Uniportal Full Endoscopic Posterolateral Transforaminal Lumbar Interbody Fusion with Endoscopic Disc Drilling Preparation Technique for Symptomatic Foraminal Stenosis Secondary to Severe Collapsed Disc Space: A Clinical and Computer Tomographic Study with Technical Note
Abstract
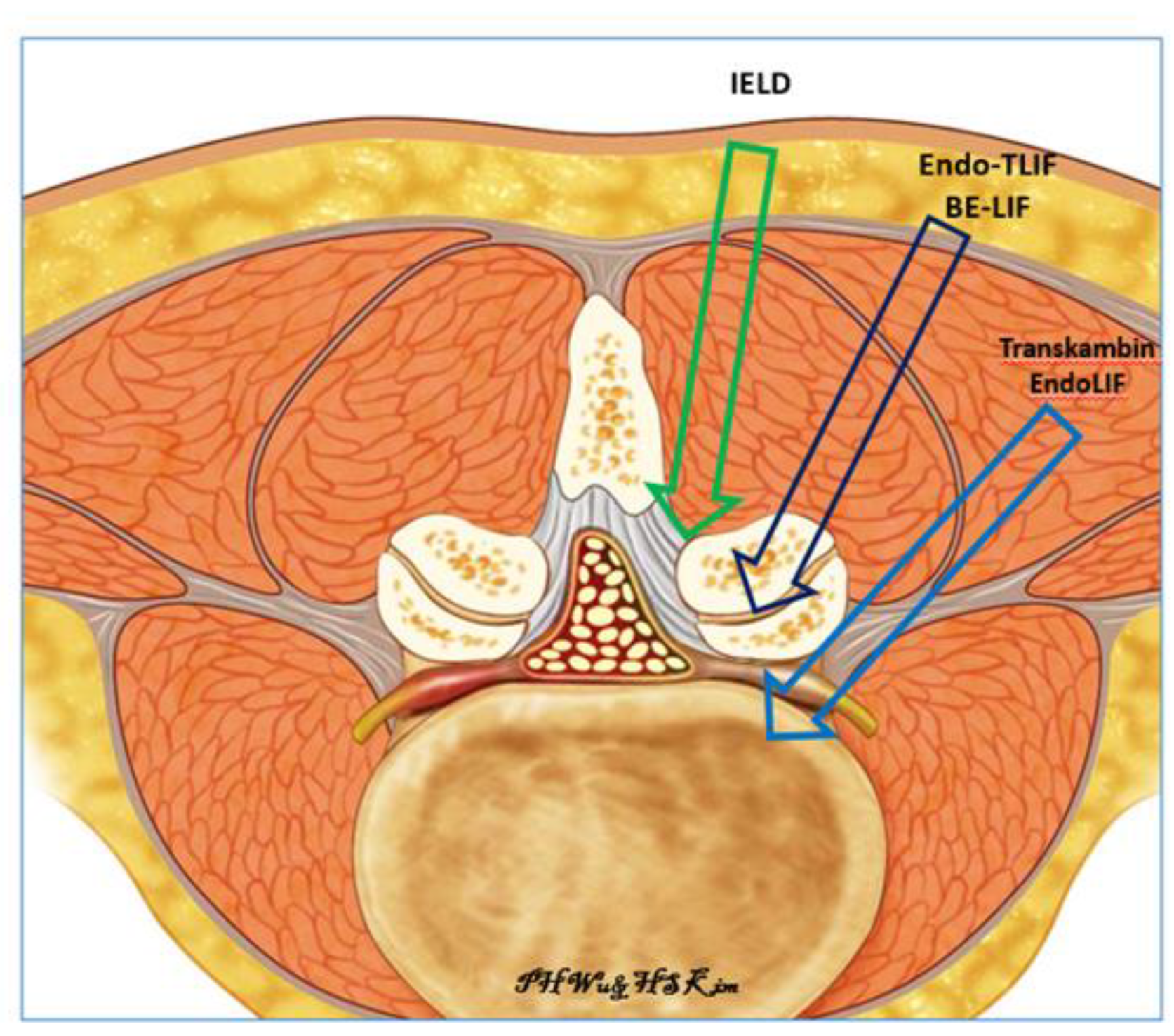
:1. Introduction
2. Materials and Methods
2.1. Indication, Inclusion and Exclusion Criteria
2.2. Surgical Technique
2.2.1. Pre-Operative Preparation
2.2.2. Anesthesia and Skin Incision
2.2.3. Insertion of Endoscope
2.2.4. Surgical Procedure
3. Pedicle Screw and Rod Insertion with Appropriate Compression and Distraction
3.1. Post-Operative Care
3.2. Statistical Analysis
4. Results
4.1. Baseline Demographics
4.2. Clinical and Radiological Outcomes
5. Discussion
6. Limitations
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vos, T.P.; Flaxman, A.D.P.; Naghavi, M.P.; Lozano, R.P.; Michaud, C.M.D.; Ezzati, M.P.; Shibuya, K.P.; Salomon, J.A.P.; Abdalla, S.M.; Aboyans, V.P.; et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2163–2196. [Google Scholar] [CrossRef]
- Wu, P.H.; Kim, H.S.; Jang, I.-T. Intervertebral Disc Diseases PART 2: A Review of the Current Diagnostic and Treatment Strategies for Intervertebral Disc Disease. Int. J. Mol. Sci. 2020, 21, 2135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weinstein, J.N.; Lurie, J.D.; Tosteson, T.D.; Zhao, W.; Blood, E.A.; Tosteson, A.N.; Birkmeyer, N.; Herkowitz, H.; Longley, M.; Lenke, L.; et al. Surgical compared with nonoperative treatment for lumbar degenerative spondylolisthesis: Four-year results in the Spine Patient Outcomes Research Trial (SPORT) randomized and observational cohorts. J. Bone Jt. Surg. Am. 2009, 91, 1295–1304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, S.I.; Javed, G.; Bareeqa, S.B.; Shah, A.; Zubair, M.; Avedia, R.F.; Rahman, N.; Samar, S.S.; Aziz, K. Comparison of Decompression Alone versus Decompression with Fusion for Stenotic Lumbar Spine: A Systematic Review and Meta-analysis. Cureus 2018, 10, e3135. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.; Kim, H.S.; Oh, S.W.; Adsul, N.M.; Singh, R.; Kashlan, O.N.; Noh, J.H.; Jang, I.T.; Oh, S.H. Evolution of Spinal Endoscopic Surgery. Neurospine 2019, 16, 6–14. [Google Scholar] [CrossRef] [Green Version]
- Ruetten, S.; Komp, M.; Merk, H.; Godolias, G. Recurrent lumbar disc herniation after conventional discectomy: A prospective, randomized study comparing full-endoscopic interlaminar and transforaminal versus microsurgical revision. J. Spinal Disord. Tech. 2009, 22, 122–129. [Google Scholar] [CrossRef]
- Ahn, Y. Percutaneous endoscopic decompression for lumbar spinal stenosis. Expert Rev. Med. Devices 2014, 11, 605–616. [Google Scholar] [CrossRef]
- Wu, P.H.; Kim, H.S.; Jang, I.-T. How I do it? Uniportal full endoscopic contralateral approach for lumbar foraminal stenosis with double crush syndrome. Acta Neurochir. 2019, 162, 305–310. [Google Scholar] [CrossRef] [Green Version]
- Ito, F.; Ito, Z.; Shibayama, M.; Nakamura, S.; Yamada, M.; Yoshimatu, H.; Takeuchi, M.; Shimizu, K.; Miura, Y. Step-by-Step Sublaminar Approach with a Newly-Designed Spinal Endoscope for Unilateral-Approach Bilateral Decompression in Spinal Stenosis. Neurospine 2019, 16, 41–51. [Google Scholar] [CrossRef] [Green Version]
- Morgenstern, R.; Morgenstern, C.; Jane, R.; Lee, S.H. Usefulness of an expandable interbody spacer for the treatment of foraminal stenosis in extremely collapsed disks: Preliminary clinical experience with endoscopic posterolateral transforaminal approach. J. Spinal Disord. Tech. 2011, 24, 485–491. [Google Scholar] [CrossRef]
- Wang, M.Y.; Grossman, J. Endoscopic minimally invasive transforaminal interbody fusion without general anesthesia: Initial clinical experience with 1-year follow-up. Neurosurg. Focus 2016, 40, E13. [Google Scholar] [CrossRef] [PubMed]
- Shen, J. Fully Endoscopic Lumbar Laminectomy and Transforaminal Lumbar Interbody Fusion under Local Anesthesia with Conscious Sedation: A Case Series. Biomed. Res. Int. 2019, 127, e745–e750. [Google Scholar] [CrossRef] [PubMed]
- Harms, J. Dorsale Repositionsspondylodese bei lumbalen Spondylolisthesis. Oper. Orthopädie Traumatol. 1999, 11, 79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heo, D.H.; Son, S.K.; Eum, J.H.; Park, C.K. Fully endoscopic lumbar interbody fusion using a percutaneous unilateral biportal endoscopic technique: Technical note and preliminary clinical results. Biomed. Res. Int. 2017, 43, E8. [Google Scholar] [CrossRef] [PubMed]
- Son, S.K.; Park, W.W.; Choi, S.H.; Ahn, Y.; Youn, M.S.; Heo, D.H. Endoscopic transforaminal lumbar interbody fusion: A comprehensive review. Neurosurg. Rev. 2019, 16, 373–380. [Google Scholar]
- Kim, H.S.; Wu, P.H.; Jang, I.-T. Technical note on Uniportal full endoscopic posterolateral approach transforaminal lumbar interbody fusion with reduction for grade 2 spondylolisthesis. Interdiscip. Neurosurg. 2020, 21, 100712. [Google Scholar] [CrossRef]
- Lee, S.; Lee, J.W.; Yeom, J.S.; Kim, K.J.; Kim, H.J.; Chung, S.K.; Kang, H.S. A practical MRI grading system for lumbar foraminal stenosis. Am. J. Roentgenol. 2010, 194, 1095–1098. [Google Scholar] [CrossRef] [Green Version]
- Panjabi, M.M. Clinical spinal instability and low back pain. J. Electromyogr. Kinesiol. 2003, 13, 371–379. [Google Scholar] [CrossRef]
- Haimoto, S.; Nishimura, Y.; Hara, M.; Nakajima, Y.; Yamamoto, Y.; Ginsberg, H.J.; Wakabayashi, T. Clinical and Radiological Outcomes of Microscopic Lumbar Foraminal Decompression: A Pilot Analysis of Possible Risk Factors for Restenosis. Neurol. Med.-Chir. 2018, 58, 49–58. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.S.; Raorane, H.D.; Hung, W.P.; Heo, D.H.; Sharma, S.B.; Jang, I.T. Incidental Durotomy during Endoscopic Stenotic Lumbar Decompression (ESLD): Incidence, classification and proposed management strategies. World Neurosurg. 2020. [Google Scholar] [CrossRef]
- Osman, S.G. Endoscopic transforaminal decompression, interbody fusion, and percutaneous pedicle screw implantation of the lumbar spine: A case series report. Int. J. Spine Surg. 2012, 6, 157–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.S.; Paudel, B.; Jang, J.S.; Lee, K.; Oh, S.H.; Jang, I.T. Percutaneous Endoscopic Lumbar Discectomy for All Types of Lumbar Disc Herniations (LDH) Including Severely Difficult and Extremely Difficult LDH Cases. Pain Physician 2018, 21, e401–e408. [Google Scholar] [PubMed]
- Zhou, Y.; Zhang, C.; Wang, J.; Chu, T.W.; Li, C.Q.; Zhang, Z.F.; Zheng, W.J. Endoscopic transforaminal lumbar decompression, interbody fusion and pedicle screw fixation—A report of 42 cases. Chin. J. Traumatol. 2008, 11, 225–231. [Google Scholar] [CrossRef] [Green Version]
- Kolcun, J.P.G.; Brusko, G.D.; Basil, G.W.; Epstein, R.; Wang, M.Y. Endoscopic transforaminal lumbar interbody fusion without general anesthesia: Operative and clinical outcomes in 100 consecutive patients with a minimum 1-year follow-up. Neurosurg. Focus 2019, 46, e14. [Google Scholar] [CrossRef] [Green Version]
- Jacquot, F.; Gastambide, D. Percutaneous endoscopic transforaminal lumbar interbody fusion: Is it worth it? Int. Orthop. 2013, 37, 1507–1510. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.E.; Choi, D.J. Biportal Endoscopic Transforaminal Lumbar Interbody Fusion with Arthroscopy. Clin. Orthop. Surg. 2018, 10, 248–252. [Google Scholar] [CrossRef]
- Cripton, P.A.; Jain, G.M.; Wittenberg, R.H.; Nolte, L.P. Load-sharing characteristics of stabilized lumbar spine segments. Spine 2000, 25, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Morgenstern, C.; Yue, J.J.; Morgenstern, R. Full Percutaneous Transforaminal Lumbar Interbody Fusion Using the Facet-sparing, Trans-Kambin Approach. Clin. Spine Surg. 2019, 33, 40–45. [Google Scholar] [CrossRef]
- Lee, K.H.; Yeo, W.; Soeharno, H.; Yue, W.M. Learning curve of a complex surgical technique: Minimally invasive transforaminal lumbar interbody fusion (MIS TLIF). J. Spinal Disord. Tech. 2014, 27, e234–e240. [Google Scholar] [CrossRef]
- Park, H.-J.; Kim, S.-K.; Lee, S.-C.; Kim, W.; Han, S.; Kang, S.-S. Dural Tears in Percutaneous Biportal Endoscopic Spine Surgery: Anatomical Location and Management. World Neurosurg. 2020, 136, e578–e585. [Google Scholar] [CrossRef]
- Lee, C.-W.; Yoon, K.-J.; Kim, S.-W. Percutaneous Endoscopic Decompression in Lumbar Canal and Lateral Recess Stenosis—The Surgical Learning Curve. Neurospine 2019, 16, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, H.S.; Kapoor, A.; Adsul, N.; Kim, K.J.; Choi, S.H.; Jang, J.-S.; Jang, I.-T.; Oh, S.-H. Feasibility of Full Endoscopic Spine Surgery in Patients over the Age of 70 Years with Degenerative Lumbar Spine Disease. Neurospine 2018, 15, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-H.; Choi, M.; Ryu, D.S.; Choi, I.; Kim, C.H.; Kim, H.S.; Sohn, M.-J. Efficacy and Safety of Full-endoscopic Decompression via Interlaminar Approach for Central or Lateral Recess Spinal Stenosis of the Lumbar Spine: A Meta-analysis. Spine 2018, 43, 1756–1764. [Google Scholar] [CrossRef]
- Hwa Eum, J.; Hwa Heo, D.; Son, S.K.; Park, C.K. Percutaneous biportal endoscopic decompression for lumbar spinal stenosis: A technical note and preliminary clinical results. J. Neurosurg. Spine 2016, 24, 602–607. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-S.M.D.P.; Paudel, B.M.D.M.S.; Jang, J.-S.M.D.P.; Oh, S.-H.M.D.P.; Lee, S.B.E.; Park, J.E.B.S.; Jang, I.-T.M.D.P. Percutaneous Full Endoscopic Bilateral Lumbar Decompression of Spinal Stenosis through Uniportal-Contralateral Approach: Techniques and Preliminary Results. World Neurosurg. 2017, 103, 201–209. [Google Scholar] [CrossRef]
- Hwang, J.H.M.D.; Park, C.W.M.D.P. Contralateral interlaminar keyhole percutaneous endoscopic surgery in patients with unilateral radiculopathy: Technical notes. World Neurosurg. 2017, 101, 33–41. [Google Scholar] [CrossRef]
- Hasan, S.; McGrath, L.B.; Sen, R.D.; Barber, J.K.; Hofstetter, C.P. Comparison of full-endoscopic and minimally invasive decompression for lumbar spinal stenosis in the setting of degenerative scoliosis and spondylolisthesis. Neurosurg. Focus 2019, 46, e16. [Google Scholar] [CrossRef] [Green Version]
- Muller, S.J.; Burkhardt, B.W.; Oertel, J.M. Management of Dural Tears in Endoscopic Lumbar Spinal Surgery: A Review of the Literature. World Neurosurg. 2018, 119, 494–499. [Google Scholar] [CrossRef]
- Knight, M.T.; Jago, I.; Norris, C.; Midwinter, L.; Boynes, C. Transforaminal endoscopic lumbar decompression & foraminoplasty: A 10 year prospective survivability outcome study of the treatment of foraminal stenosis and failed back surgery. Int. J. Spine Surg. 2014, 8, 21. [Google Scholar]
- Dusad, T.; Kundnani, V.; Dutta, S.; Patel, A.; Mehta, G.; Singh, M. Comparative Prospective Study Reporting Intraoperative Parameters, Pedicle Screw Perforation, and Radiation Exposure in Navigation-Guided versus Non-navigated Fluoroscopy-Assisted Minimal Invasive Transforaminal Lumbar Interbody Fusion. Asian Spine J. 2018, 12, 309–316. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Xu, C.; Zhou, Y.; Huang, B. Minimally Invasive Computer Navigation-Assisted Endoscopic Transforaminal Interbody Fusion with Bilateral Decompression via a Unilateral Approach: Initial Clinical Experience at One-Year Follow-Up. World Neurosurg. 2017, 106, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Fan, G.; Han, R.; Gu, X.; Zhang, H.; Guan, X.; Fan, Y.; Wang, T.; He, S. Navigation improves the learning curve of transforamimal percutaneous endoscopic lumbar discectomy. Int. Orthop. 2017, 41, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Ian, G.D.; Lawrence, G.L. Osteotomies in the posterior-only treatment of complex adult spinal deformity: A comparative review. Neurosurg. Focus 2010, 28, e4. [Google Scholar]










| Mean | Range/(SD) | p Value | Remarks | |
|---|---|---|---|---|
| Age (years) | 68 | 54 to 78 | ||
| Levels | 30 levels in 27 patients | |||
| Sex | 9 male, 18 female | |||
| Follow up (months) | 12 | 6 to 19 | ||
| Pre-operative VAS | 6.23 | 5 to 7 | ||
| Post-operative VAS at 1 week | 3.7 | 2 to 5 | ||
| Post-operative VAS at 3 months | 3.03 | 1 to 4 | ||
| Post-operative VAS at final follow up | 1.87 | 1 to 3 | ||
| Pre-op VAS-post 1 week VAS | 2.53 | 1.137 (SD) | 0.000 | |
| Pre-op VAS-post 3 month VAS | 3.20 | 0.890 (SD) | 0.000 | |
| Pre-op VAS-final VAS | 4.37 | 1.033 (SD) | 0.000 | |
| Mid coronal CT disc height (post-op-pre-op) | 7.13 | 1.90 (SD) | 0.000 | |
| Mid sagittal CT anterior disc height (post-op-pre-op) | 6.99 | 2.30 (SD) | 0.000 | |
| Mid sagittal CT middle disc height (post-op-pre-op) | 6.28 | 1.43 (SD) | 0.000 | |
| Mid sagittal CT posterior disc height (post-op-pre-op) | 5.12 | 1.79 (SD) | 0.000 | |
| Mid coronal CT Coronal Wedge Angle (post-op-pre-op) | 2.35 | 4.73 (SD) | 0.012 | |
| Mid sagittal CT focal segmental angle (post-op-pre-op) | 1.98 | 4.69 (SD) | 0.028 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, P.H.; Kim, H.S.; Lee, Y.J.; Kim, D.H.; Lee, J.H.; Jeon, J.B.; Raorane, H.D.; Jang, I.-T. Uniportal Full Endoscopic Posterolateral Transforaminal Lumbar Interbody Fusion with Endoscopic Disc Drilling Preparation Technique for Symptomatic Foraminal Stenosis Secondary to Severe Collapsed Disc Space: A Clinical and Computer Tomographic Study with Technical Note. Brain Sci. 2020, 10, 373. https://doi.org/10.3390/brainsci10060373
Wu PH, Kim HS, Lee YJ, Kim DH, Lee JH, Jeon JB, Raorane HD, Jang I-T. Uniportal Full Endoscopic Posterolateral Transforaminal Lumbar Interbody Fusion with Endoscopic Disc Drilling Preparation Technique for Symptomatic Foraminal Stenosis Secondary to Severe Collapsed Disc Space: A Clinical and Computer Tomographic Study with Technical Note. Brain Sciences. 2020; 10(6):373. https://doi.org/10.3390/brainsci10060373
Chicago/Turabian StyleWu, Pang Hung, Hyeun Sung Kim, Yeon Jin Lee, Dae Hwan Kim, Jun Hyung Lee, Jun Bok Jeon, Harshavardhan Dilip Raorane, and Il-Tae Jang. 2020. "Uniportal Full Endoscopic Posterolateral Transforaminal Lumbar Interbody Fusion with Endoscopic Disc Drilling Preparation Technique for Symptomatic Foraminal Stenosis Secondary to Severe Collapsed Disc Space: A Clinical and Computer Tomographic Study with Technical Note" Brain Sciences 10, no. 6: 373. https://doi.org/10.3390/brainsci10060373
APA StyleWu, P. H., Kim, H. S., Lee, Y. J., Kim, D. H., Lee, J. H., Jeon, J. B., Raorane, H. D., & Jang, I.-T. (2020). Uniportal Full Endoscopic Posterolateral Transforaminal Lumbar Interbody Fusion with Endoscopic Disc Drilling Preparation Technique for Symptomatic Foraminal Stenosis Secondary to Severe Collapsed Disc Space: A Clinical and Computer Tomographic Study with Technical Note. Brain Sciences, 10(6), 373. https://doi.org/10.3390/brainsci10060373






