Celiac Disease Diagnosed in an Older Adult Patient with a Complex Neuropsychiatric Involvement: A Case Report and Review of the Literature
Abstract
:1. Introduction
2. Case Report
- nerve conduction studies and electromyography (NCSs/EMG) documented a motor axonal polyneuropathy with signs of active denervation at the lower limbs;
- somatosensory and motor potentials (SEPs and MEPs) documented a complete absence of responses from the lower limbs;
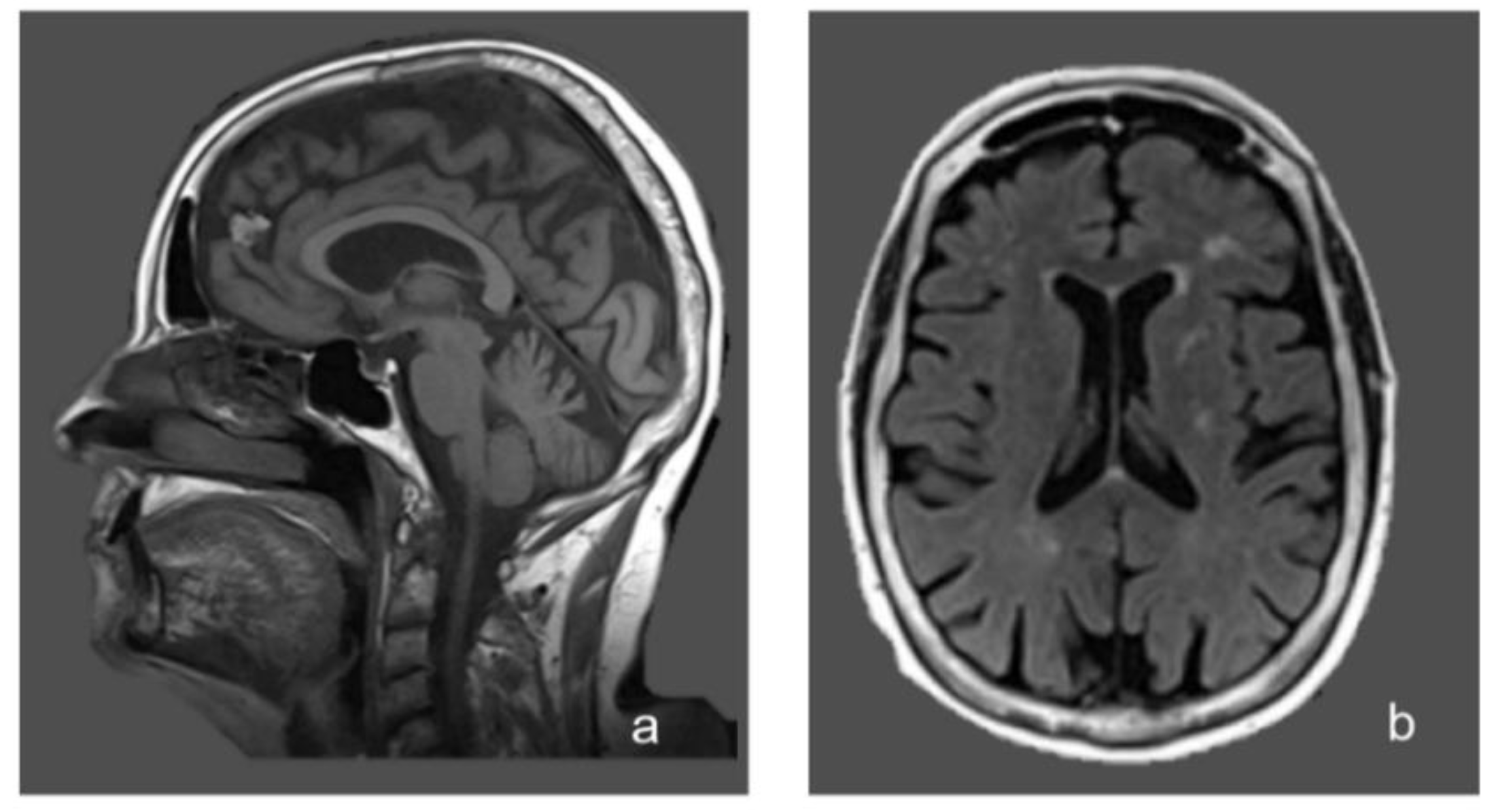
- brain magnetic resonance imaging (MRI) showed non-specific periventricular gliosis (Figure 1);
- spinal cord MRI was normal.
3. Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Al-Toma, A.; Volta, U.; Auricchio, R.; Castillejo, G.; Sanders, D.S.; Cellier, C.; Mulder, C.J.; Lundin, K. European Society for the Study of Coeliac Disease (ESsCD) guideline for coeliac disease and other gluten-related disorders. United Eur. Gastroenterol. J. 2019, 7, 583–613. [Google Scholar] [CrossRef]
- Ludvigsson, J.F.; Leffler, D.A.; Bai, J.C.; Biagi, F.; Fasano, A.; Green, P.H.; Hadjivassiliou, M.; Kaukinen, K.; Kelly, C.P.; Leonard, J.N.; et al. The Oslo definitions for coeliac disease and related terms. Gut 2013, 62, 43–52. [Google Scholar] [CrossRef]
- Lindfors, K.; Ciacci, C.; Kurppa, K.; Lundin, K.; Makharia, G.K.; Mearin, M.L.; Murray, J.A.; Verdu, E.F.; Kaukinen, K. Coeliac disease. Nat. Rev. Dis. Primers 2019, 5, 3. [Google Scholar] [CrossRef]
- Newnham, E.D. Coeliac disease in the 21st century: Paradigm shifts in the modern age. J. Gastroenterol. Hepatol. 2017, 32, 82–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lebwohl, B.; Sanders, D.S.; Green, P.H.R. Coeliac disease. Lancet 2018, 391, 70–81. [Google Scholar] [CrossRef]
- White, H. Gastrointestinal Disorders and the Nervous System. Continuum 2020, 26, 577–590. [Google Scholar] [PubMed]
- Pfeiffer, R.F. Gastroenterology and Neurology. Continuum 2017, 23, 744–761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guandalini, S.; Assiri, A. Celiac disease: A review. JAMA Pediatr. 2014, 168, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Isikay, S.; Kocamaz, H. The Neurological Face of Celiac Disease. Arq. Gastroenterol. 2015, 52, 167–170. [Google Scholar] [CrossRef]
- Hadjivassiliou, M.; Duker, A.P.; Sanders, D.S. Gluten-related neurologic dysfunction. Handb. Clin. Neurol. 2014, 120, 607–619. [Google Scholar]
- Hallert, C.; Derefeldt, T. Psychic disturbances in adult coeliac disease. I. Clinical observations. Scand. J. Gastroenterol. 1982, 17, 17–19. [Google Scholar] [CrossRef] [PubMed]
- Walsh, D. Coeliac disease and schizophrenia. Br. Med. J. 1973, 2, 242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campagna, G.; Pesce, M.; Tatangelo, R.; Rizzuto, A.; La Fratta, I.; Grilli, A. The progression of coeliac disease: Its neurological and psychiatric implications. Nutr. Res. Rev. 2017, 30, 25–35. [Google Scholar] [CrossRef] [Green Version]
- Clappison, E.; Hadjivassiliou, M.; Zis, P. Psychiatric Manifestations of Coeliac Disease, a Systematic Review and Meta-Analysis. Nutrients 2020, 12, 142. [Google Scholar] [CrossRef] [Green Version]
- Tengah, D.P.; Wills, A.J.; Holmes, G.K. Neurological complications of coeliac disease. Postgrad. Med. J. 2002, 78, 393–398. [Google Scholar] [CrossRef]
- Hadjivassiliou, M.; Rao, D.G.; Grìnewald, R.A.; Aeschlimann, D.P.; Sarrigiannis, P.G.; Hoggard, N.; Aeschlimann, P.; Mooney, P.D.; Sanders, D.S. Neurological Dysfunction in Coeliac Disease and Non-Coeliac Gluten Sensitivity. Am. J. Gastroenterol. 2016, 111, 561–567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cicarelli, G.; Della Rocca, G.; Amboni, M.; Ciacci, C.; Mazzacca, G.; Filla, A.; Barone, P. Clinical and neurological abnormalities in adult celiac disease. Neurol. Sci. 2003, 24, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Van Hees, N.J.M.; van der Does, W.; Giltay, E.J. Coeliac disease, diet adherence and depressive symptoms. J. Psychosom. Res. 2013, 74, 155–160. [Google Scholar] [CrossRef] [Green Version]
- Folstein, F.M.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Nachman, F.; Sugai, E.; Vázquez, H.; González, A.; Andrenacci, P.; Niveloni, S.; Mazure, R.; Smecuol, E.; Moreno, M.L.; Hwang, H.J.; et al. Serological tests for celiac disease as indicators of long-term compliance with the gluten-free diet. Eur. J. Gastroenterol. Hepatol. 2011, 23, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Bushara, K.O. Neurologic presentation of celiac disease. Gastroenterology 2005, 128, S92–S97. [Google Scholar] [CrossRef] [PubMed]
- Hankey, L.G.; Holmes, G.K. Coeliac disease in the elderly. Gut 1994, 35, 65–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooke, T.W.; Smith, W.T. Neurological disorders associated with adult coeliac disease. Brain 1966, 89, 683–722. [Google Scholar] [CrossRef] [PubMed]
- Trovato, C.M.; Raucci, U.; Valitutti, F.; Montuori, M.; Villa, M.P.; Cucchiara, S.; Parisi, P. Neuropsychiatric manifestations in celiac disease. Epilepsy Behav. 2019, 99, 106393. [Google Scholar] [CrossRef] [PubMed]
- Cossu, G.; Carta, M.G.; Contu, F.; Mela, Q.; Demelia, L.; Elli, L.; Dell’Osso, B. Coeliac disease and psychiatric comorbidity: Epidemiology, pathophysiological mechanisms, quality-of-life, and gluten-free diet effects. Int. Rev. Psychiatry 2017, 29, 489–503. [Google Scholar] [CrossRef]
- Addolorato, G.; Mirijello, A.; D’Angelo, C.; Leggio, L.; Ferrulli, A.; Abenavoli, L.; Vonghia, L.; Cardone, S.; Leso, V.; Cossari, A.; et al. State and trait anxiety and depression in patients affected by gastrointestinal diseases: Psychometric evaluation of 1641 patients referred to an internal medicine outpatient setting. Int. J. Clin. Pract. 2008, 62, 1063–1069. [Google Scholar] [CrossRef]
- Addolorato, G.; Leggio, L.; D’Angelo, C.; Mirijello, A.; Ferrulli, A.; Cardone, S.; Vonghia, L.; Abenavoli, L.; Leso, V.; Nesci, A.; et al. Affective and psychiatric disorders in celiac disease. Dig. Dis. 2008, 26, 140–148. [Google Scholar] [CrossRef]
- Luostarinen, L.; Pirttila, T.; Collin, P. Coeliac disease presenting with neurological disorders. Eur. Neurol. 1999, 42, 132–135. [Google Scholar] [CrossRef]
- Hadjivassiliou, M.; Chattopadhyay, A.K.; Davies-Jones, G.A.; Gibson, A.; Grunewald, R.A.; Lobo, A.J. Neuromuscular disorder as a presenting feature of coeliac disease. J. Neurol. Neurosurg. Psychiatry 1997, 63, 770–775. [Google Scholar] [CrossRef] [Green Version]
- Ludvigsson, J.F.; Reichenberg, A.; Hultman, C.M.; Murray, J.A. A nationwide study of the association between celiac disease and the risk of autistic spectrum disorders. JAMA Psychiatry 2013, 70, 1224–1230. [Google Scholar] [CrossRef] [Green Version]
- Pavone, L.; Fiumara, A.; Bottaro, G.; Mazzone, D.; Coleman, M. Autism and celiac disease: Failure to validate the hypothesis that a link might exist. Biol. Psychiatry 1997, 42, 72–75. [Google Scholar] [CrossRef]
- Smith, F.D.; Gerdes, L.U. Meta-analysis on anxiety and depression in adult celiac disease. Acta Psychiatr. Scand. 2012, 125, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Cullen, A.E.; Holmes, S.; Pollak, T.A.; Blackman, G.; Joyce, D.W.; Kempton, M.J.; Murray, R.M.; McGuire, P.; Mondelli, V. Associations Between Non-neurological Autoimmune Disorders and Psychosis: A Meta-analysis. Biol. Psychiatry 2019, 85, 35–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Croall, I.D.; Sanders, D.S.; Hadjivassiliou, M.; Hoggard, N. Cognitive Deficit and White Matter Changes in Persons With Celiac Disease: A Population-Based Study. Gastroenterology 2020, 158, 2112–2122. [Google Scholar] [CrossRef] [PubMed]
- Hadjivassiliou, M.; Sanders, D.S.; Grunewald, R.A.; Woodroofe, N.; Boscolo, S.; Aeschlimann, D. Gluten sensitivity: From gut to brain. Lancet Neurol. 2010, 9, 318–330. [Google Scholar] [CrossRef]
- Tylee, D.S.; Sun, J.; Hess, J.L.; Tahir, M.A.; Sharma, E.; Malik, R.; Worrall, B.B.; Levine, A.J.; Martinson, J.J.; Nejentsev, S. Genetic correlations among psychiatric and immune-related phenotypes based on genome-wide association data. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2018, 177, 641–657. [Google Scholar] [CrossRef]
- Severance, G.E.; Yolken, R.H.; Eaton, W.W. Autoimmune diseases, gastrointestinal disorders and the microbiome in schizophrenia: More than a gut feeling. Schizophr. Res. 2016, 176, 23–35. [Google Scholar] [CrossRef] [Green Version]
- Galland, L. The gut microbiome and the brain. J. Med. Food 2014, 17, 1261–1272. [Google Scholar] [CrossRef]
- Camara-Lemarroy, C.R.; Rodriguez-Gutierrez, R.; Monreal-Robles, R.; Marfil-Rivera, A. Gastrointestinal disorders associated with migraine: A comprehensive review. World J. Gastroenterol. 2016, 22, 8149–8160. [Google Scholar] [CrossRef] [Green Version]
- Yelland, G.W. Gluten-induced cognitive impairment ("brain fog") in coeliac disease. J. Gastroenterol. Hepatol. 2017, 32, 90–93. [Google Scholar] [CrossRef] [Green Version]
- Marazziti, D.; Poletti, M.; Dell’Osso, L.; Baroni, S.; Bonuccelli, U. Prefrontal cortex, dopamine, and jealousy endophenotype. CNS Spectr. 2013, 18, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Batinic, B.; Duisin, D.; Barisic, J. xObsessive versus delusional jealousy. Psychiatr. Danub. 2013, 25, 334–339. [Google Scholar]
- Seeman, M.V. Pathological Jealousy: An Interactive Condition. Psychiatry 2016, 79, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Cannas, A.; Solla, P.; Floris, G.; Tacconi, P.; Marrosu, F.; Marrosu, M.G. Othello syndrome in Parkinson disease patients without dementia. Neurologist 2009, 15, 34–36. [Google Scholar] [CrossRef]
- Braun, M.C.; Suffren, S. A general neuropsychological model of delusion. Cogn. Neuropsychiatry 2011, 16, 1–39. [Google Scholar] [CrossRef]
- Ortigue, S.; Bianchi-Demicheli, F. Intention, false beliefs, and delusional jealousy: Insights into the right hemisphere from neurological patients and neuroimaging studies. Med. Sci. Monit. 2011, 17, RA1–RA11. [Google Scholar]
- Graff-Radford, J.; Whitwell, J.L.; Geda, Y.E.; Josephs, K.A. Clinical and imaging features of Othello’s syndrome. Eur. J. Neurol. 2012, 19, 38–46. [Google Scholar] [CrossRef] [Green Version]
- Hadjivassiliou, M.; Sanders, D.D.; Aeschlimann, D.P. Gluten-related disorders: Gluten ataxia. Dig. Dis. 2015, 33, 264–268. [Google Scholar] [CrossRef]
- Hadjivassiliou, M.; Grünewald, R.A.; Kandler, R.H.; Chattopadhyay, A.K.; Jarratt, J.A.; Sanders, D.S.; Sharrack, B.; Wharton, S.B.; Davies-Jones, G.A. Neuropathy associated with gluten sensitivity. J. Neurol. Neurosurg. Psychiatry 2006, 77, 1262–1266. [Google Scholar] [CrossRef]
- Kieslich, M.; Errazuriz, G.; Posselt, H.G.; Moeller-Hartmann, W.; Zanella, F.; Boehles, H. Brain white-matter lesions in celiac disease: A prospective study of 75 diet-treated patients. Pediatrics 2001, 108, E21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makhlouf, S.; Msselemeni, M.; Derbali, H.; Mansour, M.; Zaouali, J.; Mrissa, R. Spastic paraparesis revealing celiac disease. Acta Gastroenterol. Belg. 2018, 81, 107–108. [Google Scholar] [PubMed]
- Frih-Ayed, M.; Boughammoura-Bouatay, A.; Fitouri, F.; Chebel, S. Coeliac disease an spastic paraplegia. Rev. Neurol. 2006, 162, 648–650. [Google Scholar] [CrossRef]
- Di Lazzaro, V.; Pilato, F.; Batocchi, A.P.; Restuccia, D.; Cammarota, G.; Profice, P. Tired legs--a gut diagnosis. Lancet 2010, 376, 1798. [Google Scholar] [CrossRef]
- Gonzalez Aleman, G.; Florenzano, N.; Padilla, E.; Bourdieu, M.; Guerrero, G.; Calvó, M.; Alberio, G.; Strejilevich, S.; de Erausquin, G.A. A 37-year-old woman with celiac disease, recurrent psychosis, and Parkinsonism. Mov. Disord. 2006, 21, 729–731. [Google Scholar] [CrossRef]
- Di Lazzaro, V.; Capone, F.; Cammarota, G.; Di Giuda, D.; Ranieri, F. Dramatic improvement of parkinsonian symptoms after gluten-free diet introduction in a patient with silent celiac disease. J. Neurol. 2014, 261, 443–445. [Google Scholar] [CrossRef]
- Arshad, I.; Javeed, A.; Ullah, U. Patient with Gluten Encephalopathy Presenting with Neuropsychiatric Symptoms. Am. J. Med. 2018, 131, e49–e50. [Google Scholar] [CrossRef]
- Ravindra, B.S.; Desai, N.; Deviprasad, S.; Bhede, V.; Ravat, S.; Sawant, P. Myotonic dystrophy in a patient of celiac disease: A new association? Trop. Gastroenterol. 2008, 29, 114–115. [Google Scholar]
- Ryan, A.M.; Ryan, J.; Wan-Ahmed, M.; Hardiman, O.; Farrell, M.A.; McNamara, B.; Sweeney, B.J. Vacuolar leucoencephalopathy and pulvinar sign in association with coeliac disease. BMJ Case Rep. 2009, 2009, bcr08.2008.0650. [Google Scholar]
- Ryan, A.M.; Ryan, J.; Wan-Ahmed, M.; Hardiman, O.; Farrell, M.A.; McNamara, B.; Sweeney, B.J. Vacuolar leucoencephalopathy and pulvinar sign in association with coeliac disease. J. Neurol. Neurosurg. Psychiatry 2007, 78, 98–99. [Google Scholar] [CrossRef] [Green Version]
- Dseplat-Jego, S.; Bernard, D.; Bagneres, D.; Frances, Y. Neuropsychiatric symptoms in the elderly: Let us not forget celiac disease. J. Am. Geriatr. Soc. 2003, 51, 884–885. [Google Scholar] [CrossRef]
- Burk, K.; Farecki, M.L.; Lamprecht, G.; Roth, G.; Decker, P.; Weller, M.; Rammensee, H.G.; Oertel, W. Neurological symptoms in patients with biopsy proven celiac disease. Mov. Disord. 2009, 24, 2358–2362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigo, L.; Hernández-Lahoz, C.; Lauret, E.; Rodriguez-Peláez, M.; Soucek, M.; Ciccocioppo, R.; Kruzliak, P. Gluten ataxia is better classified as non-celiac gluten sensitivity than as celiac disease: A comparative clinical study. Immunol. Res. 2016, 64, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Kalaydjian, A.E.; Eaton, W.; Cascella, N.; Fasano, A. The gluten connection: The association between schizophrenia and celiac disease. Acta Psychiatr. Scand. 2006, 113, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Addolorato, G.; Capristo, E.; Ghittoni, G.; Valeri, C.; Mascianà, R.; Ancona, C.; Gasbarrini, G. Anxiety but not depression decreases in coeliac patients after one-year gluten-free diet: A longitudinal study. Scand. J. Gastroenterol. 2001, 36, 502–506. [Google Scholar] [CrossRef]
- Pennisi, M.; Lanza, G.; Cantone, M.; Ricceri, R.; Ferri, R.; D’Agate, C.C.; Pennisi, G.; Di Lazzaro, V.; Bella, R. Cortical involvement in celiac disease before and after long-term gluten-free diet: A Transcranial Magnetic Stimulation study. PLoS ONE 2017, 12, e0177560. [Google Scholar] [CrossRef] [Green Version]
- Selfani, K.; Soland, V.L.; Chouinard, S.; Huot, P. Movement Disorders Induced by the “Atypical” Antipsychotic Aripiprazole. Neurologist 2017, 22, 24–28. [Google Scholar] [CrossRef]
- Ma, G.F.; Raivio, N.; Sabria, J.; Ortiz, J. Agonist and antagonist effects of aripiprazole on D(2)-like receptors controlling rat brain dopamine synthesis depend on the dopaminergic tone. Int. J. Neuropsychopharmacol. 2014, 18, pyu046. [Google Scholar] [CrossRef]
- Stahl, S.M. Stahl‘s Essential Psychopharmacology: Neuroscientific Basis and Practical Application, 4th ed.; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- Shin, W.H.; Chung, S.J. Drug-induced parkinsonism. J. Clin. Neurol. 2012, 8, 15–21. [Google Scholar] [CrossRef] [Green Version]
- Paez, M.A.; Gramelspacher, A.M.; Sinacore, J.; Winterfield, L.; Venu, M. Delay in Diagnosis of Celiac Disease in Patients Without Gastrointestinal Complaints. Am. J. Med. 2017, 130, 1318–1323. [Google Scholar] [CrossRef] [Green Version]

| Reference | Arshad et al. 2018 [57] | Ravindra et al. 2008 [58] | Ryan et al. 2007 and 2009 [59,60] | Gonzalez Aleman et al. 2006 [55] | Dseplat-Jego et al. 2003 [61] |
|---|---|---|---|---|---|
| Age at CD diagnosis | 22 | 27 | 55 | 37 | 76 |
| Gender | M | F | M | F | F |
| CD diagnosis | Duodenal biopsy | Duodenal biopsy EMA | Small bowel biopsy TTG | Duodenal biopsy | Small bowel biopsy; TTG, EMA, and anti-gliadin antibodies |
| Extraintestinal manifestations | Microcytic hypochromic anemia | Microcytic hypochromic anemia, cheilitis | None | None | Macrocytic anemia, low serum albumin, vitamin B12 deficiency, cachectic status |
| Neurological signs and symptoms | Gradual cognitive impairment, speech difficulties for 2 years, brisk lower limb reflexes, increased tone in all four limbs | Myotonic dystrophy | Rapidly progressive neurological decline: headache, seizures, ataxia, myoclonus, cognitive impairment | Parkinsonism | Mental confusion, auditory and visual hallucinations, memory impairment |
| Psychiatric symptoms | Aggressive behavior, not otherwise specified | Irritability | Anxiety | Psychosis with severe anxiety and marked psychological agitation | Depressive state, withdrawal attitude, paranoid delirium |
| Neuroimaging | Normal | N.A. | Brain MRI: leukoencephalopathy, pulvinar sign | Brain MRI: periventricular white matter hyperintensities. Tanscranial ultrasound: enlarged echogenic areas in the substantia nigra bilaterally | N.A. |
| Treatment | GFD | GFD, iron, calcium and folic acid supplements for 8 weeks | Probably already on GFD (CD diagnosis 6 months before), anticonvulsants, immunosuppressive treatment | Already on GFD (not specified for how long), clozapine | GFD, Vit B12 supplementation |
| Effect of treatment on neuropsychiatric symptoms | Improvement | None | None | Clozapine improved psychiatric symptoms. Parkinsonism did not change after clozapine discontinuation | Improvement |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Falato, E.; Capone, F.; Ranieri, F.; Florio, L.; Corbetto, M.; Taffon, C.; Niolu, C.; Di Lorenzo, G.; Di Lazzaro, V. Celiac Disease Diagnosed in an Older Adult Patient with a Complex Neuropsychiatric Involvement: A Case Report and Review of the Literature. Brain Sci. 2020, 10, 426. https://doi.org/10.3390/brainsci10070426
Falato E, Capone F, Ranieri F, Florio L, Corbetto M, Taffon C, Niolu C, Di Lorenzo G, Di Lazzaro V. Celiac Disease Diagnosed in an Older Adult Patient with a Complex Neuropsychiatric Involvement: A Case Report and Review of the Literature. Brain Sciences. 2020; 10(7):426. https://doi.org/10.3390/brainsci10070426
Chicago/Turabian StyleFalato, Emma, Fioravante Capone, Federico Ranieri, Lucia Florio, Marzia Corbetto, Chiara Taffon, Cinzia Niolu, Giorgio Di Lorenzo, and Vincenzo Di Lazzaro. 2020. "Celiac Disease Diagnosed in an Older Adult Patient with a Complex Neuropsychiatric Involvement: A Case Report and Review of the Literature" Brain Sciences 10, no. 7: 426. https://doi.org/10.3390/brainsci10070426





