Theory of Mind Performance Predicts tDCS-Mediated Effects on the Medial Prefrontal Cortex: A Pilot Study to Investigate the Role of Sex and Age
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Procedure
2.2.1. Clinical and Neuropsychological Assessment
2.2.2. Theory of Mind Assessment
2.2.2.1. Reading the Mind in the Eyes Task
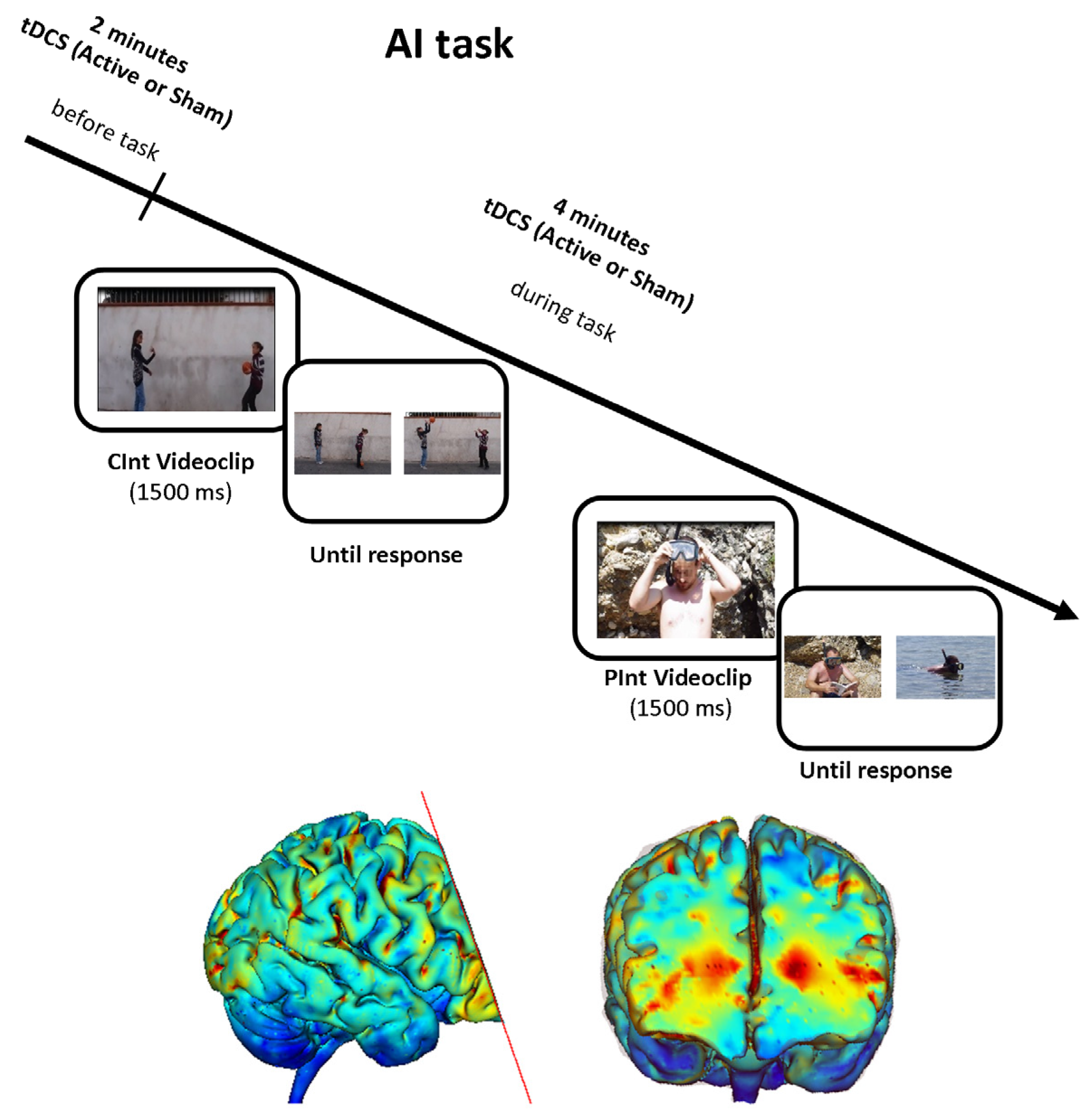
2.2.2.2. Attribution of Intentions Task
2.2.3. tDCS Procedure
2.2.4. Statistical Analyses
3. Results
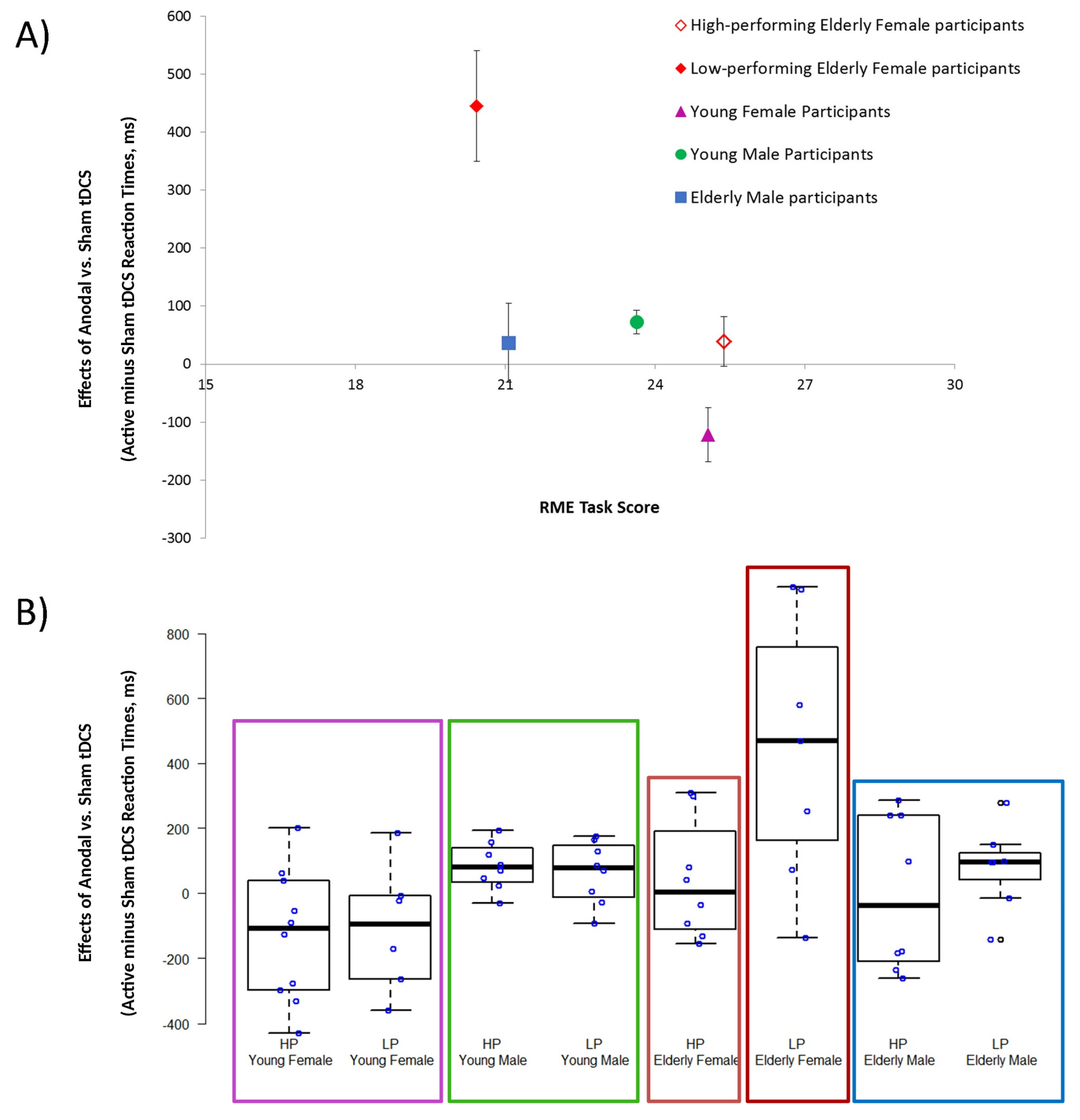
3.1. Active tDCS Effects Recorded in High- and Low-Performing Elderly and Young Participants
3.2. RME Task Score and AI Sham Performance Recorded in High- and Low-Performing Elderly and Young Participants
3.3. Clinical and Neuropsychological Performances Achieved by High- and Low-Performing Elderly Subgroups
3.4. TDCS-Sensations Questionnaire
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Enrici, I.; Bara, B.G.; Adenzato, M. Theory of Mind, pragmatics and the brain: Converging evidence for the role of intention processing as a core feature ofhuman communication. Pragmat. Cogn. 2019, 26, 5–38. [Google Scholar] [CrossRef]
- Frith, C.D.; Frith, U. How we predict what other people are going to do. Brain Res. 2006, 1079, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Adenzato, M.; Cavallo, M.; Enrici, I. Theory of mind ability in the behavioural variant of frontotemporal dementia: An analysis of the neural, cognitive, and social levels. Neuropsychologia 2010, 48, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Cavallo, M.; Enrici, I.; Adenzato, M. The comprehension of social situations in a small group of patients with frontotemporal dementia and Alzheimer’s disease. Acta Neuropsychol. 2011, 9, 167–176. [Google Scholar]
- Brune, M.; Brune-Cohrs, U. Theory of mind--evolution, ontogeny, brain mechanisms and psychopathology. Neurosci. Biobehav. Rev. 2006, 30, 437–455. [Google Scholar] [CrossRef]
- Poletti, M.; Enrici, I.; Adenzato, M. Cognitive and affective Theory of Mind in neurodegenerative diseases: Neuropsychological, neuroanatomical and neurochemical levels. Neuroscience Biobehav. Rev. 2012, 36, 2147–2164. [Google Scholar] [CrossRef]
- Ciaramidaro, A.; Adenzato, M.; Enrici, I.; Erk, S.; Pia, L.; Bara, B.G.; Walter, H. The intentional network: How the brain reads varieties of intentions. Neuropsychologia 2007, 45, 3105–3113. [Google Scholar] [CrossRef]
- Carrington, S.J.; Bailey, A.J. Are there theory of mind regions in the brain? A review of the neuroimaging literature. Human Brain Mapp. 2009, 30, 2313–2335. [Google Scholar] [CrossRef]
- Kemp, J.; Despres, O.; Sellal, F.; Dufour, A. Theory of Mind in normal ageing and neurodegenerative pathologies. Ageing Res. Rev. 2012, 11, 199–219. [Google Scholar] [CrossRef]
- Sullivan, S.; Ruffman, T. Social understanding: How does it fare with advancing years? Br. J. Psychol. (Lond. Engl.: 1953) 2004, 95 (Pt 1), 1–18. [Google Scholar] [CrossRef] [Green Version]
- Di Tella, M.; Miti, F.; Ardito, R.B.; Adenzato, M. Social cognition and sex: Are men and women really different? Personal. Individ. Differ. 2020, 162, 110045. [Google Scholar] [CrossRef]
- Baron-Cohen, S. Empathizing, systemizing, and the extreme male brain theory of autism. Prog. Brain Res. 2010, 186, 167–175. [Google Scholar] [PubMed]
- McClure, E.B. A meta-analytic review of sex differences in facial expression processing and their development in infants, children, and adolescents. Psychol. Bull. 2000, 126, 424–453. [Google Scholar] [CrossRef] [PubMed]
- Baron-Cohen, S.; O’Riordan, M.; Stone, V.; Jones, R.; Plaisted, K. Recognition of faux pas by normally developing children and children with Asperger syndrome or high-functioning autism. J. Autism Dev. Disord. 1999, 29, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Baron-Cohen, S.; Wheelwright, S. The empathy quotient: An investigation of adults with Asperger syndrome or high functioning autism, and normal sex differences. J. Autism Dev. Disord. 2004, 34, 163–175. [Google Scholar] [CrossRef]
- Brackett, M.A.; Salovey, P. Measuring emotional intelligence with the Mayer-Salovery-Caruso Emotional Intelligence Test (MSCEIT). Psicothema 2006, 18 (Suppl.), 34–41. [Google Scholar]
- Cabinio, M.; Rossetto, F.; Blasi, V.; Savazzi, F.; Castelli, I.; Massaro, D.; Valle, A.; Nemni, R.; Clerici, M.; Marchetti, A.; et al. Mind-Reading Ability and Structural Connectivity Changes in Aging. Front. Psychol. 2015, 6, 1808. [Google Scholar] [CrossRef] [Green Version]
- Moran, J.M. Lifespan development: The effects of typical aging on theory of mind. Behav. Brain Res. 2013, 237, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Dayan, E.; Censor, N.; Buch, E.R.; Sandrini, M.; Cohen, L.G. Noninvasive brain stimulation: From physiology to network dynamics and back. Nat. Neurosci. 2013, 16, 838–844. [Google Scholar] [CrossRef]
- Sellaro, R.; Nitsche, M.A.; Colzato, L.S. The stimulated social brain: Effects of transcranial direct current stimulation on social cognition. Ann. N. Y. Acad. Sci. 2016, 1369, 218–239. [Google Scholar] [CrossRef]
- Jacobson, L.; Koslowsky, M.; Lavidor, M. tDCS polarity effects in motor and cognitive domains: A meta-analytical review. Exp. Brain Res. 2012, 216, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Filmer, H.L.; Dux, P.E.; Mattingley, J.B. Applications of transcranial direct current stimulation for understanding brain function. Trends Neurosci. 2014, 37, 742–753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brunoni, A.R.; Nitsche, M.A.; Bolognini, N.; Bikson, M.; Wagner, T.; Merabet, L.; Edwards, D.J.; Valero-Cabre, A.; Rotenberg, A.; Pascual-Leone, A.; et al. Clinical research with transcranial direct current stimulation (tDCS): Challenges and future directions. Brain Stimul. 2012, 5, 175–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lefaucheur, J.P.; Antal, A.; Ayache, S.S.; Benninger, D.H.; Brunelin, J.; Cogiamanian, F.; Cotelli, M.; De Ridder, D.; Ferrucci, R.; Langguth, B.; et al. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clin Neurophysiol. 2017, 128, 56–92. [Google Scholar] [CrossRef] [PubMed]
- Conson, M.; Errico, D.; Mazzarella, E.; Giordano, M.; Grossi, D.; Trojano, L. Transcranial Electrical Stimulation over Dorsolateral Prefrontal Cortex Modulates Processing of Social Cognitive and Affective Information. PLoS ONE 2015, 10, e0126448. [Google Scholar] [CrossRef] [PubMed]
- Fumagalli, M.; Vergari, M.; Pasqualetti, P.; Marceglia, S.; Mameli, F.; Ferrucci, R.; Mrakic-Sposta, S.; Zago, S.; Sartori, G.; Pravettoni, G.; et al. Brain switches utilitarian behavior: Does gender make the difference? PLoS ONE 2010, 5, e8865. [Google Scholar] [CrossRef] [PubMed]
- Adenzato, M.; Brambilla, M.; Manenti, R.; De Lucia, L.; Trojano, L.; Garofalo, S.; Enrici, I.; Cotelli, M. Gender differences in cognitive Theory of Mind revealed by transcranial direct current stimulation on medial prefrontal cortex. Sci. Rep. 2017, 7, 41219. [Google Scholar] [CrossRef]
- Martin, A.K.; Huang, J.; Hunold, A.; Meinzer, M. Sex Mediates the Effects of High-Definition Transcranial Direct Current Stimulation on “Mind-Reading”. Neuroscience 2017, 366, 84–94. [Google Scholar] [CrossRef] [Green Version]
- Adenzato, M.; Manenti, R.; Gobbi, E.; Enrici, I.; Rusich, D.; Cotelli, M. Aging, sex and cognitive Theory of Mind: A transcranial direct current stimulation study. Sci. Rep. 2019, 9, 18064. [Google Scholar] [CrossRef] [Green Version]
- Baron-Cohen, S.; Wheelwright, S.; Hill, J.; Raste, Y.; Plumb, I. The “Reading the Mind in the Eyes” Test revised version: A study with normal adults, and adults with Asperger syndrome or high-functioning autism. J. Child Psychol. Psychiatry Allied Discip. 2001, 42, 241–251. [Google Scholar] [CrossRef]
- Adenzato, M.; Manenti, R.; Enrici, I.; Gobbi, E.; Brambilla, M.; Alberici, A.; Cotelli, M.S.; Padovani, A.; Borroni, B.; Cotelli, M. Transcranial direct current stimulation enhances theory of mind in Parkinson’s disease patients with mild cognitive impairment: A randomized, double-blind, sham-controlled study. Transl. Neurodegener. 2019, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Cotelli, M.; Adenzato, M.; Cantoni, V.; Manenti, R.; Alberici, A.; Enrici, I.; Benussi, A.; Dell’Era, V.; Bonetta, E.; Padovani, A.; et al. Enhancing theory of mind in behavioural variant frontotemporal dementia with transcranial direct current stimulation. Cogn. Affect. Behav. Neurosci. 2018, 18, 1065–1075. [Google Scholar] [CrossRef] [PubMed]
- Enrici, I.; Adenzato, M.; Cappa, S.; Bara, B.G.; Tettamanti, M. Intention processing in communication: A common brain network for language and gestures. J. Cogn. Neurosci. 2011, 23, 2415–2431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tettamanti, M.; Vaghi, M.M.; Bara, B.G.; Cappa, S.F.; Enrici, I.; Adenzato, M. Effective connectivity gateways to the Theory of Mind network in processing communicative intention. NeuroImage 2017, 155, 169–176. [Google Scholar] [CrossRef] [Green Version]
- Walter, H.; Adenzato, M.; Ciaramidaro, A.; Enrici, I.; Pia, L.; Bara, B.G. Understanding intentions in social interaction: The role of the anterior paracingulate cortex. J. Cogn. Neurosci. 2004, 16, 1854–1863. [Google Scholar] [CrossRef]
- Walter, H.; Ciaramidaro, A.; Adenzato, M.; Vasic, N.; Ardito, R.B.; Erk, S.; Bara, B.G. Dysfunction of the social brain in schizophrenia is modulated by intention type: An fMRI study. Soc. Cogn. Affect. Neurosci. 2009, 4, 166–176. [Google Scholar] [CrossRef] [Green Version]
- Schurz, M.; Radua, J.; Aichhorn, M.; Richlan, F.; Perner, J. Fractionating theory of mind: A meta-analysis of functional brain imaging studies. Neurosci. Biobehav. Rev. 2014, 42, 9–34. [Google Scholar] [CrossRef] [Green Version]
- Thye, M.D.; Ammons, C.J.; Murdaugh, D.L.; Kana, R.K. Differential recruitment of theory of mind brain network across three tasks: An independent component analysis. Behav. Brain Res. 2018, 347, 385–393. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Lezak, M.; Howieson, D.; Loring, D. Neuropsychological Assessment, 5th ed.; Oxford University Press: Oxford, NY, USA, 2012; Volume 10, ISBN 9780195395525. [Google Scholar]
- Yesavage, J.A.; Brink, T.L.; Rose, T.L.; Lum, O.; Huang, V.; Adey, M.; Leirer, V.O. Development and validation of a geriatric depression screening scale: A preliminary report. J. Psychiatr. Res. 1982, 17, 37–49. [Google Scholar] [CrossRef]
- Spielberger, C.; Gorsuch, R.; Lushene, R.; Vagg, P.; Jacobs, G. Manual for the State-trait Anxiety Inventory (form Y self-evaluation Questionnaire); Consulting Psychologists Press: Palo Alto, CA, USA, 1983. [Google Scholar]
- Davis, M.H. Measuring individual differences in empathy: Evidence for a multidimensional approach. J. Personal. Soc. Psychol. 1983, 44, 113. [Google Scholar] [CrossRef]
- Calabria, M.; Manenti, R.; Rosini, S.; Zanetti, O.; Miniussi, C.; Cotelli, M. Objective and subjective memory impairment in elderly adults: A revised version of the Everyday Memory Questionnaire. Aging Clin. Exp. Res. 2011, 23, 67–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nucci, M.; Mapelli, D.; Mondini, S. Cognitive Reserve Index questionnaire (CRIq): A new instrument for measuring cognitive reserve. Aging Clin. Exp. Res. 2012, 24, 218–226. [Google Scholar] [PubMed]
- Presentation Software, Version 16.3. Available online: www.neurobs.com (accessed on 31 January 2020).
- Noetscher, G.M.; Yanamadala, J.; Makarov, S.N.; Pascual-Leone, A. Comparison of cephalic and extracephalic montages for transcranial direct current stimulation—A numerical study. IEEE Trans. Biomed. Eng. 2014, 61, 2488–2498. [Google Scholar] [CrossRef]
- Poreisz, C.; Boros, K.; Antal, A.; Paulus, W. Safety aspects of transcranial direct current stimulation concerning healthy subjects and patients. Brain Res. Bull. 2007, 72, 208–214. [Google Scholar] [CrossRef]
- Bikson, M.; Datta, A.; Elwassif, M. Establishing safety limits for transcranial direct current stimulation. Clin. Neurophysiol. 2009, 120, 1033–1034. [Google Scholar] [CrossRef] [Green Version]
- Iyer, M.B.; Mattu, U.; Grafman, J.; Lomarev, M.; Sato, S.; Wassermann, E.M. Safety and cognitive effect of frontal DC brain polarization in healthy individuals. Neurology 2005, 64, 872–875. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Cohen, L.G.; Wassermann, E.M.; Priori, A.; Lang, N.; Antal, A.; Paulus, W.; Hummel, F.; Boggio, P.S.; Fregni, F.; et al. Transcranial direct current stimulation: State of the art 2008. Brain Stimul. 2008, 1, 206–223. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Paulus, W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J. Physiol. 2000, 527 Pt 3, 633–639. [Google Scholar] [CrossRef]
- Priori, A.; Berardelli, A.; Rona, S.; Accornero, N.; Manfredi, M. Polarization of the human motor cortex through the scalp. Neuroreport 1998, 9, 2257–2260. [Google Scholar] [CrossRef]
- Utz, K.S.; Dimova, V.; Oppenländer, K.; Kerkhoff, G. Electrified minds: Transcranial direct current stimulation (tDCS) and galvanic vestibular stimulation (GVS) as methods of non-invasive brain stimulation in neuropsychology--a review of current data and future implications. Neuropsychologia 2010, 48, 2789–2810. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Qiao, L.; Fan, D.; Zhang, S.; Turel, O.; Li, Y.; Li, J.; Xue, G.; Chen, A.; He, Q. Modulation of Brain Activity with Noninvasive Transcranial Direct Current Stimulation (tDCS): Clinical Applications and Safety Concerns. Front. Psychol. 2017, 8, 685. [Google Scholar] [CrossRef]
- Opitz, A.; Paulus, W.; Will, S.; Antunes, A.; Thielscher, A. Determinants of the electric field during transcranial direct current stimulation. NeuroImage 2015, 109, 140–150. [Google Scholar] [CrossRef] [PubMed]
- West, R.L. An application of prefrontal cortex function theory to cognitive aging. Psychol. Bull. 1996, 120, 272. [Google Scholar] [CrossRef] [PubMed]
- Filmer, H.L.; Ehrhardt, S.E.; Shaw, T.B.; Mattingley, J.B.; Dux, P.E. The efficacy of transcranial direct current stimulation to prefrontal areas is related to underlying cortical morphology. NeuroImage 2019, 196, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Lindenberger, U. Human cognitive aging: Corriger la fortune? Science 2014, 346, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Sandoz, M.; Demonet, J.F.; Fossard, M. Theory of mind and cognitive processes in aging and Alzheimer type dementia: A systematic review. Aging Ment. Health 2014, 18, 815–827. [Google Scholar] [CrossRef]
- Kirkland, R.A.; Peterson, E.; Baker, C.A.; Miller, S.; Pulos, S. Meta-analysis Reveals Adult Female Superiority in “Reading the Mind in the Eyes Test”. N. Am. J. Psychol. 2013, 15, 121–146. [Google Scholar]
- Polania, R.; Nitsche, M.A.; Ruff, C.C. Studying and modifying brain function with non-invasive brain stimulation. Nat. Neurosci. 2018, 21, 174–187. [Google Scholar] [CrossRef]
- Nord, C.L.; Forster, S.; Halahakoon, D.C.; Penton-Voak, I.S.; Munafo, M.R.; Roiser, J.P. Prefrontal cortex stimulation does not affect emotional bias, but may slow emotion identification. Soc. Cogn. Affect. Neurosci. 2017, 12, 839–847. [Google Scholar] [CrossRef] [Green Version]
- Payne, S.; Tsakiris, M. Anodal transcranial direct current stimulation of right temporoparietal area inhibits self-recognition. Cogn. Affect. Behav. Neurosci. 2017, 17, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del Maschio, N.; Sulpizio, S.; Gallo, F.; Fedeli, D.; Weekes, B.S.; Abutalebi, J. Neuroplasticity across the lifespan and aging effects in bilinguals and monolinguals. Brain Cogn. 2018, 125, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Gusnard, D.A.; Akbudak, E.; Shulman, G.L.; Raichle, M.E. Medial prefrontal cortex and self-referential mental activity: Relation to a default mode of brain function. Proc. Natl. Acad. Sci. USA 2001, 98, 4259–4264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagel, I.E.; Preuschhof, C.; Li, S.-C.; Nyberg, L.; Bäckman, L.; Lindenberger, U.; Heekeren, H.R. Performance level modulates adult age differences in brain activation during spatial working memory. Proc. Natl. Acad. Sci. USA 2009, 106, 22552–22557. [Google Scholar] [CrossRef] [Green Version]
- Nagel, I.E.; Preuschhof, C.; Li, S.-C.; Nyberg, L.; Bäckman, L.; Lindenberger, U.; Heekeren, H.R. Load modulation of BOLD response and connectivity predicts working memory performance in younger and older adults. J. Cogn. Neurosci. 2011, 23, 2030–2045. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, F.S.; Miller, L.S. Relationship between theory of mind and functional independence is mediated by executive function. Psychol. Aging 2013, 28, 293–303. [Google Scholar] [CrossRef]
- Bailey, P.E.; Henry, J.D. Growing less empathic with age: Disinhibition of the self-perspective. J. Gerontol. Ser. B Psychol. Sci. Soc. Sci. 2008, 63, P219–P226. [Google Scholar] [CrossRef] [Green Version]
- Phillips, L.H.; Bull, R.; Allen, R.; Insch, P.; Burr, K.; Ogg, W. Lifespan aging and belief reasoning: Influences of executive function and social cue decoding. Cognition 2011, 120, 236–247. [Google Scholar] [CrossRef]
- MacPherson, S.E.; Phillips, L.H.; Della Sala, S. Age, executive function, and social decision making: A dorsolateral prefrontal theory of cognitive aging. Psychol. Aging 2002, 17, 598–609. [Google Scholar] [CrossRef]
- Schilbach, L.; Eickhoff, S.B.; Rotarska-Jagiela, A.; Fink, G.R.; Vogeley, K. Minds at rest? Social cognition as the default mode of cognizing and its putative relationship to the “default system” of the brain. Conscious. Cogn. 2008, 17, 457–467. [Google Scholar] [CrossRef]
- Malpetti, M.; Ballarini, T.; Presotto, L.; Garibotto, V.; Tettamanti, M.; Perani, D. Gender differences in healthy aging and Alzheimer’s Dementia: A (18) F-FDG-PET study of brain and cognitive reserve. Hum. Brain Mapp. 2017, 38, 4212–4227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutchess, A.; Sokal, R.; Coleman, J.A.; Gotthilf, G.; Grewal, L.; Rosa, N. Age differences in self-referencing: Evidence for common and distinct encoding strategies. Brain Res. 2015, 1612, 118–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kensinger, E.A.; Gutchess, A.H. Cognitive Aging in a Social and Affective Context: Advances Over the Past 50 Years. J. Gerontol. Ser. B Psychol. Sci. Soc. Sci. 2017, 72, 61–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mather, M. The Affective Neuroscience of Aging. Annu. Rev. Psychol. 2016, 67, 213–238. [Google Scholar] [CrossRef] [Green Version]
- Reed, A.E.; Mikels, J.A.; Lockenhoff, C.E. Preferences for choice across adulthood: Age trajectories and potential mechanisms. Psychol. Aging 2013, 28, 625–632. [Google Scholar] [CrossRef]
- Urry, H.L.; Gross, J.J. Emotion regulation in older age. Curr. Dir. Psychol. Sci. 2010, 19, 352–357. [Google Scholar] [CrossRef]
- Jancke, L. The plastic human brain. Restor. Neurol. Neurosci. 2009, 27, 521–538. [Google Scholar] [CrossRef]
- Greenwood, P.M. Functional plasticity in cognitive aging: Review and hypothesis. Neuropsychology 2007, 21, 657–673. [Google Scholar] [CrossRef]
- Zollig, J.; Eschen, A. Measuring compensation and its plasticity across the lifespan. Restor. Neurol. Neurosci. 2009, 27, 421–433. [Google Scholar] [CrossRef]
- Nyberg, L.; Lovden, M.; Riklund, K.; Lindenberger, U.; Backman, L. Memory aging and brain maintenance. Trends Cogn. Sci. 2012, 16, 292–305. [Google Scholar] [CrossRef] [Green Version]
- Reuter-Lorenz, P.A.; Cappell, K.A. Neurocognitive aging and the compensation hypothesis. Curr. Dir. Psychol. Sci. 2008, 17, 177–182. [Google Scholar] [CrossRef]
- Reuter-Lorenz, P.A.; Park, D.C. How does it STAC up? Revisiting the scaffolding theory of aging and cognition. Neuropsychol. Rev. 2014, 24, 355–370. [Google Scholar] [CrossRef] [Green Version]
- Park, D.C.; Reuter-Lorenz, P. The adaptive brain: Aging and neurocognitive scaffolding. Annu. Rev. Psychol. 2009, 60, 173–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reuter-Lorenz, P.A.; Park, D.C. Human neuroscience and the aging mind: A new look at old problems. J. Gerontol. Ser. B 2010, 65, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.W.; Dennis, N.A.; Daselaar, S.M.; Fleck, M.S.; Cabeza, R. Que PASA? The posterior-anterior shift in aging. Cerebral. Cortex 2008, 18, 1201–1209. [Google Scholar] [CrossRef]
- Grady, C.L. Functional brain imaging and age-related changes in cognition. Biol. Psychol. 2000, 54, 259–281. [Google Scholar] [CrossRef]
- Morcom, A.M.; Johnson, W. Neural reorganization and compensation in aging. J. Cogn. Neurosci. 2015, 27, 1275–1285. [Google Scholar] [CrossRef]
- Park, D.C.; McDonough, I.M. The dynamic aging mind: Revelations from functional neuroimaging research. Perspect. Psychol. Sci. 2013, 8, 62–67. [Google Scholar] [CrossRef]
- Colcombe, S.J.; Kramer, A.F.; Erickson, K.I.; Scalf, P. The implications of cortical recruitment and brain morphology for individual differences in inhibitory function in aging humans. Psychol. Aging 2005, 20, 363. [Google Scholar] [CrossRef] [Green Version]
- Gold, B.T.; Kim, C.; Johnson, N.F.; Kryscio, R.J.; Smith, C.D. Lifelong bilingualism maintains neural efficiency for cognitive control in aging. J. Neurosci. 2013, 33, 387–396. [Google Scholar] [CrossRef] [Green Version]
- Rypma, B.; Berger, J.S.; Genova, H.M.; Rebbechi, D.; D’Esposito, M. Dissociating age-related changes in cognitive strategy and neural efficiency using event-related fMRI. Cortex J. Devoted Study Nerv. Syst. Behav. 2005, 41, 582–594. [Google Scholar] [CrossRef]
- Rypma, B.; Berger, J.S.; Prabhakaran, V.; Bly, B.M.; Kimberg, D.Y.; Biswal, B.B.; D’esposito, M. Neural correlates of cognitive efficiency. NeuroImage 2006, 33, 969–979. [Google Scholar] [CrossRef] [PubMed]
- Zarahn, E.; Rakitin, B.; Abela, D.; Flynn, J.; Stern, Y. Age-related changes in brain activation during a delayed item recognition task. Neurobiol. Aging 2007, 28, 784–798. [Google Scholar] [CrossRef] [PubMed]
- Daselaar, S.; Veltman, D.; Rombouts, S.; Raaijmakers, J.; Jonker, C. Neuroanatomical correlates of episodic encoding and retrieval in young and elderly subjects. Brain 2003, 126, 43–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Düzel, E.; Schütze, H.; Yonelinas, A.P.; Heinze, H.J. Functional phenotyping of successful aging in long-term memory: Preserved performance in the absence of neural compensation. Hippocampus 2011, 21, 803–814. [Google Scholar] [CrossRef] [PubMed]
- Nozari, N.; Woodard, K.; Thompson-Schill, S.L. Consequences of cathodal stimulation for behavior: When does it help and when does it hurt performance? PLoS ONE 2014, 9, e84338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monte-Silva, K.; Liebetanz, D.; Grundey, J.; Paulus, W.; Nitsche, M.A. Dosage-dependent non-linear effect of L-dopa on human motor cortex plasticity. J Physiol 2010, 588 (Pt 18), 3415–3424. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Muller-Dahlhaus, F.; Paulus, W.; Ziemann, U. The pharmacology of neuroplasticity induced by non-invasive brain stimulation: Building models for the clinical use of CNS active drugs. J Physiol. 2012, 590 (Pt 19), 4641–4662. [Google Scholar] [CrossRef]
- Paulus, W. Transcranial electrical stimulation (tES—tDCS; tACS; tRNS) methods. Neuropsychol. Rehabil. 2011, 21, 602–617. [Google Scholar] [CrossRef]
- Ruigrok, A.N.; Salimi-Khorshidi, G.; Lai, M.C.; Baron-Cohen, S.; Lombardo, M.V.; Tait, R.J.; Suckling, J. A meta-analysis of sex differences in human brain structure. Neurosci. Biobehav. Rev. 2014, 39, 34–50. [Google Scholar] [CrossRef] [Green Version]
- Hill, A.T.; Rogasch, N.C.; Fitzgerald, P.B.; Hoy, K.E. TMS-EEG: A window into the neurophysiological effects of transcranial electrical stimulation in non-motor brain regions. Neurosci. Biobehav. Rev. 2016, 64, 175–184. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cotelli, M.; Manenti, R.; Gobbi, E.; Enrici, I.; Rusich, D.; Ferrari, C.; Adenzato, M. Theory of Mind Performance Predicts tDCS-Mediated Effects on the Medial Prefrontal Cortex: A Pilot Study to Investigate the Role of Sex and Age. Brain Sci. 2020, 10, 257. https://doi.org/10.3390/brainsci10050257
Cotelli M, Manenti R, Gobbi E, Enrici I, Rusich D, Ferrari C, Adenzato M. Theory of Mind Performance Predicts tDCS-Mediated Effects on the Medial Prefrontal Cortex: A Pilot Study to Investigate the Role of Sex and Age. Brain Sciences. 2020; 10(5):257. https://doi.org/10.3390/brainsci10050257
Chicago/Turabian StyleCotelli, Maria, Rosa Manenti, Elena Gobbi, Ivan Enrici, Danila Rusich, Clarissa Ferrari, and Mauro Adenzato. 2020. "Theory of Mind Performance Predicts tDCS-Mediated Effects on the Medial Prefrontal Cortex: A Pilot Study to Investigate the Role of Sex and Age" Brain Sciences 10, no. 5: 257. https://doi.org/10.3390/brainsci10050257
APA StyleCotelli, M., Manenti, R., Gobbi, E., Enrici, I., Rusich, D., Ferrari, C., & Adenzato, M. (2020). Theory of Mind Performance Predicts tDCS-Mediated Effects on the Medial Prefrontal Cortex: A Pilot Study to Investigate the Role of Sex and Age. Brain Sciences, 10(5), 257. https://doi.org/10.3390/brainsci10050257




