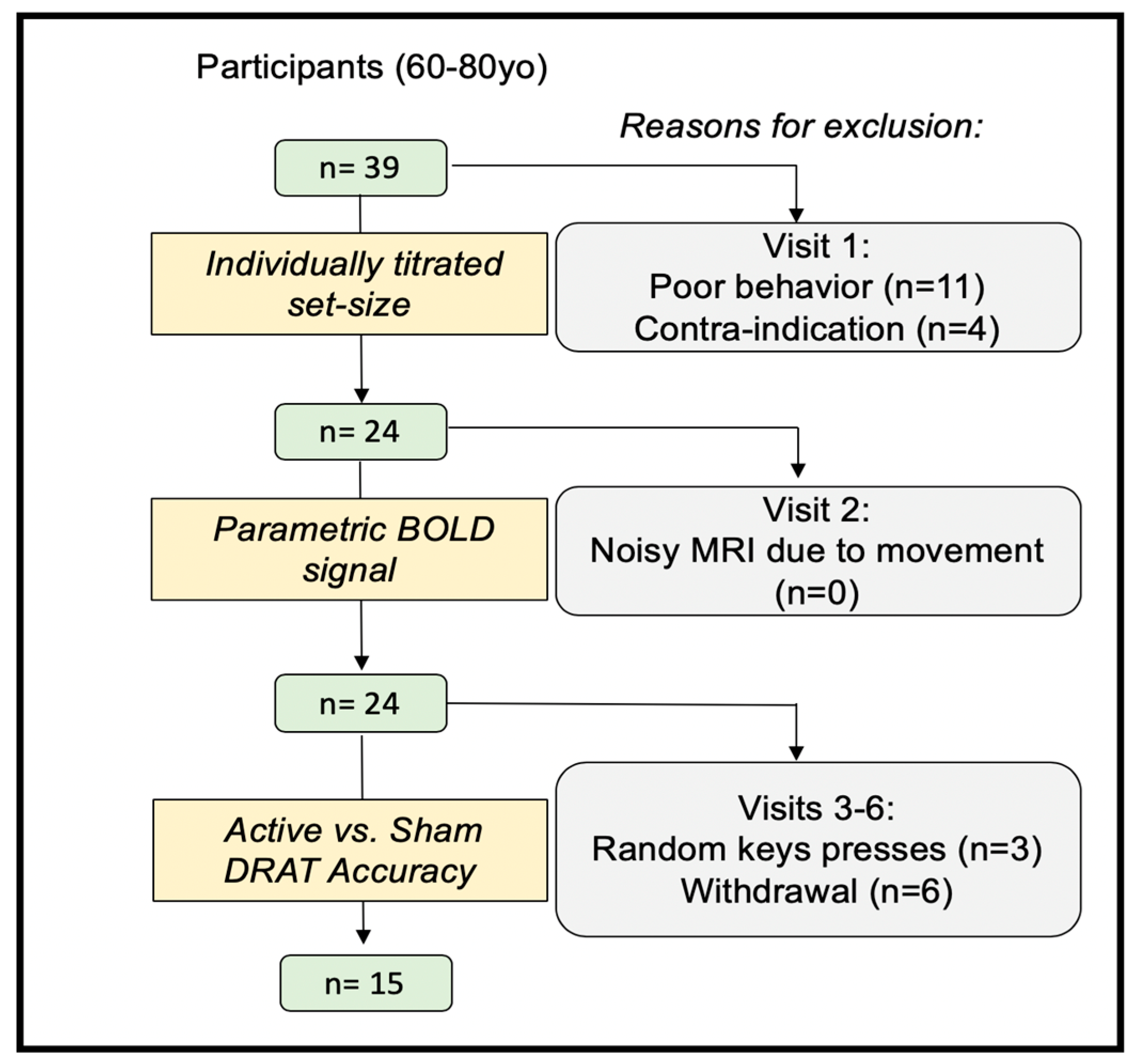
2.1. Participants
2.2. Experimental Protocol
2.3. Delayed-Response Alphabetization Task (DRAT)
2.4. Targeting Approach
2.5. Stimulation Amplitude Approach
2.6. TMS Procedure
2.7. Statistical Analyses
3. Results
3.1. fMRI Activation during the DRAT
3.2. Performance without rTMS
3.3. TMS Dosing Results
3.3.1. TMS Coil Position and Orientation
3.3.2. Stimulation Amplitude
3.4. rTMS Effects
3.4.1. Cumulative rTMS Effects
3.4.2. Omnibus rTMS Effects on the DRAT
3.4.3. Follow-Up Tests on the Effects of Stimulation Amplitude
3.5. Effect Sizes Comparison with Prior Study
4. Discussion
4.1. Site- and Timing-Specific rTMS Effects on WM Manipulation
4.2. Moderate Effect Sizes
4.3. Electric-Field-Based TMS Dosing
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Data Available
References
- Baddeley, A. Working memory. Mémoire de travail. Comptes Rendus l’Académie Des Sciences Series III Sci. Vie 1998, 321, 137–173. [Google Scholar]
- Kane, M.J.; Brown, L.H.; McVay, J.C.; Silvia, P.J.; Myin-Germeys, I.; Kwapil, T. For whom the mind wanders, and when. Psychol. Sci. 2007, 18, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Pasula, E.Y.; Brown, G.G.; McKenna, B.S.; Mellor, A.; Turner, T.; Anderson, C.; Drummond, S. Effects of sleep deprivation on component processes of working memory in younger and older adults. Sleep 2018, 41. [Google Scholar] [CrossRef]
- Cappell, K.A.; Gmeindl, L.; Reuter-Lorenz, P. Age differences in prefontal recruitment during verbal working memory maintenance depend on memory load. Cortex 2009, 46, 462–473. [Google Scholar] [CrossRef]
- Kirova, A.-M.; Bays, R.B.; Lagalwar, S. Working memory and executive function decline across normal aging, mild cognitive impairment, and Alzheimer’s Disease. BioMed Res. Int. 2015, 2015, 748212. [Google Scholar] [CrossRef] [PubMed]
- Walsh, V.; Pascual-Leone, A. Transcranial Magnetic Stimulation; MIT Press: Cambridge, MA, USA, 2003. [Google Scholar]
- Luber, B.; Lisanby, S.H. Enhancement of human cognitive performance using transcranial magnetic stimulation (TMS). NeuroImage 2013, 85, 961–970. [Google Scholar] [CrossRef]
- Hamidi, M.; Tononi, G.; Postle, B.R. Evaluating frontal and parietal contributions to spatial working memory with repetitive transcranial magnetic stimulation. Brain Res. 2008, 1230, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Luber, B.; Kinnunen, L.; Rakitin, B.; Ellsasser, R.; Stern, Y.; Lisanby, S.H. Facilitation of performance in a working memory task with rTMS stimulation of the precuneus: Frequency- and time-dependent effects. Brain Res. 2007, 1128, 120–129. [Google Scholar] [CrossRef]
- Johnson, J.S.; Hamidi, M.; Postle, B.R. Using EEG to explore how rTMS produces its effects on behavior. Brain Topogr. 2009, 22, 281–293. [Google Scholar] [CrossRef][Green Version]
- Roux, F.; Uhlhaas, P. Working memory and neural oscillations: Alpha–gamma versus theta–gamma codes for distinct WM information? Trends Cogn. Sci. 2014, 18, 16–25. [Google Scholar] [CrossRef]
- Hanslmayr, S.; Axmacher, N.; Inman, C.S. Modulating human memory via entrainment of brain oscillations. Trends Neurosci. 2019, 42, 485–499. [Google Scholar] [CrossRef] [PubMed]
- Violante, I.; Li, L.; Carmichael, D.; Lorenz, R.; Leech, R.; Hampshire, A.; Rothwell, J.C.; Sharp, D.J. Externally induced frontoparietal synchronization modulates network dynamics and enhances working memory performance. eLife 2017, 6, 111. [Google Scholar] [CrossRef] [PubMed]
- Albouy, P.; Weiss, A.; Baillet, S.; Zatorre, R.J. Selective entrainment of theta oscillations in the dorsal stream causally enhances auditory working memory performance. Neuron 2017, 94, 193–206.e5. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Jin, J.-N.; Wang, X.; Qi, H.-Z.; Liu, Z.-P.; Yin, T. Theta and alpha oscillations during the retention period of working memory by rTMS stimulating the parietal lobe. Front. Behav. Neurosci. 2017, 11. [Google Scholar] [CrossRef]
- Hoy, K.E.; Bailey, N.W.; Michael, M.; FitzGibbon, B.M.; Rogasch, N.C.; Saeki, T.; Fitzgerald, P.B. Enhancement of working memory and task-related oscillatory activity following intermittent theta burst stimulation in healthy controls. Cereb. Cortex 2015, 26, 4563–4573. [Google Scholar] [CrossRef]
- Chung, S.W.; Rogasch, N.C.; Hoy, K.E.; Fitzgerald, P.B. The effect of single and repeated prefrontal intermittent theta burst stimulation on cortical reactivity and working memory. Brain Stimul. 2018, 11, 566–574. [Google Scholar] [CrossRef]
- Beynel, L.; Appelbaum, L.G.; Luber, B.; Crowell, C.A.; Hilbig, S.A.; Lim, W.; Nguyen, D.; Chrapliwy, N.A.; Davis, S.W.; Cabeza, R.; et al. Effects of online repetitive transcranial magnetic stimulation (rTMS) on cognitive processing: A meta-analysis and recommendations for future studies. Neurosci. Biobehav. Rev. 2019, 107, 47–58. [Google Scholar] [CrossRef]
- Hortobágyi, T.; Del Olmo, M.F.; Rothwell, J.C.; Olmo, M.F. Age reduces cortical reciprocal inhibition in humans. Exp. Brain Res. 2005, 171, 322–329. [Google Scholar] [CrossRef]
- Salat, D.H.; Buckner, R.L.; Snyder, A.Z.; Greve, D.N.; Desikan, R.S.R.; Busa, E.; Morris, J.C.; Dale, A.M.; Fischl, B. Thinning of the cerebral cortex in aging. Cereb. Cortex 2004, 14, 721–730. [Google Scholar] [CrossRef]
- Hutton, C.; Draganski, B.; Ashburner, J.; Weiskopf, N. A comparison between voxel-based cortical thickness and voxel-based morphometry in normal aging. NeuroImage 2009, 48, 371–380. [Google Scholar] [CrossRef]
- Stokes, M.G.; Chambers, C.D.; Gould, I.; English, T.; McNaught, E.; McDonald, O.; Mattingley, J.B. Distance-adjusted motor threshold for transcranial magnetic stimulation. Clin. Neurophysiol. 2007, 118, 1617–1625. [Google Scholar] [CrossRef] [PubMed]
- Oliviero, A.; Profice, P.; Tonali, P.; Pilato, F.; Saturno, E.; Dileone, M.; Ranieri, F.; Di Lazzaro, V. Effects of aging on motor cortex excitability. Neurosci. Res. 2006, 55, 74–77. [Google Scholar] [CrossRef] [PubMed]
- Beynel, L.; Davis, S.W.; Crowell, C.A.; Hilbig, S.A.; Lim, W.; Nguyen, D.; Palmer, H.; Brito, A.; Peterchev, A.V.; Luber, B.; et al. Online repetitive transcranial magnetic stimulation during working memory in younger and older adults: A randomized within-subject comparison. PLoS ONE 2019, 14, e0213707. [Google Scholar] [CrossRef] [PubMed]
- Koenigs, M.; Barbey, A.; Postle, B.R.; Grafman, J. Superior parietal cortex is critical for the manipulation of information in working memory. J. Neurosci. 2009, 29, 14980–14986. [Google Scholar] [CrossRef] [PubMed]
- Postle, B.R. Working memory as an emergent property of the mind and brain. Neurosci. 2005, 139, 23–38. [Google Scholar] [CrossRef]
- Davis, S.W.; Crowell, C.A.; Beynel, L.; Deng, L.; Lakhlani, D.; Hilbig, S.A.; Lim, W.; Nguyen, D.; Peterchev, A.V.; Luber, B.M.; et al. Complementary topology of maintenance and manipulation brain networks in working memory. Sci. Rep. 2018, 8, 17827. [Google Scholar] [CrossRef]
- Beck, S.; Hallett, M. Surround inhibition is modulated by task difficulty. Clin. Neurophysiol. 2009, 121, 98–103. [Google Scholar] [CrossRef]
- Muggleton, N.G.; Juan, C.-H.; Cowey, A.; Walsh, V. Human frontal eye fields and visual search. J. Neurophysiol. 2003, 89, 3340–3343. [Google Scholar] [CrossRef]
- Jurica, P.J.; Leitten, C.L.; Mattis, S. DRS-2: Dementia Rating Scale-2: Professional Manual; PAR Psychological Assessment: Lutz, FL, USA, 2001. [Google Scholar]
- Janssen, A.; Oostendorp, T.F.; Stegeman, D.F. The coil orientation dependency of the electric field induced by TMS for M1 and other brain areas. J. Neuroeng. Rehabil. 2015, 12, 47. [Google Scholar] [CrossRef]
- Kammer, T.; Beck, S.; Erb, M.; Grodd, W. The influence of current direction on phosphene thresholds evoked by transcranial magnetic stimulation. Clin. Neurophysiol. 2001, 112, 2015–2021. [Google Scholar] [CrossRef]
- Fischl, B.; Van Der Kouwe, A.; Destrieux, C.; Cash, S.S.S.; Ségonne, F.; Salat, D.H.; Busa, E.; Seidman, L.J.; Goldstein, J.; Kennedy, D.; et al. Automatically parcellating the human cerebral cortex. Cereb. Cortex 2004, 14, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Aberra, A.S.; Wang, B.; Grill, W.M.; Peterchev, A.V. Simulation of transcranial magnetic stimulation in head model with morphologically-realistic cortical neurons. Brain Stimul. 2020, 13, 175–189. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.M.; Jenkinson, M.; Woolrich, M.W.; Beckmann, C.F.; Behrens, T.E.; Johansen-Berg, H.; Bannister, P.R.; De Luca, M.; Drobnjak, I.; Flitney, D.E.; et al. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage 2004, 23, S208–S219. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.; Hallett, M.; Rossini, P.M.; Pascual-Leone, A. The Safety of TMS Consensus Group Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin. Neurophysiol. 2009, 120, 2008–2039. [Google Scholar] [CrossRef] [PubMed]
- Güllmar, D.; Haueisen, J.; Reichenbach, J.R. Influence of anisotropic electrical conductivity in white matter tissue on the EEG/MEG forward and inverse solution. A high-resolution whole head simulation study. NeuroImage 2010, 51, 145–163. [Google Scholar] [CrossRef]
- Luber, B.; Stanford, A.D.; Bulow, P.; Nguyen, T.; Rakitin, B.C.; Habeck, C.; Basner, R.; Stern, Y.; Lisanby, S.H. Remediation of sleep-deprivation–induced working memory impairment with fMRI-guided transcranial magnetic stimulation. Cereb. Cortex 2008, 18, 2077–2085. [Google Scholar] [CrossRef]
- Luber, B.; Steffener, J.; Tucker, A.; Habeck, C.; Peterchev, A.V.; Deng, Z.-D.; Basner, R.C.; Stern, Y.; Lisanby, S.H. Extended remediation of sleep deprived-induced working memory deficits using fMRI-guided transcranial magnetic stimulation. Sleep 2013, 36, 857–871. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.E.; Peterchev, A.V. Electric field measurement of two commercial active/sham coils for transcranial magnetic stimulation. J. Neural Eng. 2018, 15, 054001. [Google Scholar] [CrossRef]
- Dehaene, S.; Piazza, M.; Pinel, P.; Cohen, L. Three parietal circuits for number processing. Cogn. Neuropsychol. 2003, 20, 487–506. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Park, D.C.; Polk, T.A. Parietal functional connectivity in numerical cognition. Cereb. Cortex 2012, 23, 2127–2135. [Google Scholar] [CrossRef]
- Piazza, M.; Pinel, P.; Le Bihan, D.; Dehaene, S. A Magnitude code common to numerosities and number symbols in human intraparietal cortex. Neuron 2007, 53, 293–305. [Google Scholar] [CrossRef] [PubMed]
- Jensen, O.; Tesche, C.D. Frontal theta activity in humans increases with memory load in a working memory task. Eur. J. Neurosci. 2002, 15, 1395–1399. [Google Scholar] [CrossRef] [PubMed]
- Hanslmayr, S.; Matuschek, J.; Fellner, M.-C. Entrainment of prefrontal beta oscillations induces an endogenous echo and impairs memory formation. Curr. Boil. 2014, 24, 904–909. [Google Scholar] [CrossRef] [PubMed]
- Roberts, B.M.; Clarke, A.; Addante, R.; Ranganath, C. Entrainment enhances theta oscillations and improves episodic memory. Cogn. Neurosci. 2018, 9, 181–193. [Google Scholar] [CrossRef]
- Benwell, C.S.; Learmonth, G.; Miniussi, C.; Harvey, M.; Thut, G. Non-linear effects of transcranial direct current stimulation as a function of individual baseline performance: Evidence from biparietal tDCS influence on lateralized attention bias. Cortex 2015, 69, 152–165. [Google Scholar] [CrossRef]
- Hsu, T.-Y.; Juan, C.-H.; Tseng, P. Individual differences and state-dependent responses in transcranial direct current stimulation. Front. Hum. Neurosci. 2016, 10, 84. [Google Scholar] [CrossRef]
- Albouy, P.; Baillet, S.; Zatorre, R.J. Driving working memory with frequency-tuned noninvasive brain stimulation. Ann. N. Y. Acad. Sci. 2018, 1423, 126–137. [Google Scholar] [CrossRef]
- Silvanto, J.; Muggleton, N.; Walsh, V. State-dependency in brain stimulation studies of perception and cognition. Trends Cogn. Sci. 2008, 12, 447–454. [Google Scholar] [CrossRef]
- Thut, G.; Bergmann, T.O.; Fröhlich, F.; Soekadar, S.R.; Brittain, J.-S.; Valero-Cabré, A.; Sack, A.T.; Miniussi, C.; Antal, A.; Siebner, H.R.; et al. Guiding transcranial brain stimulation by EEG/MEG to interact with ongoing brain activity and associated functions: A position paper. Clin. Neurophysiol. 2017, 128, 843–857. [Google Scholar] [CrossRef]
- Brasil-Neto, J.P.; Cohen, L.G.; Panizza, M.; Nilsson, J.; Roth, B.J.; Hallett, M. Optimal focal transcranial magnetic activation of the human motor cortex: Effects of coil orientation, shape of the induced current pulse, and stimulus intensity. J. Clin. Neurophysiol. 1992, 9, 132–136. [Google Scholar] [CrossRef]
- Richter, L.; Neumann, G.; Oung, S.; Schweikard, A.; Trillenberg, P. Optimal Coil Orientation for Transcranial Magnetic Stimulation. PLoS ONE 2013, 8, e60358. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.C.; Davey, N.J.; Kennard, C. Current orientation induced by magnetic stimulation influences a cognitive task. NeuroReport 2000, 11, 3257–3259. [Google Scholar] [CrossRef] [PubMed]





| Number of completers | 15 |
| Age (Mean ± SD) | 66.13 ± 5.50 years old |
| Gender: | |
| Number of Females | 4 |
| Number of Males | 11 |
| Number of education years (Mean ± SD) | 17.33 ± 1.79 years |
| Easy | Hard | |
|---|---|---|
| Active rTMS | 87.97 ± 3.70% | 55.42 ± 5.50% |
| Sham rTMS | 87.00 ± 3.71% | 57.76 ± 5.70% |
| p-values | 1.00 | 0.045 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beynel, L.; Davis, S.W.; Crowell, C.A.; Dannhauer, M.; Lim, W.; Palmer, H.; Hilbig, S.A.; Brito, A.; Hile, C.; Luber, B.; et al. Site-Specific Effects of Online rTMS during a Working Memory Task in Healthy Older Adults. Brain Sci. 2020, 10, 255. https://doi.org/10.3390/brainsci10050255
Beynel L, Davis SW, Crowell CA, Dannhauer M, Lim W, Palmer H, Hilbig SA, Brito A, Hile C, Luber B, et al. Site-Specific Effects of Online rTMS during a Working Memory Task in Healthy Older Adults. Brain Sciences. 2020; 10(5):255. https://doi.org/10.3390/brainsci10050255
Chicago/Turabian StyleBeynel, Lysianne, Simon W. Davis, Courtney A. Crowell, Moritz Dannhauer, Wesley Lim, Hannah Palmer, Susan A. Hilbig, Alexandra Brito, Connor Hile, Bruce Luber, and et al. 2020. "Site-Specific Effects of Online rTMS during a Working Memory Task in Healthy Older Adults" Brain Sciences 10, no. 5: 255. https://doi.org/10.3390/brainsci10050255
APA StyleBeynel, L., Davis, S. W., Crowell, C. A., Dannhauer, M., Lim, W., Palmer, H., Hilbig, S. A., Brito, A., Hile, C., Luber, B., Lisanby, S. H., Peterchev, A. V., Cabeza, R., & Appelbaum, L. G. (2020). Site-Specific Effects of Online rTMS during a Working Memory Task in Healthy Older Adults. Brain Sciences, 10(5), 255. https://doi.org/10.3390/brainsci10050255





