Effort-Based Decision-Making and Gross Motor Performance: Are They Linked?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Participants
2.3. Procedure
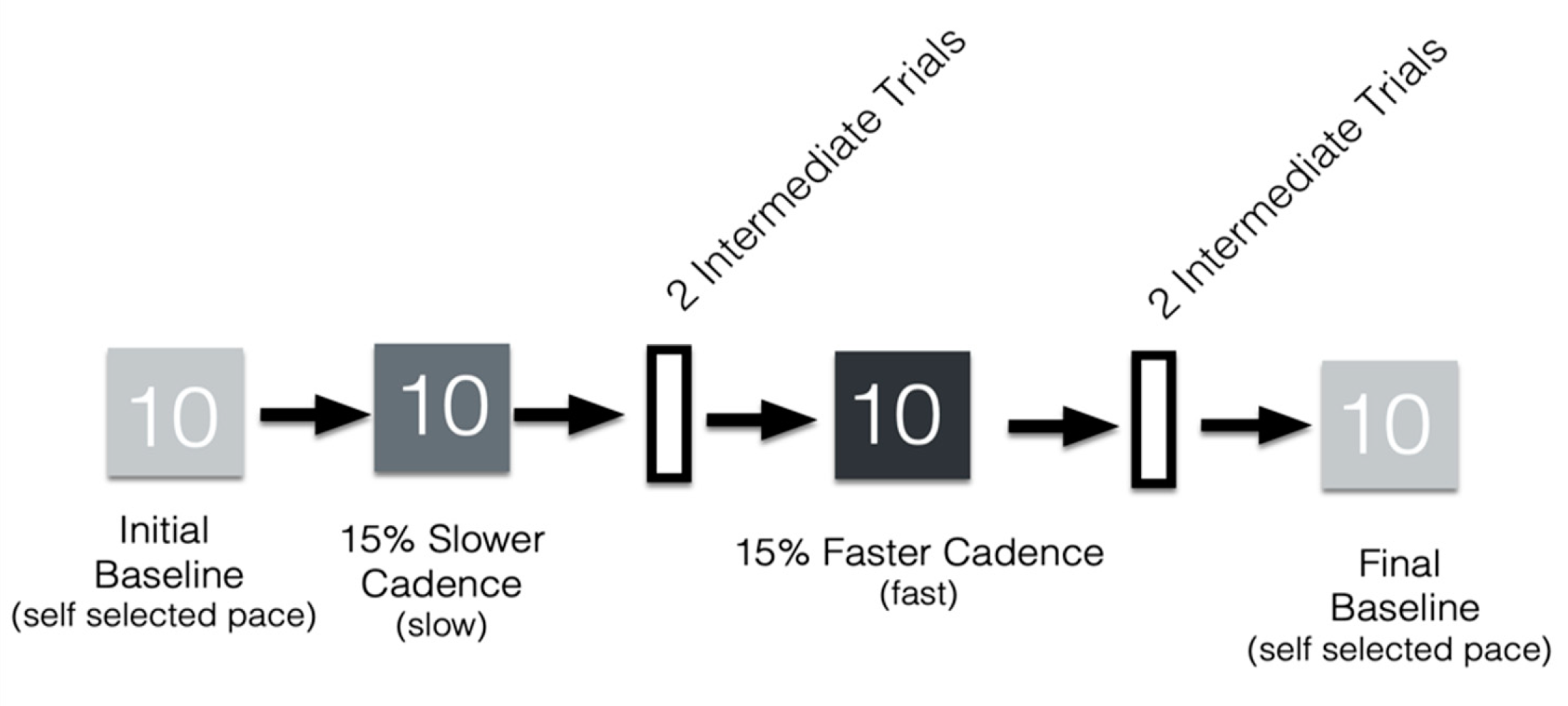
2.4. Metronome Walking Task
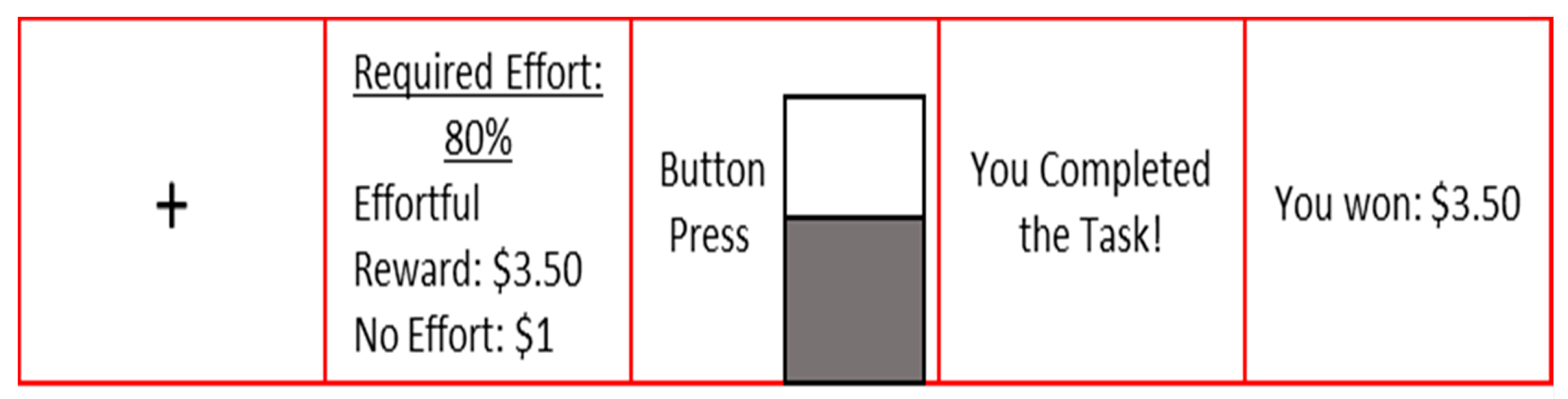
2.5. Effort-Based Decision-Making Task
2.6. Data Analysis
3. Results
3.1. Bivariate Correlations
3.2. Multilevel Models
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gill, S.V.; Hung, Y.C. Effects of overweight and obese body mass on motor planning and motor skills during obstacle crossing in children. Res. Dev. Disabil. 2014, 35, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.V.; Walsh, M.K.; Pratt, J.A.; Toosizadeh, N.; Najafi, B.; Travison, T.G. Changes in spatio-temporal gait patterns during flat ground walking and obstacle crossing one year after bariatric surgery. Surg. Obes. Relat. Dis. 2016, 12, 1080–1085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogamba, M.I.; Loverro, K.L.; Laudicina, N.M.; Gill, S.V.; Lewis, C.L. Changes in Gait with Anteriorly Added Mass: A Pregnancy Simulation Study. J. Appl. Biomech. 2016. [Google Scholar] [CrossRef] [PubMed]
- Kayser, A.S.; Buchsbaum, B.R.; Erickson, D.T.; D’Esposito, M. The functional anatomy of a perceptual decision in the human brain. J. Neurophysiol. 2010, 103, 1179–1194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kayser, A.S.; Erickson, D.T.; Buchsbaum, B.R.; D’Esposito, M. Neural representation of relevant and irrelevant features in perceptual decision making. J. Neurosci. 2010, 30, 15778–15789. [Google Scholar] [CrossRef]
- Noppeney, U.; Ostwald, D.; Werner, S. Perceptual decisions formed by accumulation of audiovisual evidence in prefrontal cortex. J. Neurosci. 2010, 30, 7434–7446. [Google Scholar] [CrossRef] [Green Version]
- Philiastides, M.G.; Sajda, P. EEG-informed fMRI reveals spatio-temporal characteristics of perceptual decision making. J. Neurosci. 2007, 27, 13082–13091. [Google Scholar] [CrossRef] [Green Version]
- Ernst, M.; Paulus, M.P. A selective review from a neurocognitive and clinical perspective. Biol. Psychiatry. 2005, 58, 597–604. [Google Scholar] [CrossRef]
- Gallivan, J.P.; Chapman, C.S.; Wolpert, D.M.; Flanagan, J.R. Decision-making in sensorimotor control. Nat. Rev. Neurosci. 2018, 19, 519–534. [Google Scholar] [CrossRef]
- Scott, S.H. Optimal feedback control and the neural basis of volitional motor control. Nat. Rev. Neurosci. 2004, 5, 532–546. [Google Scholar] [CrossRef] [PubMed]
- Todorov, E. Optimality principles in sensorimotor control. Nat. Neurosci. 2004, 7, 907–915. [Google Scholar] [CrossRef] [Green Version]
- Todorov, E.; Jordan, M.I. Optimal feedback control as a theory of motor coordination. Nat. Neurosci. 2002, 5, 1226–1235. [Google Scholar] [CrossRef]
- Heekeren, H.R.; Marrett, S.; Ungerleider, L.G. The neural systems that mediate human perceptual decision making. Nat. Rev. Neurosci. 2008, 9, 467–479. [Google Scholar] [CrossRef]
- Glover, S. Separate visual representation in the planning and control of action. Behav. Brain Res. 2004, 27, 3–24. [Google Scholar] [CrossRef] [Green Version]
- Jeannerod, M. The Neural and Behavioural Organization of Goal-Directed Movements; Oxford University Press: Oxford, England, 1990; Volume 15, pp. 1–40. [Google Scholar]
- Rosenbaum, D.A. Human Motor Control; Cambridge Academic Press: Cambridge, UK, 1991; Volume 2, pp. 4–65. [Google Scholar]
- Woodworth, R.S. The accuracy of voluntary movement. Psychol. Rev. 1899, 3, i114. [Google Scholar]
- Filimon, F.; Philiastides, M.G.; Nelson, J.D.; Kloosterman, N.A.; Heekeren, H.R. How embodied is perceptual decision making? Evidence for separate processing of perceptual and motor decisions. J. Neurosci. 2013, 33, 2121–2136. [Google Scholar] [CrossRef] [Green Version]
- Kiani, R.; Shadlen, M.N. Representation of confidence associated with a decision by neurons in the parietal cortex. Science. 2009, 324, 759–764. [Google Scholar] [CrossRef] [Green Version]
- Treadway, M.T.; Zald, D.H. Reconsidering anhedonia in depression: Lessons from translational neuroscience. Neurosci. Biobehav. Rev. 2011, 35, 537–555. [Google Scholar] [CrossRef] [Green Version]
- Treadway, M.T.; Buckholtz, J.W.; Schwartzman, A.N.; Lambert, W.E.; Zald, D.H. Worth the ‘EEfRT’? The effort expenditure for rewards task as an objective measure of motivation and anhedonia. PLoS ONE 2009, 4, e6598. [Google Scholar] [CrossRef] [Green Version]
- Gold, J.M.; Waltz, J.A.; Frank, M.J. Effort Cost Computation in Schizophrenia: A Commentary on the Recent Literature. Biol. Psychiatry 2015, 78, 747–753. [Google Scholar] [CrossRef] [Green Version]
- Gill, S.V. Walking to the beat of their own drum: How children and adults meet task constraints. PLoS ONE 2015, 10, e0127894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gill, S.V.; Narain, A. Quantifying the Effects of Body Mass Index on Safety: Reliability of a Video Coding Procedure and Utility of a Rhythmic Walking Task. Arch. Phys. Med. Rehabil. 2012, 93, 728–730. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.V. The impact of weight classification on safety: Timing steps to adapt to external constraints. J. Musculoskelet Neuronal Interact. 2015, 15, 103–108. [Google Scholar] [PubMed]
- Mehmet, H.; Robinson, S.R.; Yang, A.W.H. Assessment of Gait Speed in Older Adults. J. Geriatr. Phys. Ther. 2001. 2020, 43, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Corzani, M.; Ferrari, A.; Ginis, P.; Nieuwboer, A.; Chiari, L. Motor Adaptation in Parkinson’s Disease During Prolonged Walking in Response to Corrective Acoustic Messages. Front. Aging. Neurosci. 2019, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muller, F.; Canal-Bruland, R. Motivation in the wild: A critical review of the relationship between motives and motor performance. Motiv. Sci. 2020, 6, 93–109. [Google Scholar] [CrossRef]
- Umesh, A.; Sanawu Kutten, K.; Hogan, P.S.; Ratnanather, J.T.; Chiib, V.S. Motor cortical thickness is related to effort-based decision-making in humans. J. Neurophysiol. (in press). [CrossRef]
- Abplanalp, S.J.; Fulford, D. Physical effort exertion and pain: Links with trait-based risk for psychopathology. Psychiatry Res. 2019, 271, 46–51. [Google Scholar] [CrossRef]
- Arulpragasam, A.R.; Cooper, J.A.; Nuutinen, M.R.; Treadway, M.T. Corticoinsular circuits encode subjective value expectation and violation for effortful goal-directed behavior. Proc. Natl. Acad. Sci. 2018, 115, E5233–E5242. [Google Scholar] [CrossRef] [Green Version]
- Raudenbush, S.; Bryk, A.; Cheong, Y.; Congdon, R., Jr. HLM for Windows; Scientific Software International: Chicago, IL, USA, 2013; Volume 6, pp. 140–148. [Google Scholar]
- Gelman, A.; Hill, J.; Yajima, M. Why We (Usually) Don’t Have to Worry About Multiple Comparisons. J. Res. Educ. Eff. 2012, 5, 189–211. [Google Scholar] [CrossRef] [Green Version]
- Ben-Sasson, A.; Gill, S.V. Motor and language abilities from early to late toddlerhood: Using formalized assessments to capture continuity and discontinuity in development. Res. Dev. Disabil. 2014, 35, 1425–1432. [Google Scholar] [CrossRef]
- Dominguez-Zamora, F.J.; Marigold, D.S. Motor cost affects the decision of when to shift gaze for guiding movement. J. Neurophysiol. 2019, 122, 378–388. [Google Scholar] [CrossRef]
- Gill, S.V. Optimising motor adaptation in childhood obesity. Aust. Occup. Ther. J. 2011, 58, 386–389. [Google Scholar] [CrossRef]
- Johansson, R.S.; Flanagan, J.R. Coding and use of tactile signals from the fingertips in object manipulation tasks. Nat. Neurosci. 2009, 10, 345–359. [Google Scholar] [CrossRef]
- Andersen, R.A.; Buneo, C.A. Intentional maps in posterior parietal cortex. Annu. Rev. Neurosci. 2002, 25, 189–220. [Google Scholar] [CrossRef] [Green Version]
- Bennur, S.; Gold, J.I. Distinct representation of a perceptual decision and the associated oculomotor plan in the monkey lateral intraparietal area. J. Neurosci. 2011, 31, 913–921. [Google Scholar] [CrossRef] [Green Version]
- Gold, J.I.; Shadlen, M.N. The influence of behavioral context on the representation of a perceptual decision in developing oculomotor commands. J. Neurosci. 2003, 23, 632–651. [Google Scholar] [CrossRef]
- Faisal, A.A.; Selen, L.P.J.; Wolpert, D.M. Noise in the nervous system. Nat. Rev. Neurosci. vol. 2008, 9, 292–303. [Google Scholar] [CrossRef]
- Lopez-Gamundi, P.; Wardle, M.C. The cognitive effort expenditure for rewards task (C-EEfRT): A novel measure of willingness to expend cognitive effort. Psychol. Assess. 2018, 30, 1237–1248. [Google Scholar] [CrossRef]
- Gill, S.V.; Yang, Z.; Hung, Y.C. Effects of singular and dual task constraints on motor skill variability in childhood. Gait Posture 2017, 53, 121–126. [Google Scholar] [CrossRef] [Green Version]
- Hagura, N.; Haggard, P.; Diedrichsen, J. Perceptual decisions are biased by the cost to act. eLife 2017, 6, e18422. [Google Scholar] [CrossRef] [Green Version]
- Proffitt, D.R.; Stefanucci, J.; Banton, I.; Epstein, W. The role of effort in perceiving distance. Psychol Sci. 2003, 14, 106–112. [Google Scholar] [CrossRef]
- Ramenzoni, V.C.; Riley, M.A.; Shockley, K.; Davis, T. Carrying the height of the world on your ankles: Encumbering observers reduces estimates of how high an actor can jump. Q. J. Exp. Psychol. 2008, 61, 1487–1495. [Google Scholar] [CrossRef]
- Nashed, J.Y.; Crevecoeur, F.; Scott, S.H. Influence of the behavioural goal and environmental obstacles on rapid feedback responses. J. Neurophysiol. 2012, 108, 999–1009. [Google Scholar] [CrossRef] [Green Version]
- Gill, S.; Hicks, G.; Zhang, Y.; Niu, J.; Apovian, C.; White, D.K. The association of waist circumference with community walking ability in knee osteoarthritis: The osteoarthritis initiative. Osteoarthr. Cartil. 2017, 25, 60–66. [Google Scholar] [CrossRef] [Green Version]
- Lick, D.J.; Johnson, K.L.; Gill, S.V. Why do they have to flaunt it? Perceptions of communicative intent predict antigay prejudice based upon brief exposure to nonverbal cues. Soc. Psychol. Personal. Sci. 2014, 5, 927–935. [Google Scholar] [CrossRef]
- Lick, D.J.; Johnson, K.L.; Gill, S.V. Deliberate Changes to Gendered Body Motion Influence Basic Social Perceptions. Soc. Cogn. 2013, 31, 656–671. [Google Scholar] [CrossRef]
- Shadmehr, R.; Huang, H.J.; Ahmed, A.A. A representation of effort in decision-making and motor control. Curr Biol. 2016, 26, 1929–1934. [Google Scholar] [CrossRef] [Green Version]
| Mean (%) | |
|---|---|
| Age | 23.74 (8.45; 18–65) |
| Gender | |
| Female | 48.0 |
| Male | 52.0 |
| Race | |
| Non-Hispanic Caucasian | 62.0 |
| Asian-American | 30.0 |
| African-American | 4.0 |
| Multiracial | 2.0 |
| Other not listed | 2.0 |
| Mean | Standard Deviation | Range | |
|---|---|---|---|
| Cadence * | |||
| Baseline | 107.20 | 7.18 | 92–132 |
| 15% slower | 91.12 | 6.43 | 76–112 |
| 15% faster | 122.56 | 8.19 | 104–152 |
| Effort based-decision making † | 0.71 | 0.12 | 0.43–0.93 |
| 1. | 2. | 3. | 4. | 5. | |
|---|---|---|---|---|---|
| 1. Age | |||||
| 2. Gender | .24 | ||||
| 3. Baseline cadence | .15 | −.33 * | |||
| 4. 15% slower cadence | .14 | −.31 * | .97 ** | ||
| 5. 15% faster cadence | .13 | −.37 ** | .98 ** | .97 ** | |
| 6. Effort-based decision-making | .15 | −.19 | .27 † | .25 † | .24 † |
| Baseline Cadence Model | 15% Slower Cadence Model | 15% Faster Cadence Model | |
|---|---|---|---|
| Coefficient (SE) p | Coefficient (SE) p | Coefficient (SE) p | |
| Within subject level | |||
| Effort level | −6.49 (2.30) <.01 | −6.69 (2.26) <.01 | −6.66 (2.34) <.01 |
| Reward amount | 1.65 (0.42) <.01 | 1.65 (0.41) <.01 | 1.67 (0.43) <.01 |
| Between subject level | |||
| Age | 0.03 (0.07) .64 | 0.04 (0.07) .60 | 0.03 (0.07) .63 |
| Age X effort level | 0.02 (0.01) .77 | 0.02 (0.08) .89 | 0.02 (0.08) .79 |
| Age X reward amount | −0.01 (0.02) .88 | −0.01 (0.01) .52 | −0.01 (0.02) .54 |
| Sex | 0.40 (0.07) .74 | 0.29 (1.18) .81 | 0.35 (1.21) .77 |
| Sex X effort level | −0.33 (1.46) .82 | −0.20 (1.43) .89 | −0.22 (1.48) .88 |
| Sex X reward amount | −0.20 (0.26) .44 | −0.21 (0.26) .43 | −0.22 (0.27) .42 |
| Baseline cadence | −0.03 (0.08) .68 | ||
| Baseline cadence X effort level | 0.07 (0.10) .51 | ||
| Baseline cadence X reward amount | 0.01 (0.02) .88 | ||
| 15% slower cadence | −0.07 (0.10) .37 | ||
| 15% slower cadence X effort level | 0.12 (0.11) .31 | ||
| 15% slower cadence X reward amount | 0.01 (0.02) .92 | ||
| 15% faster cadence | −0.03 (0.07) .64 | ||
| 15% faster cadence X effort level | 0.07 (0.09) .43 | ||
| 15% faster cadence X reward amount | −0.01 (0.02) .98 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gill, S.V.; Abplanalp, S.J.; Keegan, L.; Fulford, D. Effort-Based Decision-Making and Gross Motor Performance: Are They Linked? Brain Sci. 2020, 10, 347. https://doi.org/10.3390/brainsci10060347
Gill SV, Abplanalp SJ, Keegan L, Fulford D. Effort-Based Decision-Making and Gross Motor Performance: Are They Linked? Brain Sciences. 2020; 10(6):347. https://doi.org/10.3390/brainsci10060347
Chicago/Turabian StyleGill, Simone V., Samuel J. Abplanalp, Laura Keegan, and Daniel Fulford. 2020. "Effort-Based Decision-Making and Gross Motor Performance: Are They Linked?" Brain Sciences 10, no. 6: 347. https://doi.org/10.3390/brainsci10060347
APA StyleGill, S. V., Abplanalp, S. J., Keegan, L., & Fulford, D. (2020). Effort-Based Decision-Making and Gross Motor Performance: Are They Linked? Brain Sciences, 10(6), 347. https://doi.org/10.3390/brainsci10060347




