Intracranial Carotid Artery Aneurysm Treatment: First Reported Case of DERIVO®Flow-Diverter Placement by Direct Carotid Artery Puncture
Abstract
:1. Introduction
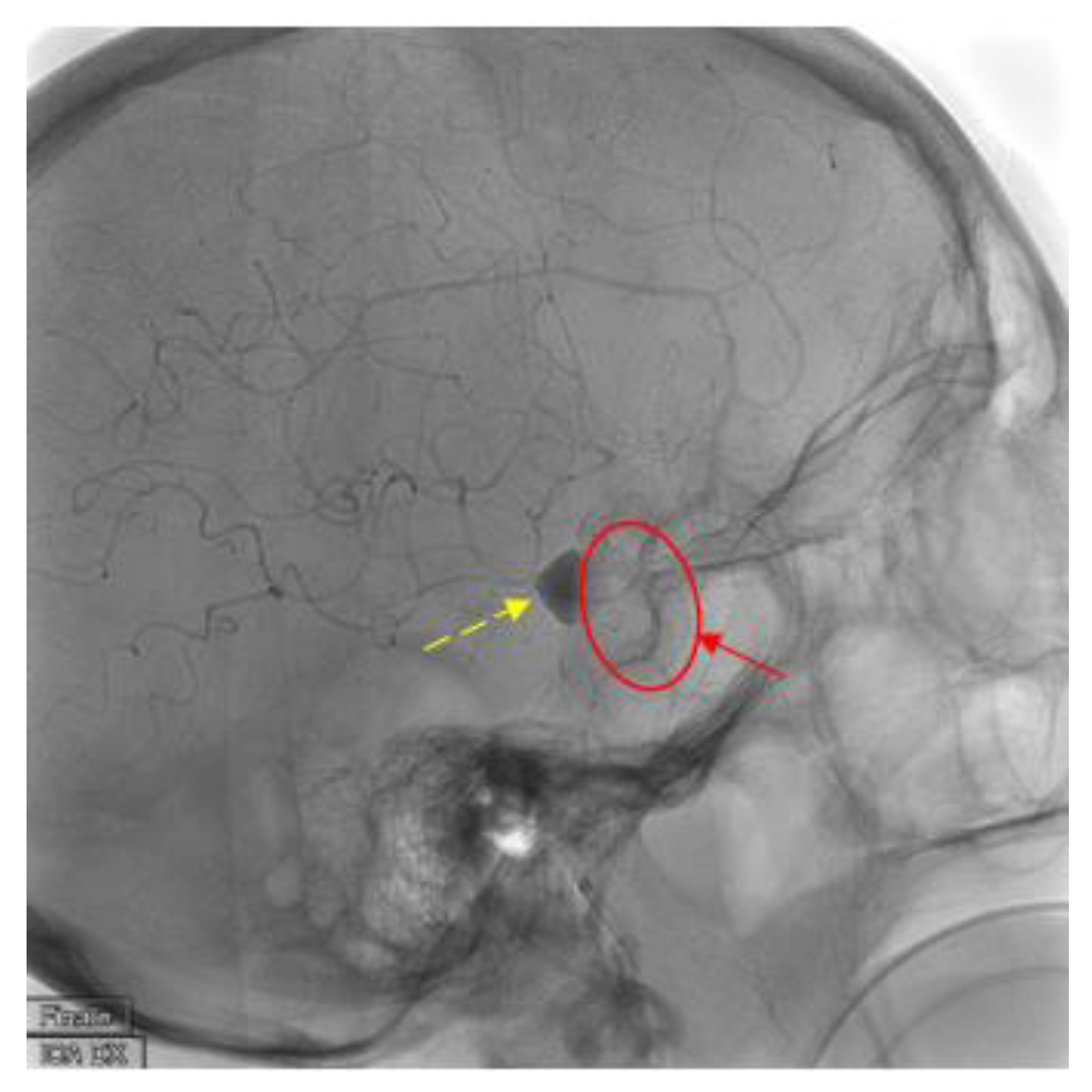
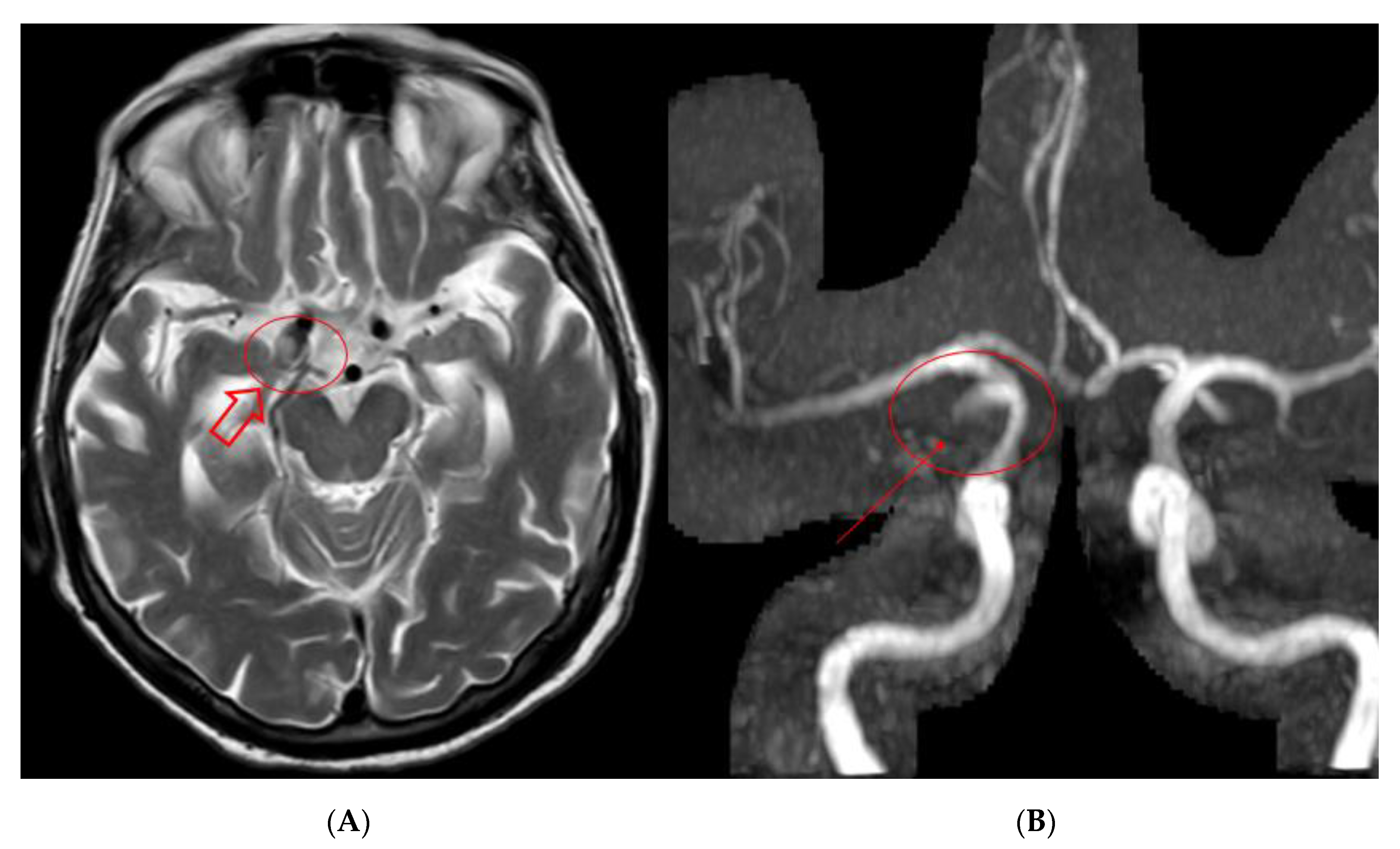
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Molyneux, A.J.; Kerr, R.S.C.; Stratton, I.; Sandercock, P.; Clarke, M.; Shrimpton, J.; Holman, R.; International Subarachnoid Aneurysm Trial (ISAT) Collaborative Group. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: A randomised trial. Lancet 2002, 360, 1267–1274. [Google Scholar] [CrossRef]
- Molyneux, A.J.; Kerr, R.S.C.; Birks, J.; Ramzi, N.; Yarnold, J.; Sneade, M.; Rischmiller, J.; ISAT Collaborators. Risk of recurrent subarachnoid haemorrhage, death, or dependence and standardised mortality ratios after clipping or coiling of an intracranial aneurysm in the International Subarachnoid Aneurysm Trial (ISAT): Long-term follow-up. Lancet Neurol. 2009, 8, 427–433. [Google Scholar] [CrossRef] [Green Version]
- McDougall, C.G.; Spetzler, R.F.; Zabramski, J.M.; Partovi, S.; Hills, N.K.; Nakaji, P.; Albuquerque, F.C. The Barrow Ruptured Aneurysm Trial. J. Neurosurg. 2012, 116, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Brinjikji, W.; Cloft, H.J.; Fiorella, D.; Lanzino, G.; Kallmes, D.F. Estimating the proportion of intracranial aneurysms likely to be amenable to treatment with the pipeline embolization device. J. Neurointerv. Surg. 2013, 5, 45–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazur, M.D.; Taussky, P.; Park, M.S.; Couldwell, W.T. Contemporary endovascular and openaneurysm treatment in the era of flow diversion. J. Neurol. Neurosurg. Psychiatry 2018, 89, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Briganti, F.; Leone, G.; Marseglia, M.; Mariniello, G.; Caranci, F.; Brunetti, A.; Maiuri, F. Endovascular treatment of cerebral aneurysms using flow-diverter devices: A systematic review. Neuroradiol. J. 2015, 28, 365–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levy, E.I.; Boulos, A.S.; Fessler, R.D.; Bendok, B.R.; Ringer, A.J.; Kim, S.H.; Qureshi, A.I.; Guterman, L.R.; Hopkins, L.N. Transradial cerebral angiography: An alternative route. Neurosurgery 2002, 51, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Wiesmann, M.; Kalder, J.; Reich, A.; Brockmann, M.A.; Othman, A.; Greiner, A.; Nikoubashman, O. Feasibility of combined surgical and endovascular carotid access for interventional treatment of ischemic stroke. J. Neurointerv. Surg. 2016, 8, 571–575. [Google Scholar] [CrossRef]
- Mokin, M.; Snyder, K.V.; Levy, E.I.; Hopkins, L.N.; Siddiqui, A.H. Direct carotid artery puncture access for endovascular treatment of acute ischemic stroke: Technical aspects, advantages, and limitations. J. Neurointerv. Surg. 2015, 7, 108–113. [Google Scholar] [CrossRef]
- Nii, K.; Kazekawa, K.; Onizuka, M.; Aikawa, H.; Tsutsumi, M.; Tomokiyo, M.; Iko, M.; Kodama, T.; Matsubara, S.; Go, Y.; et al. Direct carotid puncture for the endovascular treatment of anterior circulation aneurysms. Am. J. Neuroradiol. 2006, 27, 1502–1504. [Google Scholar]
- Matsuda, Y.; Terada, T.; Masuo, O.; Matsumoto, H.; Ohshima, K.; Tsumoto, T.; Tsuura, M. The clinical results of transcervical carotid artery stenting and frequency chosen as the approach route of carotid artery stenting in 1,067 consecutive cases. Acta Neurochir. 2013, 155, 1575–1581. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Park, J.H.; Jeon, H.J.; Yoon, D.Y.; Park, S.W.; Cho, B.M. Transcervical access via direct neck exposure for neurointerventional procedures in the hybrid angiosuite. Neuroradiology 2018, 60, 565. [Google Scholar] [CrossRef] [PubMed]
- Larrazabal, R.; Klurfan, P.; Sarma, D.; Gunnarsson, T. Surgical exposure of the carotid artery for endovascular interventional procedures. Acta Neurochir. 2010, 152, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Dorfer, C.; Standhardt, H.; Gruber, A.; Ferraz-Leite, H.; Knosp, E.; Bavinzski, G. Direct Percutaneous Puncture Approach versus Surgical Cutdown Technique for Intracranial Neuroendovascular Procedures: Technical Aspects. World Neurosurg. 2012, 77, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Cuellar, H.; Guimaraens, L.; Ambekar, S.; Vivas, E.; Theron, J. Angioseal as a hemostatic device for direct carotid puncture during endovascular procedures. Interv. Neuroradiol. 2015, 21, 273–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brunozzi, D.; Shakur, S.F.; Alaraj, A. Pipeline Embolization of Giant Cavernous Internal Carotid Artery Aneurysm with Direct Carotid Puncture and Arteriotomy Closure Device: Neuroendovascular Surgical Video. World Neurosurg. 2019, 123, 40. [Google Scholar] [CrossRef] [PubMed]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guzzardi, G.; Del Sette, B.; Stanca, C.; Paladini, A.; Galbiati, A.; Spinetta, M.; Cernigliaro, M.; Leigheb, M.; Carriero, A. Intracranial Carotid Artery Aneurysm Treatment: First Reported Case of DERIVO®Flow-Diverter Placement by Direct Carotid Artery Puncture. Brain Sci. 2020, 10, 320. https://doi.org/10.3390/brainsci10050320
Guzzardi G, Del Sette B, Stanca C, Paladini A, Galbiati A, Spinetta M, Cernigliaro M, Leigheb M, Carriero A. Intracranial Carotid Artery Aneurysm Treatment: First Reported Case of DERIVO®Flow-Diverter Placement by Direct Carotid Artery Puncture. Brain Sciences. 2020; 10(5):320. https://doi.org/10.3390/brainsci10050320
Chicago/Turabian StyleGuzzardi, Giuseppe, Bruno Del Sette, Carmelo Stanca, Andrea Paladini, Andrea Galbiati, Marco Spinetta, Massimiliano Cernigliaro, Massimiliano Leigheb, and Alessandro Carriero. 2020. "Intracranial Carotid Artery Aneurysm Treatment: First Reported Case of DERIVO®Flow-Diverter Placement by Direct Carotid Artery Puncture" Brain Sciences 10, no. 5: 320. https://doi.org/10.3390/brainsci10050320
APA StyleGuzzardi, G., Del Sette, B., Stanca, C., Paladini, A., Galbiati, A., Spinetta, M., Cernigliaro, M., Leigheb, M., & Carriero, A. (2020). Intracranial Carotid Artery Aneurysm Treatment: First Reported Case of DERIVO®Flow-Diverter Placement by Direct Carotid Artery Puncture. Brain Sciences, 10(5), 320. https://doi.org/10.3390/brainsci10050320






