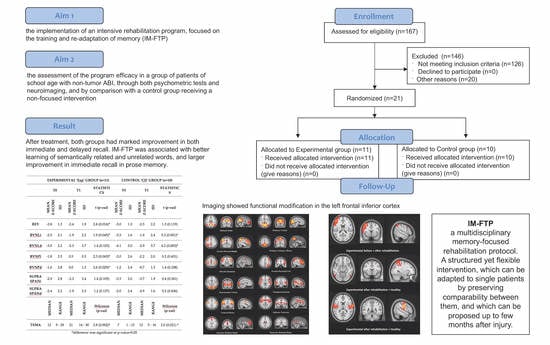
Feasibility Randomized Trial for an Intensive Memory-Focused Training Program for School-Aged Children with Acquired Brain Injury
Mild and Moderate Traumatic Brain Injuries: Diagnosis, Assessment Tools, Management and Factors Influencing Recovery
)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Clinical and Neuropsychological Measures
2.3. Psychometric Evaluation (Primary Measures)
2.4. Theoretical Framework for the Pediatric Adaptation of Rehabilitation Programs
- (1)
- The developmental needs of the child (need to play, need of novelty, acceptance of the challenge and of the failure/mistake, need to understand their own difficulty while overcoming frustration and avoiding giving up).
- (2)
- The different cognitive functioning compared to the adult. The child approaches tasks in ipo-strategic and impulsive manner, due to the immaturity of the executive functions. Also, they can only deploy reduced wealth of experience and limited procedure automation.
- (3)
- The immature self-motivation and self-determination, which negatively impact on the compliance to the rehabilitation work.
2.5. Rehabilitation Program
2.5.1. Intensive Memory-Focused Training Program (IM-FTP)
- (1)
- Stimulation of the short-term visuo-spatial memory. We trained the patient to work around their specific deficit and discover strategies for memorizing through visualization, acting on the patient’s short-term memory through empowerment. We introduced the concept of an “internal camera” for memorizing selected images and situations [29], and we personalized the training through a selection of activities to be continuously adapted to the patient’s need. Then patients solved logical matrices and visual memory exercises taken from the Test of Visual Perception Skills v.3 (TVPS-3) protocol [30], and exercises of object permanence.
- (2)
- Stimulation of the long-term visuo-spatial memory. In this block the therapist administered exercises of visual memory with interference, and training exercises from the TVPS-3 protocol. Then, exercises were carried out, divided in sections, to train the recall of image series, recall of object position inside a scenery, and learning of simple visuo-spatial sequences [29]. Each section administered exercises grouped according to type and graded according to increasing difficulty. Then the therapist carried out exercises for the memorization of 3 objects and their respective spatial location; the task was followed by the patient’s involvement in a distracting skill, and the subsequent request to recall the positions of the objects and to find them inside the room. Lastly, patients were administered exercises of supra-span visual memory.
- (3)
- Stimulation of the visual working-memory (visuo-spatial notepad). In this block the staff administered n-back exercises, Sudoku, solo card games (e.g., Spider and similar), updating exercises and exercises from Marzocchi et al. [31], which includes different visual and audio activities, aiming at the development and empowerment of working-memory.
- (1)
- Stimulation of the short-term verbal memory. Repetition exercises (continuous reiteration of a given span of numbers or words) were initially administered, based on Rudland et al. [29]. Then the therapist administered memory timing exercises [32,33]. Patients were requested to remember numbers and words for short time (e.g., store and recall, by typing a phone number or writing a car number plate).
- (2)
- Stimulation of the long-term verbal memory. Patients were asked to recall information, also through the method in Powell, Gollin et al. [32,33], lists of words, newspaper articles, names and numbers. Then, exercises of text recall with and without interference were delivered, tasks for the learning and retention of words lists, tasks for learning and retention of couples of linked and non-linked words, and exercises for face-name association.
- (3)
- Stimulation of the verbal working-memory. Therapists administered logical grids, the Paced Auditory Serial Addition Task and the Children’s Paced Auditory Serial Addition Task [34,35], exercises on acronyms, and tasks for the memorization of the first, second or third element contained in a sentence. Additionally patients took on tasks of the generation of sentences for learning couples of words.
2.5.2. Standard Training Program
2.6. Statistical Analysis
2.7. Magnetic Resonance Imaging (MRI) Acquisition and Functional MRI Analysis
3. Results
3.1. Study Sample
3.2. Clinical and Behavioral Scores before and after Rehabilitation
3.3. Neuropsychological Scores before and after Rehabilitation
3.4. Resting State Functional MRI (rsMRI)
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Giacino, J.T.; Katz, D.I.; Schiff, N.D.; Whyte, J.; Ashman, E.J.; Ashwal, S.; Barbano, R.; Hammond, F.M.; Laureys, S.; Ling, G.S.F.; et al. Comprehensive Systematic Review Update Summary: Disorders of Consciousness: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology; the American Congress of Rehabilitation Medicine; And the National Institute on Disability, Independent Living, and Rehabilitation Research. Arch. Phys. Med. Rehabil. 2018, 99, 1710–1719. [Google Scholar] [PubMed]
- Gómez-Pérez, E.; Ostrosky-Solís, F. Attention and memory evaluation across the life span: Heterogeneous effects of age and education. J. Clin. Exp. Neuropsychol. 2006, 28, 477–494. [Google Scholar] [CrossRef]
- Carney, N.; Chesnut, R.M.; Maynard, H.; Mann, N.C.; Patterson, P.; Helfand, M. Effect of cognitive rehabilitation on outcomes for persons with traumatic brain injury: A systematic review. J. Head Trauma Rehabil. 1999, 14, 277–307. [Google Scholar] [CrossRef] [PubMed]
- Cicerone, K.D.; Dahlberg, C.; Kalmar, K.; Langenbahn, D.M.; Malec, J.F.; Bergquist, T.F.; Felicetti, T.; Giacino, J.T.; Harley, J.P.; Harrington, D.E.; et al. Evidence-based cognitive rehabilitation: Recommendations for clinical practice. Arch. Phys. Med. Rehabil. 2000, 81, 1596–1615. [Google Scholar] [CrossRef]
- Dennis, M.; Wilkinson, M.; Koski, L.; Humphreys, R.P. Attention deficits in the long term after childhood head injury. In Traumatic Head Injury in Children; Oxford University Press: Oxford, UK, 1995; pp. 165–187. ISBN 0-19-509428-X. [Google Scholar]
- Sohlberg, M.M.; Mateer, C. Introduction to Cognitive Rehabilitation; Guilford Press: New York, NY, USA, 1989. [Google Scholar]
- Hendriks, C.M.; van den Broek, T.M. Amat-c Manual and Workbook; Swets & Zeitlinger: Lisse, The Netherlands, 1996. [Google Scholar]
- Van’t Hooft, I.; Andersson, K.; Bergman, B.; Sejersen, T.; von Wendt, L.; Bartfai, A. Beneficial effect from a cognitive training programme on children with acquired brain injuries demonstrated in a controlled study. Brain Inj. 2005, 19, 511–518. [Google Scholar] [CrossRef]
- Van’t Hooft, I.; Andersson, K.; Sejersen, T.; Bartfai, A.; Von Wendt, L. Attention and memory training in children with acquired brain injuries. Acta Paediatr. 2003, 92, 935–940. [Google Scholar]
- Van’t Hooft, I.; Andersson, K.; Bergman, B.; Sejersen, T. Sustained favorable effects of cognitive training in children with acquired brain injuries. NeuroRehabilitation 2007, 22, 109–116. [Google Scholar] [CrossRef]
- Sjö, N.M.; Spellerberg, S.; Weidner, S.; Kihlgren, M. Training of attention and memory deficits in children with acquired brain injury. Acta Paediatr. Int. J. Paediatr. 2010, 99, 230–236. [Google Scholar] [CrossRef]
- Catroppa, C.; Stone, K.; Rosema, S.; Soo, C.; Anderson, V. Preliminary efficacy of an attention and memory intervention post-childhood brain injury. Brain Inj. 2014, 28, 252–260. [Google Scholar] [CrossRef]
- Catroppa, C.; Stone, K.; Hearps, S.J.C.; Soo, C.; Anderson, V.; Rosema, S. Evaluation of an attention and memory intervention post-childhood acquired brain injury: Preliminary efficacy, immediate and 6 months post-intervention. Brain Inj. 2015, 29, 1317–1324. [Google Scholar] [CrossRef]
- Conklin, H.M.; Ashford, J.M.; Clark, K.N.; Martin-Elbahesh, K.; Hardy, K.K.; Merchant, T.E.; Ogg, R.J.; Jeha, S.; Huang, L.; Zhang, H. Long-term efficacy of computerized cognitive training among survivors of childhood cancer: A single-blind randomized controlled trial. J. Pediatr. Psychol. 2017, 42, 220–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conklin, H.M.; Ogg, R.J.; Ashford, J.M.; Scoggins, M.A.; Zou, P.; Clark, K.N.; Martin-Elbahesh, K.; Hardy, K.K.; Merchant, T.E.; Jeha, S.; et al. Computerized cognitive training for amelioration of cognitive late effects among childhood cancer survivors: A randomized controlled trial. J. Clin. Oncol. 2015, 33, 3894–3902. [Google Scholar] [CrossRef] [Green Version]
- Lambregts, S.A.M.; Smetsers, J.E.M.; Verhoeven, I.M.A.J.; de Kloet, A.J.; van de Port, I.G.L.; Ribbers, G.M.; Catsman-Berrevoets, C.E. Cognitive function and participation in children and youth with mild traumatic brain injury two years after injury. Brain Inj. 2018, 32, 230–241. [Google Scholar] [CrossRef]
- Phillips, N.L.; Parry, L.; Mandalis, A.; Lah, S. Working memory outcomes following traumatic brain injury in children: A systematic review with meta-analysis. Child. Neuropsychol. 2017, 23, 26–66. [Google Scholar] [CrossRef] [PubMed]
- Viot, S.; Câmara-Costa, H.; Laurence, W.; Francillette, L.; Toure, H.; Brugel, D.; Laurent-Vannier, A.; Dellatolas, G.; Gillibert, A.; Meyer, P.; et al. Assessment of memory functioning over two years following severe childhood traumatic brain injury: Results of the TGE cohort. Brain Inj. 2019, 33, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Irimia, A.; Van Horn, J.D. Functional neuroimaging of traumatic brain injury: Advances and clinical utility. Neuropsychiatr. Dis. Treat. 2015, 11, 2355–2365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dennis, E.L.; Babikian, T.; Giza, C.C.; Thompson, P.M.; Asarnow, R.F. Neuroimaging of the Injured Pediatric Brain: Methods and New Lessons. Neuroscientist 2018, 24, 652–670. [Google Scholar] [CrossRef]
- Strangman, G.; O’Neil-Pirozzi, T.M.; Burke, D.; Cristina, D.; Goldstein, R.; Rauch, S.L.; Savage, C.R.; Glenn, M.B. Functional neuroimaging and cognitive rehabilitation for people with traumatic brain injury. Am. J. Phys. Med. Rehabil. 2005, 84, 62–75. [Google Scholar] [CrossRef]
- Bayona, N.A.; Bitensky, J.; Salter, K.; Teasell, R. The role of task-specific training in rehabilitation therapies. Top. Stroke Rehabil. 2005, 12, 58–65. [Google Scholar] [CrossRef]
- Rocca, M.A.; Turconi, A.C.; Strazzer, S.; Absinta, M.; Valsasina, P.; Beretta, E.; Copetti, M.; Cazzagon, M.; Falini, A.; Filippi, M. MRI predicts efficacy of constraint-induced movement therapy in children with brain injury. Neurotherapeutics 2013, 10, 511–519. [Google Scholar] [CrossRef] [Green Version]
- Kilgard, M.P.; Merzenich, M.M. Cortical map reorganization enabled by nucleus basalis activity. Science 1998, 279, 1714–1718. [Google Scholar] [CrossRef] [PubMed]
- Nudo, R. Adaptive plasticity in motor cortex: Implications for rehabilitation after brain injury. J. Rehabil. Med. 2003, 35, 7–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teasdale, G.; Jennett, B. Assessment Of Coma And Impared Conciousness. Lancet 1974, 304, 81–84. [Google Scholar] [CrossRef]
- Working Party of the Royal College of Physicians. Permanent Vegetative State: Guidance on Diagnosis and Management. Clin. Med. 2003, 3, 249–254. [Google Scholar] [CrossRef]
- Giacino, J.T.; Ashwal, S.; Childs, N.; Cranford, R.; Jennett, B.; Katz, D.I.; Kelly, J.P.; Rosenberg, J.H.; Whyte, J.; Zafonte, R.D.; et al. The minimally conscious state: Definition and diagnostic criteria. Neurology 2002, 58, 349–353. [Google Scholar] [CrossRef]
- Rudland, J. Potenziare la Memoria a Breve Termine; Erickson: Trento, Italy, 2008. [Google Scholar]
- Brown, T.; Rodger, S. An evaluation of the validity of the Test of Visual Perceptual Skills—Revised (TVPS-R) using the Rasch measurement model. Br. J. Occup. Ther. 2009, 72, 65–78. [Google Scholar] [CrossRef]
- Marzocchi, G.M.; Portolan, S.; Usilla, A. Autoregolare L’attenzione; Erickson: Trento, Italy, 2006. [Google Scholar]
- Powell, T.; Malia, K. Training di Riabilitazione Cognitiva. Esercizi di Memoria, Abilità di Pensiero e Funzioni Esecutive Dopo una Lesione Cerebrale; Erickson: Trento, Italy, 2009. [Google Scholar]
- Gollin, D.; Ferrari, A.; Peruzzi, A. Una Palestra per la Mente; Erickson: Trento, Italy, 2011. [Google Scholar]
- Johnson, D.A.; Roethig-Johnston, K.; Middleton, J. Development and evaluation of an attentional test for head injured children—1. Information processing capacity in a normal sample. J. Child. Psychol. Psychiatry 1988, 29, 199–208. [Google Scholar] [CrossRef]
- Dyche, G.M.; Johnson, D.A. Development and evaluation of CHIPASAT, an attention test for children: II. Test-retest reliability and practice effect for a normal sample. Percept. Mot. Skills 1991, 72, 563–572. [Google Scholar] [CrossRef]
- L’Ecuyer-Giguère, F.; Greffou, S.; Tabet, S.; Frenette, L.C.; Tinawi, S.; Feyz, M.; de Guise, E. Visual memory performance following mild traumatic brain injury and its relationship with intellectual functioning. Appl. Neuropsychol. Adult 2020, 27, 219–231. [Google Scholar] [CrossRef]
- Bigler, E.D.; McCauley, S.R.; Wu, T.C.; Yallampalli, R.; Shah, S.; MacLeod, M.; Chu, Z.; Hunter, J.V.; Clifton, G.L.; Levin, H.S.; et al. The temporal stem in traumatic brain injury: Preliminary findings. Brain Imaging Behav. 2010, 4, 270–282. [Google Scholar] [CrossRef]
- Molteni, E.; Bianchi, A.M.; Butti, M.; Reni, G.; Zucca, C. Combined behavioral and EEG power analysis in DAI improve accuracy in the assessment of sustained attention deficit. Ann. Biomed. Eng. 2008, 36, 1216–1227. [Google Scholar] [CrossRef] [PubMed]
- Tyler, L.K.; Marslen-Wilson, W.D.; Randall, B.; Wright, P.; Devereux, B.J.; Zhuang, J.; Papoutsi, M.; Stamatakis, E.A. Left inferior frontal cortex and syntax: Function, structure and behaviour in patients with left hemisphere damage. Brain 2011, 134, 415–431. [Google Scholar] [CrossRef] [Green Version]
- Python, G.; Glize, B.; Laganaro, M. The involvement of left inferior frontal and middle temporal cortices in word production unveiled by greater facilitation effects following brain damage. Neuropsychologia 2018, 121, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Yerys, B.E.; White, D.A.; Salorio, C.F.; McKinstry, R.; Moinuddin, A.; DeBaun, M. Memory strategy training in children with cerebral infarcts related to sickle cell disease. J. Pediatr. Hematol. Oncol. 2003, 25, 495–498. [Google Scholar] [CrossRef] [PubMed]
- Van’T Hooft, I.; Norberg, A.L. SMART cognitive training combined with a parental coaching programme for three children treated for medulloblastoma. NeuroRehabilitation 2010, 26, 105–113. [Google Scholar] [CrossRef]
- Resch, C.; Rosema, S.; Hurks, P.; de Kloet, A.; van Heugten, C. Searching for effective components of cognitive rehabilitation for children and adolescents with acquired brain injury: A systematic review. Brain Inj. 2018, 32, 679–692. [Google Scholar] [CrossRef] [Green Version]
- Galbiati, S.; Recla, M.; Pastore, V.; Liscio, M.; Bardoni, A.; Castelli, E.; Strazzer, S. Attention Remediation Following Traumatic Brain Injury in Childhood and Adolescence. Neuropsychology 2009, 23, 40–49. [Google Scholar] [CrossRef]
- Malia, K.; Law, P.; Sidebottom, L.; Bewick, K.; Danziger, S.; Schold-Davis, E.; Martin-Scull, R.; Murphy, K.; Vaidya, A. Recommendations for Best Practice in Cognitive Rehabilitation Therapy: Acquired Brain Injury. Pract. Innov. Cogn. Rehabil. Ther. 2004, 38, 1–57. [Google Scholar]
- Dams-O’Connor, K.; Gordon, W.A. Role and impact of cognitive rehabilitation. Psychiatr. Clin. N. Am. 2010, 33, 893–904. [Google Scholar] [CrossRef]
- Laatsch, L.; Harrington, D.; Hotz, G.; Marcantuono, J.; Mozzoni, M.P.; Walsh, V.; Hersey, K.P. An evidence-based review of cognitive and behavioral rehabilitation treatment studies in children with acquired brain injury. J. Head Trauma Rehabil. 2007, 22, 248–256. [Google Scholar] [CrossRef]
- Slomine, B.; Locascio, G. Cognitive rehabilitation for children with acquired brain injury. Dev. Disabil. Res. Rev. 2009, 15, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Barman, A.; Chatterjee, A.; Bhide, R. Cognitive impairment and rehabilitation strategies after traumatic brain injury. Indian J. Psychol. Med. 2016, 38, 172–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prigatano, G.P. Challenging dogma in neuropsychology and related disciplines. Arch. Clin. Neuropsychol. 2003, 18, 811–825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Limond, J.; Adlam, A.L.R.; Cormack, M. A model for pediatric neurocognitive interventions: Considering the role of development and maturation in rehabilitation planning. Clin. Neuropsychol. 2014, 28, 181–198. [Google Scholar] [CrossRef] [Green Version]
- Ryan, N.P.; Catroppa, C.; Godfrey, C.; Noble-Haeusslein, L.J.; Shultz, S.R.; O’Brien, T.J.; Anderson, V.; Semple, B.D. Social dysfunction after pediatric traumatic brain injury: A translational perspective. Neurosci. Biobehav. Rev. 2016, 64, 196–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Total Sample (n = 21) | Experimental Group (n = 11) | Control Group (n = 10) | Test | ||
|---|---|---|---|---|---|
| N | % | N | % | χ2 (p-Value) | |
| Gender | |||||
| Male | 5 | 45.4 | 6 | 60.0 | 0.44 (0.505) |
| Female | 6 | 54.5 | 4 | 40.0 | |
| Etiology | |||||
| Traumatic Brain Injury (TBI) | 7 | 63.7 | 7 | 70.0 | 0.10 (0.757) |
| Non Traumatic Brain Injury (NTBI) | 4 | 36.3 | 3 | 30.0 | |
| of Which: | |||||
| Ictal Stroke | 2 | 1 | |||
| Hemorrhagic Stroke | 1 | 1 | |||
| Immune Encephalitis | 1 | 1 | |||
| Mean | SD | Mean | SD | Wilcoxon(p-value) | |
| Age at Injury [months] | 146.4 | 41.4 | 165.9 | 27.6 | 1.41 (0.159) |
| Age at Admission [months] | 148.6 | 42.8 | 169.4 | 29.3 | 1.33 (0.184) |
| Days of Coma | 16.9 | 14.2 | 28.6 | 24.4 | 1.17 (0.244) |
| Days from Injury/Illness to Treatment | 30.1 | 14.6 | 35.1 | 18.9 | 0.44 (0.503) |
| N | % | N | % | χ2(p-value) | |
| Need of Neurosurgery | 5 | 45.5 | 3 | 30.0 | 0.53 (0.466) |
| Epileptic Seizures | 1 | 9.1 | 0 | 0.0 | 0.96 (0.329) |
| Motor Impairment | |||||
| Quadriparesis | 0 | 0.0 | 2 | 20.0 | 3.96 (0.266) |
| Hemiparesis | 7 | 63.6 | 7 | 70.0 | |
| Ataxia | 1 | 9.1 | 0 | 0.0 | |
| None | 3 | 27.3 | 1 | 10.0 | |
| Brain Lesions at Magnetic Resonance Imaging | |||||
| Diffuse Axonal Injury | 4 | 36.3 | 5 | 50.0 | 2.07 (0.356) |
| Multifocal Damage | 5 | 45.5 | 5 | 50.0 | |
| Damage Due to Extradural Hematoma | 2 | 18.2 | 0 | 0.0 | |
| Mean | SD | Mean | SD | Wilcoxon(p-value) | |
| Wechsler Intelligence Scale for Children or Wechsler Adult Intelligence Scale | |||||
| Total Intelligence Quotient | 67.8 | 15.4 | 60.5 | 13.7 | 1.09 (0.275) |
| Verbal Intelligence Quotient | 79.7 | 16.8 | 79.9 | 16.1 | 0.01 (0.998) |
| Performance Intelligence Quotient | 66.0 | 17.8 | 66.5 | 20.2 | 0.08 (0.940) |
| Experimental Group (n = 11) | Control Group (n = 10) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| T0 | T1 | Statistics | T0 | T1 | Statistics | |||||
| Median | Range | Median | Range | Wilcoxon (p-Value) | Median | Range | Median | Range | Wilcoxon (p-Value) | |
| GCS Score | 5 | 3–8 | - | - | 6 | 3–8 | - | - | ||
| GOS Score | 3 | 2–4 | 4 | 3–5 | 2.4 (0.015) * | 3 | 2–3 | 4 | 3–5 | 2.6 (0.010) * |
| GOS-E Score | 3 | 2–5 | 6 | 4–7 | 2.8 (0.004) * | 3 | 2–4 | 6 | 4–7 | 2.8 (0.005) * |
| LCF Score | 6 | 2–8 | 8 | 7–8 | 2.7 (0.007) * | 5 | 2–7 | 8 | 8 | 2.8 (0.005) * |
| Mean | SD | Mean | SD | t (p-value) | Mean | SD | Mean | SD | t (p-value) | |
| DRS | 13.2 | 5.2 | 4.6 | 1.8 | 5.8 (<0.001) * | 15.8 | 6.6 | 4.2 | 1.8 | 5.3 (0.001) * |
| FIM | 35.7 | 24.4 | 78.0 | 30.9 | 4.6 (0.001) * | 36.3 | 28.6 | 97.5 | 22.5 | 6.8 (<0.001) * |
| Experimental Group (n = 11) | Control Group (n = 10) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| T0 | T1 | Statistics | T0 | T1 | Statistics | |||||
| Mean z-Score | SD | Mean z-Score | SD | t (p-Value) | Mean z-Score | SD | Mean z-Score | SD | t (p-Value) | |
| REY | −2.8 | 1.3 | −2.4 | 1.9 | 2.8 (0.016) * | −3.0 | 1.3 | −2.5 | 2.2 | 1.3 (0.119) |
| BVNLi | −2.5 | 2.1 | −1.9 | 2.2 | 1.9 (0.045) * | −3.3 | 1.6 | −1.9 | 2.4 | 5.3 (0.001) * |
| BVNLd | −3.0 | 2.2 | −2.3 | 2.7 | 1.4 (0.103) | −4.1 | 3.0 | −2.9 | 3.7 | 4.2 (0.003) * |
| BVNPi | −1.8 | 2.5 | 0.5 | 0.5 | 2.3 (0.043) * | −2.0 | 2.6 | −2.2 | 2.0 | 0.2 (0.431) |
| BVNPd | −1.6 | 2.8 | 0.0 | 1.3 | 2.6 (0.029) * | −1.2 | 2.4 | −0.7 | 1.3 | 1.4 (0.108) |
| SUPRA SPANi | −2.9 | 2.8 | −2.3 | 3.4 | 1.4 (0.105) | −3.3 | 2.0 | −3.7 | 1.9 | 0.4 (0.341) |
| SUPRA SPANd | v2.4 | 2.2 | −1.9 | 2.3 | 1.2 (0.137) | −2.0 | 2.4 | −2.9 | 1.6 | 0.2 (0.436) |
| Median | Range | Median | Range | Wilcoxon(p-value) | Median | Range | Median | Range | Wilcoxon(p-value) | |
| TEMA | 12 | 9–29 | 21 | 14–30 | 2.8 (0.002) * | 7 | 1–13 | 12 | 5–16 | 2.0 (0.021) * |
| Sample Size | |
|---|---|
| REY | 6 |
| BVNLi | 31 |
| BVNLd | 14 |
| BVNPi | 7 |
| BVNPd | 11 |
| SUPRASPANi | 26 |
| SUPRASPANd | 27 |
| TEMA | 3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Recla, M.; Molteni, E.; Manfredi, V.; Arrigoni, F.; Nordio, A.; Galbiati, S.; Pastore, V.; Modat, M.; Strazzer, S. Feasibility Randomized Trial for an Intensive Memory-Focused Training Program for School-Aged Children with Acquired Brain Injury. Brain Sci. 2020, 10, 430. https://doi.org/10.3390/brainsci10070430
Recla M, Molteni E, Manfredi V, Arrigoni F, Nordio A, Galbiati S, Pastore V, Modat M, Strazzer S. Feasibility Randomized Trial for an Intensive Memory-Focused Training Program for School-Aged Children with Acquired Brain Injury. Brain Sciences. 2020; 10(7):430. https://doi.org/10.3390/brainsci10070430
Chicago/Turabian StyleRecla, Monica, Erika Molteni, Valentina Manfredi, Filippo Arrigoni, Andrea Nordio, Susanna Galbiati, Valentina Pastore, Marc Modat, and Sandra Strazzer. 2020. "Feasibility Randomized Trial for an Intensive Memory-Focused Training Program for School-Aged Children with Acquired Brain Injury" Brain Sciences 10, no. 7: 430. https://doi.org/10.3390/brainsci10070430
APA StyleRecla, M., Molteni, E., Manfredi, V., Arrigoni, F., Nordio, A., Galbiati, S., Pastore, V., Modat, M., & Strazzer, S. (2020). Feasibility Randomized Trial for an Intensive Memory-Focused Training Program for School-Aged Children with Acquired Brain Injury. Brain Sciences, 10(7), 430. https://doi.org/10.3390/brainsci10070430