Investigating Influences of Medial Olivocochlear Efferent System on Central Auditory Processing and Listening in Noise: A Behavioral and Event-Related Potential Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants and Qualification Testing
2.2. OAEs
2.3. Listening Conditions for Word Recognition Testing and Event-Related Potentials
2.4. Word Recognition in Noise Test
2.5. ERPs
3. Results
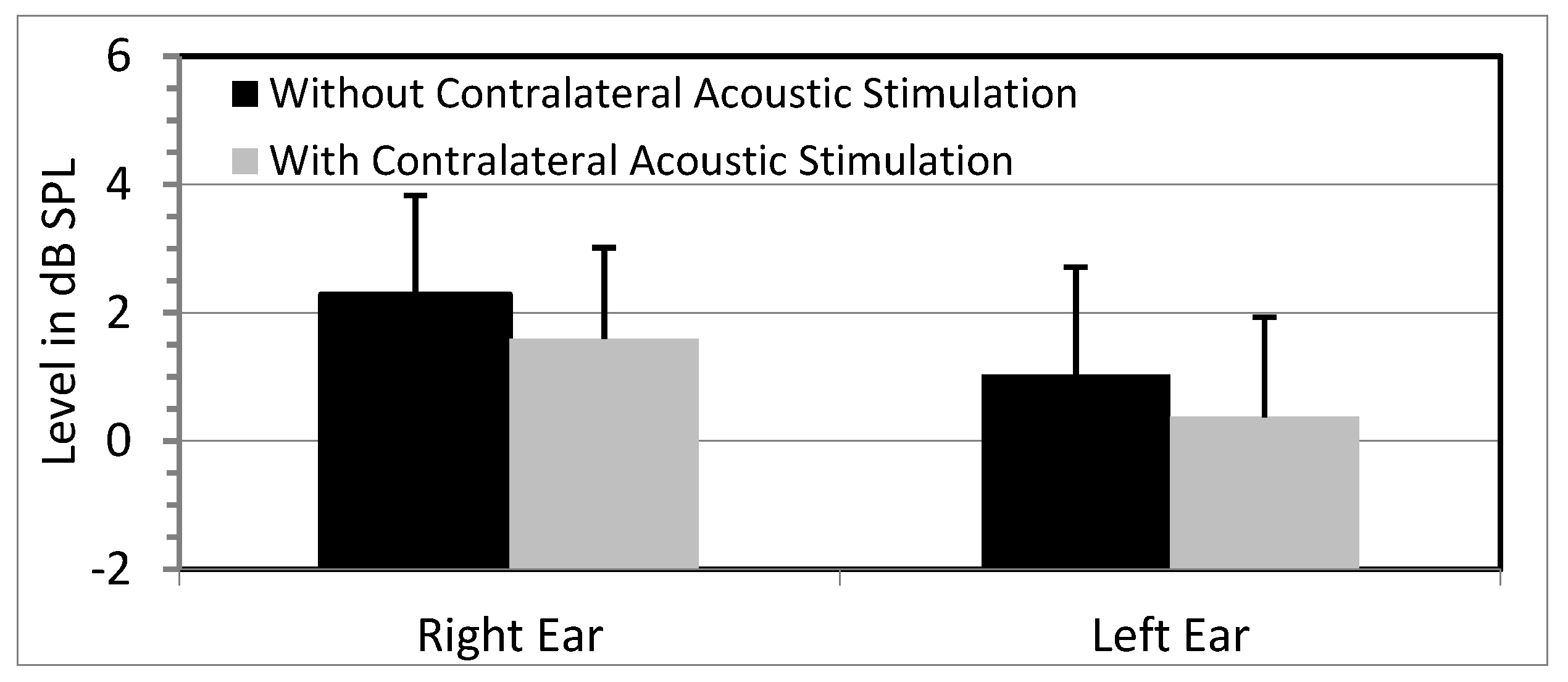
3.1. OAE Data
3.2. WRS Data
3.3. MMN Data for Passive Listening Condition
3.4. P300 Data for Active Listening Condition
4. Discussion
4.1. OAE Inhibition
4.2. Word Recognition Testing
4.3. ERPs
4.4. Methodological Challenges and Lessons
4.5. Clinical Relevance and Future Directions
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of variance |
| BBN | Broadband noise |
| CAS | Contralateral acoustic stimulation |
| CID | Central Institute for the Deaf |
| ER | Etymotic research |
| ERP | Event-related potentials |
| MOC | Medial olivocochlear |
| MMN | Mismatch negativity |
| NST | Nonsense Syllable Test |
| P300 | Positive peak at 300 ms |
| OHC | Outer hair cell |
| OAE | Otoacoustic emission |
| SL | Sensation level |
| SPL | Sound pressure level |
| SNR | Signal-to-noise ratio |
| SRT | Speech reception threshold |
| TEOAE | Transient-evoked otoacoustic emissions |
| WRS | Word recognition score |
References
- Smith, D.W.; Keil, A. The biological role of the medial olivocochlear efferents in hearing: Separating evolved function from exaptation. Front. Syst. Neurosci. 2015, 9, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez-Poveda, E.A. Olivocochlear efferents in animals and humans: From anatomy to clinical relevance. Front. Neurol. 2018, 9, 197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, Z.; Suga, N. Modulation of cochlear hair cells by the auditory cortex in the mustached bat. Nat. Neurosci. 2002, 5, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Guinan, J.J.J. Olivocochlear efferents: Anatomy, physiology, function, and the measurement of efferent effects in humans. Ear Hear. 2006, 27, 589–607. [Google Scholar] [CrossRef] [PubMed]
- Cooper, N.P.; Guinan, J.J.J. Separate mechanical processes underlie fast and slow effects of medial olivocochlear efferent activity. J. Physiol. 2003, 548, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Cooper, N.P.; Guinan, J.J.J. Efferent-mediated control of basilar membrane motion. J. Physiol. 2006, 576, 49–54. [Google Scholar] [CrossRef]
- Murugasu, E.; Russell, I.J. The effect of efferent stimulation on basilar membrane displacement in the basal turn of the guinea pig cochlea. J. Neurosci. 1996, 16, 325–332. [Google Scholar] [CrossRef]
- Dolan, D.F.; Nuttall, A.L. Masked cochlear whole-nerve response intensity functions altered by electrical stimulation of the crossed olivocochlear bundle. J. Acoust. Soc. Am. 1988, 83, 1081–1086. [Google Scholar] [CrossRef]
- Kawase, T.; Delgutte, B.; Liberman, M.C. Antimasking effects of the olivocochlear reflex. II. Enhancement of auditory-nerve response to masked tones. J. Neurophysiol. 1993, 70, 2533–2549. [Google Scholar] [CrossRef]
- Winslow, R.L.; Sachs, M.B. Single-tone intensity discrimination based on auditory-nerve rate responses in backgrounds of quiet, noise, and with stimulation of the crossed olivocochlear bundle. Hear. Res. 1988, 35, 165–189. [Google Scholar] [CrossRef]
- Liberman, M.C.; Guinan, J.J.J. Feedback control of the auditory periphery: Anti-masking effects of middle ear muscles vs. olivocochlear efferents. J. Commun. Disord. 1998, 31, 471–482. [Google Scholar] [CrossRef]
- Micheyl, C.; Collet, L. Involvement of the olivocochlear bundle in the detection of tones in noise. J. Acoust. Soc. Am. 1996, 99, 1604–1610. [Google Scholar] [CrossRef] [PubMed]
- Micheyl, C.; Morlet, T.; Giraud, A.L.; Collet, L.; Morgon, A. Contralateral suppression of evoked otoacoustic emissions and detection of a multi-tone complex in noise. Acta Oto-Laryngol. 1995, 115, 178–182. [Google Scholar] [CrossRef]
- Micheyl, C.; Perrot, X.; Collet, L. Relationship between auditory intensity discrimination in noise and olivocochlear efferent system activity in humans. Behav. Neurosci. 1997, 111, 801–807. [Google Scholar] [CrossRef] [PubMed]
- De Boer, J.; Thornton, A.R. Neural correlates of perceptual learning in the auditory brainstem: Efferent activity predicts and reflects improvement at a speech-in-noise discrimination task. J. Neurosci. 2008, 28, 4929–4937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Irving, S.; Moore, D.R.; Liberman, M.C.; Sumner, C.J. Olivocochlear efferent control in sound localization and experience-dependent learning. J. Neurosci. 2011, 31, 2493–2501. [Google Scholar] [CrossRef] [PubMed]
- Maison, S.; Micheyl, C.; Collet, L. Influence of focused auditory attention on cochlear activity in humans. Psychophysiology 2001, 38, 35–40. [Google Scholar] [CrossRef]
- Veuillet, E.; Magnan, A.; Ecalle, J.; Thai-Van, H.; Collet, L. Auditory processing disorder in children with reading disabilities: Effect of audiovisual training. Brain 2007, 130, 2915–2928. [Google Scholar] [CrossRef] [Green Version]
- Smith, S.B.; Cone, B. The medial olivocochlear reflex in children during active listening. Int. J. Audiol. 2015, 54, 518–523. [Google Scholar] [CrossRef] [Green Version]
- Maison, S.F.; Liberman, M.C. Predicting vulnerability to acoustic injury with a noninvasive assay of olivocochlear reflex strength. J. Neurosci. 2000, 20, 4701–4707. [Google Scholar] [CrossRef] [Green Version]
- Maison, S.F.; Usubuchi, H.; Liberman, M.C. Efferent feedback minimizes cochlear neuropathy from moderate noise exposure. J. Neurosci. 2013, 33, 5542–5552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Boer, J.; Thornton, A.R.; Krumbholz, K. What is the role of the medial olivocochlear system in speech-in-noise processing? J. Neurophysiol. 2012, 107, 1301–1312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giraud, A.L.; Garnier, S.; Micheyl, C.; Lina, G.; Chays, A.; Chery-Croze, S. Auditory efferents involved in speech-in-noise intelligibility. Neuroreport 1997, 8, 1779–1783. [Google Scholar] [CrossRef] [PubMed]
- Kumar, U.A.; Vanaja, C.S. Functioning of olivocochlear bundle and speech perception in noise. Ear Hear. 2004, 25, 142–146. [Google Scholar] [CrossRef]
- Mishra, S.K.; Lutman, M.E. Top-down influences of the medial olivocochlear efferent system in speech perception in noise. PLoS ONE 2014, 9, e85756. [Google Scholar] [CrossRef] [Green Version]
- Scharf, B.; Magnan, J.; Collet, L.; Ulmer, E.; Chays, A. On the role of the olivocochlear bundle in hearing: A case study. Hear. Res. 1994, 75, 11–26. [Google Scholar] [CrossRef]
- Stuart, A.; Butler, A.K. Contralateral suppression of transient otoacoustic emissions and sentence recognition in noise in young adults. J. Am. Acad. Audiol. 2012, 23, 686–696. [Google Scholar] [CrossRef]
- Zeng, F.G.; Martino, K.M.; Linthicum, F.H.; Soli, S.D. Auditory perception in vestibular neurectomy subjects. Hear. Res. 2000, 142, 102–112. [Google Scholar] [CrossRef]
- Mishra, S. The role of medial efferents in human auditory development: Efferent inhibition predicts frequency discrimination in noise for children. J. Neurophysiol. 2020. [Google Scholar] [CrossRef]
- Luck, S.J. An Introduction to the Event-Related Potential Technique, 2nd ed.; MIT Press: Cambridge, MA, USA, 2014. [Google Scholar]
- Koerner, T.K.; Zhang, Y. Effects of background noise on inter-trial phase coherence and auditory N1-P2 responses to speech stimuli. Hear. Res. 2015, 328, 113–119. [Google Scholar] [CrossRef]
- Koerner, T.K.; Zhang, Y. Differential effects of hearing impairment and age on electrophysiological and behavioral measures of speech in noise. Hear. Res. 2018, 370, 130–142. [Google Scholar] [CrossRef]
- Koerner, T.K.; Zhang, Y.; Nelson, P.B.; Wang, B.; Zou, H. Neural indices of phonemic discrimination and sentence-level speech intelligibility in quiet and noise: A mismatch negativity study. Hear. Res. 2016, 339, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Koerner, T.K.; Zhang, Y.; Nelson, P.B.; Wang, B.; Zou, H. Neural indices of phonemic discrimination and sentence-level speech intelligibility in quiet and noise: A P3 study. Hear. Res. 2017, 350, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, D.; Lodhia, V.; Hautus, M.J. Electrophysiological indices of amplitude modulated sounds and sensitivity to noise. Int. J. Psychophysiol. 2019, 139, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, S.J.; Billings, C.J.; Hornsby, B.W.Y.; Key, A.P. Effect of competing noise on cortical auditory evoked potentials elicited by speech sounds in 7- to 25-year-old listeners. Hear. Res. 2019, 373, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Billings, C.J.; Tremblay, K.L.; Stecker, G.C.; Tolin, W.M. Human evoked cortical activity to signal-to-noise ratio and absolute signal level. Hear. Res. 2009, 254, 15–24. [Google Scholar] [CrossRef] [Green Version]
- Kozou, H.; Kujala, T.; Shtyrov, Y.; Toppila, E.; Starck, J.; Alku, P.; Naatanen, R. The effect of different noise types on the speech and non-speech elicited mismatch negativity. Hear. Res. 2005, 199, 31–39. [Google Scholar] [CrossRef]
- Martin, B.A.; Kurtzberg, D.; Stapells, D.R. The effects of decreased audibility produced by high-pass noise masking on N1 and the mismatch negativity to speech sounds /ba/and/da. J. Speech Lang. Hear. Res. 1999, 42, 271–286. [Google Scholar] [CrossRef]
- McCullagh, J.; Shinn, J.B. Auditory P300 in noise in younger and older adults. J. Am. Acad. Audiol. 2018, 29, 909–916. [Google Scholar] [CrossRef]
- Naatanen, R.; Paavilainen, P.; Rinne, T.; Alho, K. The mismatch negativity (MMN) in basic research of central auditory processing: A review. Clin. Neurophysiol 2007, 118, 2544–2590. [Google Scholar] [CrossRef]
- Polich, J. Updating P300: An integrative theory of P3a and P3b. Clin. Neurophysiol. 2007, 118, 2128–2148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donchin, E.; Coles, M.G.H. Is the P300 component a manifestation of context updating. Behav. Brain Sci. 1988, 11, 357–374. [Google Scholar] [CrossRef]
- Mott, J.B.; Norton, S.J.; Neely, S.T.; Warr, W.B. Changes in spontaneous otoacoustic emissions produced by acoustic stimulation of the contralateral ear. Hear. Res. 1989, 38, 229–242. [Google Scholar] [CrossRef]
- Collet, L.; Kemp, D.T.; Veuillet, E.; Duclaux, R.; Moulin, A.; Morgon, A. Effect of contralateral auditory stimuli on active cochlear micro-mechanical properties in human subjects. Hear. Res. 1990, 43, 251–261. [Google Scholar] [CrossRef]
- Henin, S.; Thompson, S.; Abdelrazeq, S.; Long, G.R. Changes in amplitude and phase of distortion-product otoacoustic emission fine-structure and separated components during efferent activation. J. Acoust. Soc. Am. 2011, 129, 2068–2079. [Google Scholar] [CrossRef] [PubMed]
- Lilaonitkul, W.; Guinan, J.J.J. Human medial olivocochlear reflex: Effects as functions of contralateral, ipsilateral, and bilateral elicitor bandwidths. J. Assoc. Res. Otolaryngol. 2009, 10, 459–470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishra, S.K.; Lutman, M.E. Repeatability of click-evoked otoacoustic emission-based medial olivocochlear efferent assay. Ear Hear. 2013, 34, 789–798. [Google Scholar] [CrossRef]
- Guinan, J.J.J. Cochlear efferent innervation and function. Curr. Opin. Otolaryngol. Head Neck Surg. 2010, 18, 447–453. [Google Scholar] [CrossRef] [Green Version]
- Oldfield, R.C. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 1971, 9, 97–113. [Google Scholar] [CrossRef]
- American Speech-Language-Hearing Association. Guidelines for determining the threshold levels for speech. ASHA 1988, 30, 85–89. [Google Scholar]
- Hirsh, I.J.; Davis, H.; Silverman, S.R.; Reynolds, E.G.; Eldert, E.; Benson, R.W. Development of materials for speech audiometry. J. Speech Hear. Disord. 1952, 17, 321–337. [Google Scholar] [CrossRef] [PubMed]
- American National Standards Institute. Specification for Audiometers. In ANSI S3.6-2010; ANSI: Washington, DC, USA, 2010. [Google Scholar]
- Johnsen, N.J.; Terkildsen, K. The normal middle-ear reflex thresholds towards white noise and acoustic clicks in young-adults. Scand. Audiol. 1980, 9, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Edgerton, B.J.; Danhauer, J.L. Clinical Implications of Speech Discrimination Testing Using Nonsense Stimuli; University Park Press: Baltimore, MD, USA, 1979. [Google Scholar]
- Macmillan, N.A.; Creelman, C.D. Detection Theory: A User’s Guide; Cambridge University Press: New York, NY, USA, 1991. [Google Scholar]
- Rao, A.; Zhang, Y.; Miller, S. Selective listening of concurrent auditory stimuli: An event-related potential study. Hear. Res. 2010, 268, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Stevens, J.; Zhang, Y. Relative distance and gaze in the use of entity-referring spatial demonstratives: An event-related potential study. J. Neurolinguist 2013, 26, 31–45. [Google Scholar] [CrossRef]
- Zhang, Y.; Koerner, T.; Miller, S.; Grice-Patil, Z.; Svec, A.; Akbari, D.; Tusler, L.; Carney, E. Neural coding of formant-exaggerated speech in the infant brain. Dev. Sci. 2011, 14, 566–581. [Google Scholar] [CrossRef]
- Miller, S.; Zhang, Y. Neural coding of phonemic fricative contrast with and without hearing aid. Ear Hear. 2014, 35, e122–e133. [Google Scholar] [CrossRef]
- Giraud, A.L.; Collet, L.; Chery-Croze, S.; Magnan, J.; Chays, A. Evidence of a medial olivocochlear involvement in contralateral suppression of otoacoustic emissions in humans. Brain Res. 1995, 705, 15–23. [Google Scholar] [CrossRef]
- Kemp, D.T. Otoacoustic emissions, their origin in cochlear function, and use. Brit. Med. Bull. 2002, 63, 223–241. [Google Scholar] [CrossRef]
- Norton, S.J.; Neely, S.T. Tone-burst-evoked otoacoustic emissions from normal-hearing subjects. J. Acoust. Soc. Am. 1987, 81, 1860–1872. [Google Scholar] [CrossRef]
- Mertes, I.B. Medial olivocochlear reflex effects on synchronized spontaneous otoacoustic emissions. J. Acoust. Soc. Am. 2020, 147, EL235. [Google Scholar] [CrossRef] [Green Version]
- Garinis, A.C.; Glattke, T.; Cone, B.K. The MOC reflex during active listening to speech. J. Speech Lang. Hear. Res. 2011, 54, 1464–1476. [Google Scholar] [CrossRef]
- Khalfa, S.; Veuillet, E.; Collet, L. Influence of handedness on peripheral auditory asymmetry. Eur. J. Neurosci. 1998, 10, 2731–2737. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.J.; Ferry, R.T.; Meddis, R. A computer model of auditory efferent suppression: Implications for the recognition of speech in noise. J. Acoust. Soc. Am. 2010, 127, 943–954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mertes, I.B.; Wilbanks, E.C.; Leek, M.R. Olivocochlear efferent activity is associated with the slope of the psychometric function of speech recognition in noise. Ear Hear. 2018, 39, 583–593. [Google Scholar] [CrossRef] [PubMed]
- Alain, C.; Woods, D.L.; Knight, R.T. A distributed cortical network for auditory sensory memory in humans. Brain Res. 1998, 812, 23–37. [Google Scholar] [CrossRef]
- May, B.J.; Budelis, J.; Niparko, J.K. Behavioral studies of the olivocochlear efferent system: Learning to listen in noise. Arch. Otolaryngol. Head Neck Surg. 2004, 130, 660–664. [Google Scholar] [CrossRef] [Green Version]
- Baer, T.; Moore, B.C. Effects of spectral smearing on the intelligibility of sentences in the presence of interfering speech. J. Acoust. Soc. Am. 1994, 95, 2277–2280. [Google Scholar] [CrossRef] [PubMed]
- Mertes, I.B.; Johnson, K.M.; Dinger, Z.A. Olivocochlear efferent contributions to speech-in-noise recognition across signal-to-noise ratios. J. Acoust. Soc. Am. 2019, 145, 1529. [Google Scholar] [CrossRef] [Green Version]
- Mertes, I.B.; Goodman, S.S. Within- and across-subject variability of repeated measurements of medial olivocochlear-induced changes in transient-evoked otoacoustic emissions. Ear Hear. 2016, 37, e72–e84. [Google Scholar] [CrossRef]
- Guinan, J.J.J.; Backus, B.C.; Lilaonitkul, W.; Aharonson, V. Medial olivocochlear efferent reflex in humans: Otoacoustic emission (OAE) measurement issues and the advantages of stimulus frequency OAEs. J. Assoc. Res. Otolaryngol. 2003, 4, 521–540. [Google Scholar] [CrossRef] [Green Version]
- Goodman, S.S.; Mertes, I.B.; Lewis, J.D.; Weissbeck, D.K. Medial olivocochlear-induced transient-evoked otoacoustic emission amplitude shifts in individual subjects. J. Assoc. Res. Otolaryngol. 2013, 14, 829–842. [Google Scholar] [CrossRef] [Green Version]
- Mertes, I.B. Establishing critical differences in ear-canal stimulus amplitude for detecting middle ear muscle reflex activation during olivocochlear efferent measurements. Int. J. Audiol. 2020, 59, 140–147. [Google Scholar] [CrossRef]
- Martin, B.A.; Stapells, D.R. Effects of low-pass noise masking on auditory event-related potentials to speech. Ear Hear. 2005, 26, 195–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muller-Gass, A.; Marcoux, A.; Logan, J.; Campbell, K.B. The intensity of masking noise affects the mismatch negativity to speech sounds in human subjects. Neurosci. Lett. 2001, 299, 197–200. [Google Scholar] [CrossRef]
- Bennett, K.O.; Billings, C.J.; Molis, M.R.; Leek, M.R. Neural encoding and perception of speech signals in informational masking. Ear Hear. 2012, 33, 231–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Li, X.; Chen, J.; Gong, Q. Background suppression and its relation to foreground processing of speech versus non-speech streams. Neuroscience 2018, 373, 60–71. [Google Scholar] [CrossRef]
- Wittekindt, A.; Kaiser, J.; Abel, C. Attentional modulation of the inner ear: A combined otoacoustic emission and EEG study. J. Neurosci. 2014, 34, 9995–10002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lagace, J.; Jutras, B.; Gagne, J.P. Auditory processing disorder and speech perception problems in noise: Finding the underlying origin. Am. J. Audiol. 2010, 19, 17–25. [Google Scholar] [CrossRef]
- Sanches, S.G.; Carvallo, R.M. Contralateral suppression of transient evoked otoacoustic emissions in children with auditory processing disorder. Audiol. Neurotol. 2006, 11, 366–372. [Google Scholar] [CrossRef]
- Micheyl, C.; Carbonnel, O.; Collet, L. Medial olivocochlear system and loudness adaptation: Differences between musicians and non-musicians. Brain Cognit. 1995, 29, 127–136. [Google Scholar] [CrossRef] [Green Version]
- Strait, D.L.; Parbery-Clark, A.; Hittner, E.; Kraus, N. Musical training during early childhood enhances the neural encoding of speech in noise. Brain Lang. 2012, 123, 191–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, P.C.; Skoe, E.; Russo, N.M.; Dees, T.; Kraus, N. Musical experience shapes human brainstem encoding of linguistic pitch patterns. Nat. Neurosci. 2007, 10, 420–422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bar-Haim, Y.; Henkin, Y.; Ari-Even-Roth, D.; Tetin-Schneider, S.; Hildesheimer, M.; Muchnik, C. Reduced auditory efferent activity in childhood selective mutism. Biol. Psychiatry 2004, 55, 1061–1068. [Google Scholar] [CrossRef] [PubMed]
- Mishra, J.; Gazzaley, A. Harnessing the neuroplastic potential of the human brain & the future of cognitive rehabilitation. Front. Hum. Neurosci. 2014, 8. [Google Scholar] [CrossRef] [Green Version]


| Ear | 250 Hz | 500 Hz | 1000 Hz | 2000 Hz | 4000 Hz | 8000 Hz |
|---|---|---|---|---|---|---|
| Right | 5 (4.9) | 4.2 (3.9) | 2.3 (4.7) | 2.3 (7.5) | 3.8 (7.2) | 2.6 (7.8) |
| Left | 5 (8.3) | 4.6 (5.7) | 3.5 (4.3) | 2.3 (7.9) | 1.9 (6.1) | 2.3 (8.5) |
| Ear | Condition | Word Recognition Score in Percentage (SD) |
|---|---|---|
| Right | Without CAS | 78.3 (3.6) |
| With CAS | 76.8 (3.7) | |
| Left | Without CAS | 77.4 (5.2) |
| With CAS | 77 (3.3) |
| Ear | Condition | MMN | |
|---|---|---|---|
| Amplitude (µV) (SD) | Latency (ms) (SD) | ||
| Right | Without CAS | −1.22 (0.47) | 227.3 (23.5) |
| With CAS | −1.15 (0.34) | 219.65 (18.63) | |
| Left | Without CAS | −1.21 (0.33) | 247.72 (20.93) |
| With CAS | −1.1 (0.52) | 228.95 (18.84) | |
| Main Effects and Interactions | MMN Peak Amplitude | MMN Peak Latency |
|---|---|---|
| Ear | F(1,10) = 0.145 p = 0.71 ηp2 = 0.014 | F(1,10) = 6.71 p = 0.03 * ηp2 = 0.40 |
| Condition | F(1,10) = 1.25 p = 0.29 ηp2 = 0.11 | F(1,10) = 2.11 p = 0.17 ηp2 = 0.175 |
| Ear × Condition | F(1,10) = 0.058 p = 0.81 ηp2 = 0.006 | F(1,10) = 1.4 p = 0.26 ηp2 = 0.263 |
| Ear | Condition | MMN | P300 | ||
|---|---|---|---|---|---|
| Amplitude (µV) (SD) | Latency (ms) (SD) | Amplitude (µV) (SD) | Latency (ms) (SD) | ||
| Right | Without CAS | −1.22 (0.47) | 227.3 (23.5) | 2.09 (0.99) | 420.5 (54.97) |
| With CAS | −1.15 (0.34) | 219.65 (18.63) | 1.8 (0.95) | 417.03 (64.5) | |
| Left | Without CAS | −1.21 (0.33) | 247.72 (20.93) | 1.99 (0.98) | 409.52 (49.83) |
| With CAS | −1.1 (0.52) | 228.95 (18.84) | 1.97 (0.98) | 444.12 (50.45) | |
| Main Effects and Interactions | P300 Peak Amplitude | P300 Peak Latency |
|---|---|---|
| Ear | F(1,10) = 0.022 p = 0.88 ηp2 = 0.002 | F(1,10) = 0.72 p = 0.416 ηp2 = 0.067 |
| Condition | F(1,10) = 1.20 p = 0.298 ηp2 = 0.107 | F(1,10) = 0.85 p = 0.378 ηp2 = 0.078 |
| Ear × Condition | F(1,10) = 0.533 p = 0.482 ηp2 = 0.051 | F(1,10) = 1.65 p = 0.228 ηp2 = 0.142 |
| Ear | Condition | d’ (SD) | Criterion (SD) | Reaction Times (SD) |
|---|---|---|---|---|
| Right | Without CAS | 2.07 (1.12) | −0.69 (0.38) | 556.01 (115.66) |
| With CAS | 2.09 (1.18) | −0.76 (0.34) | 567.29 (134.59) | |
| Left | Without CAS | 2.59 (1.34) | −0.66 (0.5) | 543.18 (121.04) |
| With CAS | 2.64 (1.42) | −0.67 (0.43) | 551.19 (110.43) |
| Main Effects and Interactions | d’ | Criterion | Reaction Time |
|---|---|---|---|
| Ear | F(1,10) = 7.133 p = 0.02 * ηp2 = 0.41 | F(1,10) = 0.702 p = 0.42 ηp2 = 0.06 | F(1,10) = 1.24 p = 0.292 ηp2 = 0.11 |
| Condition | F(1,10) = 0.62 p = 0.80 ηp2 = 0.006 | F(1,10) = 1.81 p = 0.208 ηp2 = 0.153 | F(1,10) = 0.420 p = 0.53 ηp2 = 0.04 |
| Ear × Condition | F(1,10) = 0.121 p = 0.735 ηp2 = 0.012 | F(1,10) = 0.708 p = 0.420 ηp2 = 0.066 | F(1,10) = 0.015 p = 0.91 ηp2 = 0.001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rao, A.; Koerner, T.K.; Madsen, B.; Zhang, Y. Investigating Influences of Medial Olivocochlear Efferent System on Central Auditory Processing and Listening in Noise: A Behavioral and Event-Related Potential Study. Brain Sci. 2020, 10, 428. https://doi.org/10.3390/brainsci10070428
Rao A, Koerner TK, Madsen B, Zhang Y. Investigating Influences of Medial Olivocochlear Efferent System on Central Auditory Processing and Listening in Noise: A Behavioral and Event-Related Potential Study. Brain Sciences. 2020; 10(7):428. https://doi.org/10.3390/brainsci10070428
Chicago/Turabian StyleRao, Aparna, Tess K. Koerner, Brandon Madsen, and Yang Zhang. 2020. "Investigating Influences of Medial Olivocochlear Efferent System on Central Auditory Processing and Listening in Noise: A Behavioral and Event-Related Potential Study" Brain Sciences 10, no. 7: 428. https://doi.org/10.3390/brainsci10070428
APA StyleRao, A., Koerner, T. K., Madsen, B., & Zhang, Y. (2020). Investigating Influences of Medial Olivocochlear Efferent System on Central Auditory Processing and Listening in Noise: A Behavioral and Event-Related Potential Study. Brain Sciences, 10(7), 428. https://doi.org/10.3390/brainsci10070428






