Mouse Tracking to Explore Motor Inhibition Processes in Go/No-Go and Stop Signal Tasks
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Procedure
2.2.1. Proactive Inhibition (Cued GNG Task)
2.2.2. Reactive Inhibition (Stop Signal Task)
2.2.3. Feedback on Response Speed
2.3. Data Analysis
3. Results
3.1. Behavioural Performance
3.2. Movement Profiles
3.3. Reaction Times
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Duque, J.; Greenhouse, I.; Labruna, L.; Ivry, R.B. Physiological markers of motor inhibition during human behavior. Trends Neurosci. 2017, 40, 219–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braver, T.S. The variable nature of cognitive control: A dual mechanisms framework. Trends Cogn. Sci. 2012, 16, 106–113. [Google Scholar] [CrossRef] [Green Version]
- Aron, A.R. From reactive to proactive and selective control: Developing a richer model for stopping inappropriate responses. Biol. Psychiatry 2011, 69, e55–e68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyer, H.C.; Bucci, D.J. Neural and behavioral mechanisms of proactive and reactive inhibition. Learn. Mem. 2016, 23, 504–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raud, L.; Westerhausen, R.; Dooley, N.; Huster, R.J. Differences in unity: The go/no-go and stop signal tasks rely on different mechanisms. NeuroImage 2020, 210, 116582. [Google Scholar] [CrossRef]
- Verbruggen, F.; Logan, G.D. Automatic and controlled response inhibition: Associative learning in the go/no-go and stop-signal paradigms. J. Exp. Psychol. Gen. 2008, 137, 649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, J.P.; Kar, B.R. Effect of language proficiency on proactive occulo-motor control among bilinguals. PLoS ONE 2018, 13, e0207904. [Google Scholar] [CrossRef] [PubMed]
- Cunillera, T.; Fuentemilla, L.; Brignani, D.; Cucurell, D.; Miniussi, C. A simultaneous modulation of reactive and proactive inhibition processes by anodal tDCS on the right inferior frontal cortex. PLoS ONE 2014, 9, e113537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.-S.R.; Yan, P.; Sinha, R.; Lee, T.-W. Subcortical processes of motor response inhibition during a stop signal task. Neuroimage 2008, 41, 1352–1363. [Google Scholar]
- Picazio, S.; Ponzo, V.; Caltagirone, C.; Brusa, L.; Koch, G. Dysfunctional inhibitory control in Parkinson’s disease patients with levodopa-induced dyskinesias. J. Neurol. 2018, 265, 2088–2096. [Google Scholar] [CrossRef]
- Wang, C.-H.; Chang, C.-C.; Liang, Y.-M.; Shih, C.-M.; Chiu, W.-S.; Tseng, P.; Hung, D.L.; Tzeng, O.J.; Muggleton, N.G.; Juan, C.-H. Open vs. closed skill sports and the modulation of inhibitory control. PLoS ONE 2013, 8, e55773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coulacoglou, C.; Saklofske, D.H. Psychometrics and psychological Assessment: Principles and Applications; Academic Press, Elsevier: London, UK, 2017. [Google Scholar]
- Wilkowski, B.; Robinson, M. Cognitive control processes underlying individual differences in self-control. In Self-Regulation and Ego Control; Academic Press, Elsevier: London, UK, 2016; pp. 301–324. [Google Scholar]
- Allain, S.; Carbonnell, L.; Burle, B.; Hasbroucq, T.; Vidal, F. On-line executive control: An electromyographic study. Psychophysiology 2004, 41, 113–116. [Google Scholar] [CrossRef]
- Cohen, M.X.; van Gaal, S. Subthreshold muscle twitches dissociate oscillatory neural signatures of conflicts from errors. Neuroimage 2014, 86, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Coles, M.G.; Scheffers, M.K.; Fournier, L. Where did you go wrong? Errors, partial errors, and the nature of human information processing. Acta Psychol. 1995, 90, 129–144. [Google Scholar] [CrossRef]
- Giovannelli, F.; Mastrolorenzo, B.; Rossi, A.; Gavazzi, G.; Righi, S.; Zaccara, G.; Viggiano, M.; Cincotta, M. Relationship between impulsivity traits and awareness of motor intention. Eur. J. Neurosci. 2016, 44, 2455–2459. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.; Giovannelli, F.; Gavazzi, G.; Righi, S.; Cincotta, M.; Viggiano, M.P. Electrophysiological Activity Prior to Self-initiated Movements is Related to Impulsive Personality Traits. Neuroscience 2018, 372, 266–272. [Google Scholar] [CrossRef]
- Gavazzi, G.; Rossi, A.; Orsolini, S.; Diciotti, S.; Giovannelli, F.; Salvadori, E.; Pantoni, L.; Mascalchi, M.; Viggiano, M.P. Impulsivity trait and proactive cognitive control: An fMRI study. Eur. J. Neurosci. 2019, 49, 1171–1179. [Google Scholar] [CrossRef]
- Ficarella, S.C.; Rochet, N.; Burle, B. Becoming aware of subliminal responses: An EEG/EMG study on partial error detection and correction in humans. Cortex 2019, 120, 443–456. [Google Scholar] [CrossRef] [Green Version]
- Neely, K.A.; Wang, P.; Chennavasin, A.P.; Samimy, S.; Tucker, J.; Merida, A.; Perez-Edgar, K.; Huang-Pollock, C. Deficits in inhibitory force control in young adults with ADHD. Neuropsychologia 2017, 99, 172–178. [Google Scholar] [CrossRef] [Green Version]
- Spivey, M.J.; Dale, R. Continuous dynamics in real-time cognition. Curr. Dir. Psychol. Sci. 2006, 15, 207–211. [Google Scholar] [CrossRef]
- Spivey, M.J.; Grosjean, M.; Knoblich, G. Continuous attraction toward phonological competitors. Proc. Natl. Acad. Sci. USA 2005, 102, 10393–10398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freeman, J.B. Doing psychological science by hand. Curr. Dir. Psychol. Sci. 2018, 27, 315–323. [Google Scholar] [CrossRef] [Green Version]
- Leontyev, A.; Sun, S.; Wolfe, M.; Yamauchi, T. Augmented Go/No-Go task: Mouse cursor motion measures improve ADHD symptom assessment in healthy college students. Front. Psychol. 2018, 9, 496. [Google Scholar] [CrossRef] [PubMed]
- Leontyev, A.; Yamauchi, T. Mouse movement measures enhance the stop-signal task in adult ADHD assessment. PLoS ONE 2019, 14, e0225437. [Google Scholar] [CrossRef] [PubMed]
- Freeman, J.B.; Ambady, N. MouseTracker: Software for studying real-time mental processing using a computer mouse-tracking method. Behav. Res. Methods 2010, 42, 226–241. [Google Scholar] [CrossRef] [PubMed]
- Berret, B.; Chiovetto, E.; Nori, F.; Pozzo, T. Evidence for composite cost functions in arm movement planning: An inverse optimal control approach. PLoS Comput. Biol. 2011, 7, e1002183. [Google Scholar] [CrossRef]
- Bisio, A.; Stucchi, N.; Jacono, M.; Fadiga, L.; Pozzo, T. Automatic versus voluntary motor imitation: Effect of visual context and stimulus velocity. PLoS ONE 2010, 5, e13506. [Google Scholar] [CrossRef] [Green Version]
- Gavazzi, G.; Bisio, A.; Pozzo, T. Time perception of visual motion is tuned by the motor representation of human actions. Sci. Rep. 2013, 3, 1–8. [Google Scholar] [CrossRef]
- Sciutti, A.; Bisio, A.; Nori, F.; Metta, G.; Fadiga, L.; Pozzo, T.; Sandini, G. Measuring human-robot interaction through motor resonance. Int. J. Soc. Robot. 2012, 4, 223–234. [Google Scholar] [CrossRef] [Green Version]
- Soechting, J.; Lacquaniti, F. Invariant characteristics of a pointing movement in man. J. Neurosci. 1981, 1, 710–720. [Google Scholar] [CrossRef] [Green Version]
- Atkeson, C.G.; Hollerbach, J.M. Kinematic features of unrestrained vertical arm movements. J. Neurosci. 1985, 5, 2318–2330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fillmore, M.T.; Rush, C.R.; Hays, L. Acute effects of cocaine in two models of inhibitory control: Implications of non-linear dose effects. Addiction 2006, 101, 1323–1332. [Google Scholar] [CrossRef]
- Logan, G.D.; Cowan, W.B. On the ability to inhibit thought and action: A theory of an act of control. Psychol. Rev. 1984, 91, 295. [Google Scholar] [CrossRef]
- Mathôt, S.; Schreij, D.; Theeuwes, J. OpenSesame: An open-source, graphical experiment builder for the social sciences. Behav. Res. Methods 2012, 44, 314–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bissett, P.G.; Logan, G.D. Post-stop-signal adjustments: Inhibition improves subsequent inhibition. J. Exp. Psychol. Learn. Mem. Cogn. 2012, 38, 955. [Google Scholar] [CrossRef] [Green Version]
- Logan, G.D.; Schachar, R.J.; Tannock, R. Impulsivity and inhibitory control. Psychol. Sci. 1997, 8, 60–64. [Google Scholar] [CrossRef] [Green Version]
- Osman, A.; Kornblum, S.; Meyer, D.E. The point of no return in choice reaction time: Controlled and ballistic stages of response preparation. J. Exp. Psychol. Hum. Percept. Perform. 1986, 12, 243. [Google Scholar] [CrossRef] [PubMed]
- Verbruggen, F.; Aron, A.R.; Band, G.P.; Beste, C.; Bissett, P.G.; Brockett, A.T.; Brown, J.W.; Chamberlain, S.R.; Chambers, C.D.; Colonius, H. A consensus guide to capturing the ability to inhibit actions and impulsive behaviors in the stop-signal task. Elife 2019, 8, e46323. [Google Scholar] [CrossRef]
- Sharp, D.; Bonnelle, V.; De Boissezon, X.; Beckmann, C.; James, S.; Patel, M.; Mehta, M.A. Distinct frontal systems for response inhibition, attentional capture, and error processing. Proc. Natl. Acad. Sci. USA 2010, 107, 6106–6111. [Google Scholar] [CrossRef] [Green Version]
- Benikos, N.; Johnstone, S.J.; Roodenrys, S.J. Varying task difficulty in the Go/Nogo task: The effects of inhibitory control, arousal, and perceived effort on ERP components. Int. J. Psychophysiol. 2013, 87, 262–272. [Google Scholar] [CrossRef] [Green Version]
- Morasso, P. Spatial control of arm movements. Exp. Brain Res. 1981, 42, 223–227. [Google Scholar] [CrossRef]
- Georgopoulos, A.P.; Kalaska, J.F.; Massey, J.T. Spatial trajectories and reaction times of aimed movements: Effects of practice, uncertainty, and change in target location. J. Neurophysiol. 1981, 46, 725–743. [Google Scholar] [CrossRef] [PubMed]
- Flash, T.; Hogan, N. The coordination of arm movements: An experimentally confirmed mathematical model. J. Neurosci. 1985, 5, 1688–1703. [Google Scholar] [CrossRef] [PubMed]
- Bullock, D.; Grossberg, S. Neural dynamics of planned arm movements: Emergent invariants and speed-accuracy properties during trajectory formation. Psychol. Rev. 1988, 95, 49. [Google Scholar] [CrossRef] [PubMed]
- Beggs, W.; Howarth, C. The movement of the hand towards a target. Q. J. Exp. Psychol. 1972, 24, 448–453. [Google Scholar] [CrossRef]
- Abend, W.; Bizzi, E.; Morasso, P. Human arm trajectory formation. Brain A J. Neurol. 1982, 105, 331–348. [Google Scholar] [CrossRef]
- Mirabella, G. Should I stay or should I go? Conceptual underpinnings of goal-directed actions. Front. Syst. Neurosci. 2014, 8, 206. [Google Scholar] [CrossRef] [Green Version]
- Cisek, P.; Kalaska, J.F. Neural mechanisms for interacting with a world full of action choices. Annu. Rev. Neurosci. 2010, 33, 269–298. [Google Scholar] [CrossRef] [Green Version]
- Andersen, R.A.; Cui, H. Intention, action planning, and decision making in parietal-frontal circuits. Neuron 2009, 63, 568–583. [Google Scholar] [CrossRef] [Green Version]
- Fishbach, A.; Roy, S.A.; Bastianen, C.; Miller, L.E.; Houk, J.C. Kinematic properties of on-line error corrections in the monkey. Exp. Brain Res. 2005, 164, 442–457. [Google Scholar] [CrossRef]
- Criaud, M.; Wardak, C.; Ben Hamed, S.; Ballanger, B.; Boulinguez, P. Proactive inhibitory control of response as the default state of executive control. Front Psychol. 2012, 3, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verbruggen, F.; McLaren, I.P.; Chambers, C.D. Banishing the Control Homunculi in Studies of Action Control and Behavior Change. Perspect Psychol. Sci. 2014, 9, 497–524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, J.; McCandliss, B.D.; Fossella, J.; Flombaum, J.I.; Posner, M.I. The activation of attentional networks. Neuroimage 2005, 26, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Kinomura, S.; Larsson, J.; Gulyás, B.; Roland, P.E. Activation by attention of the human reticular formation and thalamic intralaminar nuclei. Science 1996, 271, 512–515. [Google Scholar] [CrossRef]
- Sturm, W.; Willmes, K. On the functional neuroanatomy of intrinsic and phasic alertness. Neuroimage 2001, 14, S76–S84. [Google Scholar] [CrossRef]
- Gavazzi, G.; Giovannelli, F.; Currò, T.; Mascalchi, M. Contiguity of proactive and reactive inhibitory brain areas: A cognitive model based on ALE meta-analyses. Brain Imaging Behav. 2020. (In press) [Google Scholar]
- Stoodley, C.J.; Schmahmann, J.D. Functional topography in the human cerebellum: A meta-analysis of neuroimaging studies. Neuroimage 2009, 44, 489–501. [Google Scholar] [CrossRef]
- Picazio, S.; Ponzo, V.; Koch, G. Cerebellar Control on Prefrontal-Motor Connectivity During Movement Inhibition. Cerebellum 2016, 15, 680–687. [Google Scholar] [CrossRef]
- Clark, S.V.; King, T.Z.; Turner, J.A. Cerebellar Contributions to Proactive and Reactive Control in the Stop Signal Task: A Systematic Review and Meta-Analysis of Functional Magnetic Resonance Imaging Studies. Available online: https://pubmed.ncbi.nlm.nih.gov/32189178/ (accessed on 12 July 2020).
- Eagle, D.M.; Bari, A.; Robbins, T.W. The neuropsychopharmacology of action inhibition: Cross-species translation of the stop-signal and go/no-go tasks. Psychopharmacol. (Berl) 2008, 199, 439–456. [Google Scholar] [CrossRef] [Green Version]
- Swick, D.; Ashley, V.; Turken, U. Are the neural correlates of stopping and not going identical? Quantitative meta-analysis of two response inhibition tasks. Neuroimage 2011, 56, 1655–1665. [Google Scholar] [CrossRef]
- Aron, A.R.; Behrens, T.; Smith, S.; Frank, M.J.; Poldrack, R.A. Triangulating a cognitive control network using diffusion-weighted magnetic resonance imaging (MRI) and functional MRI. J Neurosci. 2007, 27, 3743–3752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Hulst, B.M.; de Zeeuw, P.; Vlaskamp, C.; Rijks, Y.; Zandbelt, B.; Durston, S. Children with ADHD symptoms show deficits in reactive but not proactive inhibition, irrespective of their formal diagnosis. Psychol. Med. 2018, 48, 2515–2521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirabella, G.; Pani, P.; Parè, M.; Ferraina, S. Inhibitory Control of Reaching Movements in Humans. Exp. Brain Res. 2006, 174, 240–255. [Google Scholar] [CrossRef] [PubMed]
- Di Caprio, V.; Modugno, N.; Mancini, C.; Olivola, E.; Mirabella, G. Early-stage Parkinson’s patients show selective impairment in reactive but not proactive inhibition. Mov. Disord. 2020, 35, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Mirabella, G.; Pani, P.; Ferraina, S. Context influences on the preparation and execution of reaching movements. Cogn. Neuropsychol. 2008, 25, 996–1010. [Google Scholar] [CrossRef]
- Brevers, D.; Dubuisson, E.; Dejonghe, F.; Dutrieux, J.; Petieau, M.; Cheron, G.; Verbanck, P.; Foucart, J. Proactive and Reactive Motor Inhibition in Top Athletes Versus Nonathletes. Percept Mot. Ski. 2018, 125, 289–312. [Google Scholar] [CrossRef]
- Herman, D.C.; Barth, J.T. Drop-Jump Landing Varies With Baseline Neurocognition: Implications for Anterior Cruciate Ligament Injury Risk and Prevention. Am. J. Sports Med. 2016, 44, 2347–2353. [Google Scholar] [CrossRef]
- McGowan, A.L.; Bretzin, A.C.; Savage, J.L.; Petit, K.M.; Covassin, T.; Pontifex, M.B. Acute and protracted disruptions to inhibitory control following sports-related concussion. Neuropsychologia 2019, 131, 223–232. [Google Scholar] [CrossRef]
- Brevers, D.; Noël, X. Pathological gambling and the loss of willpower: A neurocognitive perspective. Socioaffect. Neurosci. Psychol. 2013, 3, 21592. [Google Scholar] [CrossRef]
- Sharif-Razi, M.; Hodgins, D.C.; Goghari, V.M. Reactive and proactive control mechanisms of response inhibition in gambling disorder. Psychiatry Res. 2019, 272, 114–121. [Google Scholar] [CrossRef]


| Cued GNG | SST | |||
|---|---|---|---|---|
| Total | High GO-Stimulus | Low GO-Stimulus | ||
| Go conditions | ||||
| Correct responses (%) | 98.8 ± 5.0 | 98.6 ± 7.1 | 99.1 ± 2.4 | 99.4 ± 3.7 |
| Reaction times (ms) | 404.3 ± 42.2 | 400.2 ± 41.8 | 414.1 ± 45.2 | 411.8 ± 49.7 |
| No-go/Stop conditions | ||||
| Inhibitory failures (%) | 8.1 ± 7.8 | 10.0 ± 10.7 | 7.2 ± 7.6 | 74.1 ± 11.9 |
| Reaction times (ms) | – | – | – | 394.7± 41.6 |
| SSD (ms) | – | – | – | 162.0 ± 72.3 |
| One-Shot Movements (%) | |
|---|---|
| Go conditions | |
| GNG (high GO-stimulus) | 97.6 ± 3.5 |
| GNG (low GO-stimulus) | 94.3 ± 15.7 |
| SST | 97.9 ± 2.1 |
| No-go/Stop conditions (inhibition failures) | |
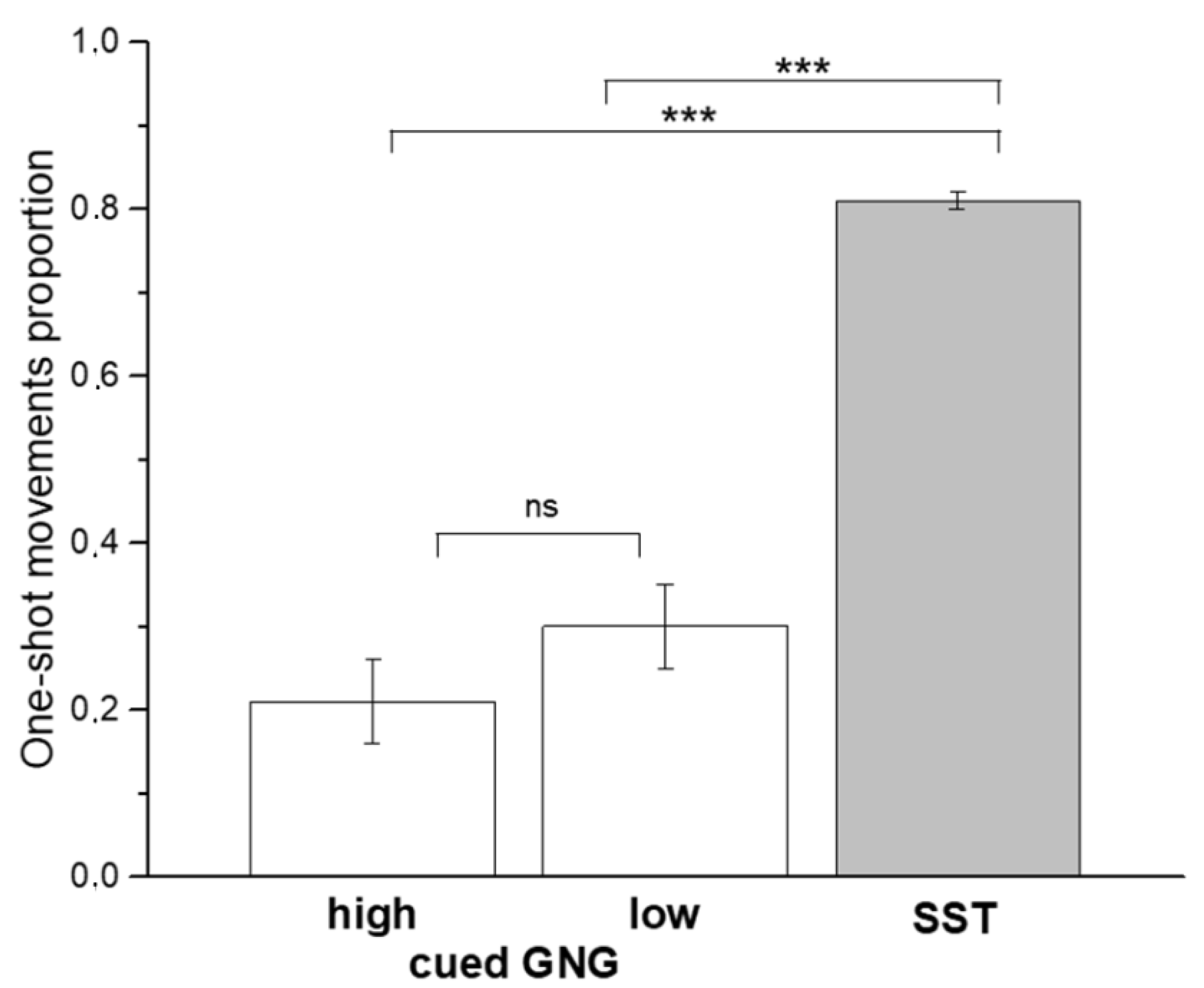
| GNG (high GO-stimulus) | 21.2 ± 34.2 |
| GNG (low GO-stimulus) | 30.1 ± 33.5 |
| SST | 81.3 ± 9.1 |
| One-Shot Movement | Non-One-Shot Movement | |
|---|---|---|
| Go conditions | ||
| GNG (high GO-stimulus) | 401.8 ± 69.9 | 387.7 ± 71.0 |
| GNG (low GO-stimulus) | 417.7 ± 74.6 | 392.4 ± 60.8 |
| SST | 411.9 ± 95.7 | 412.6 ± 106.2 |
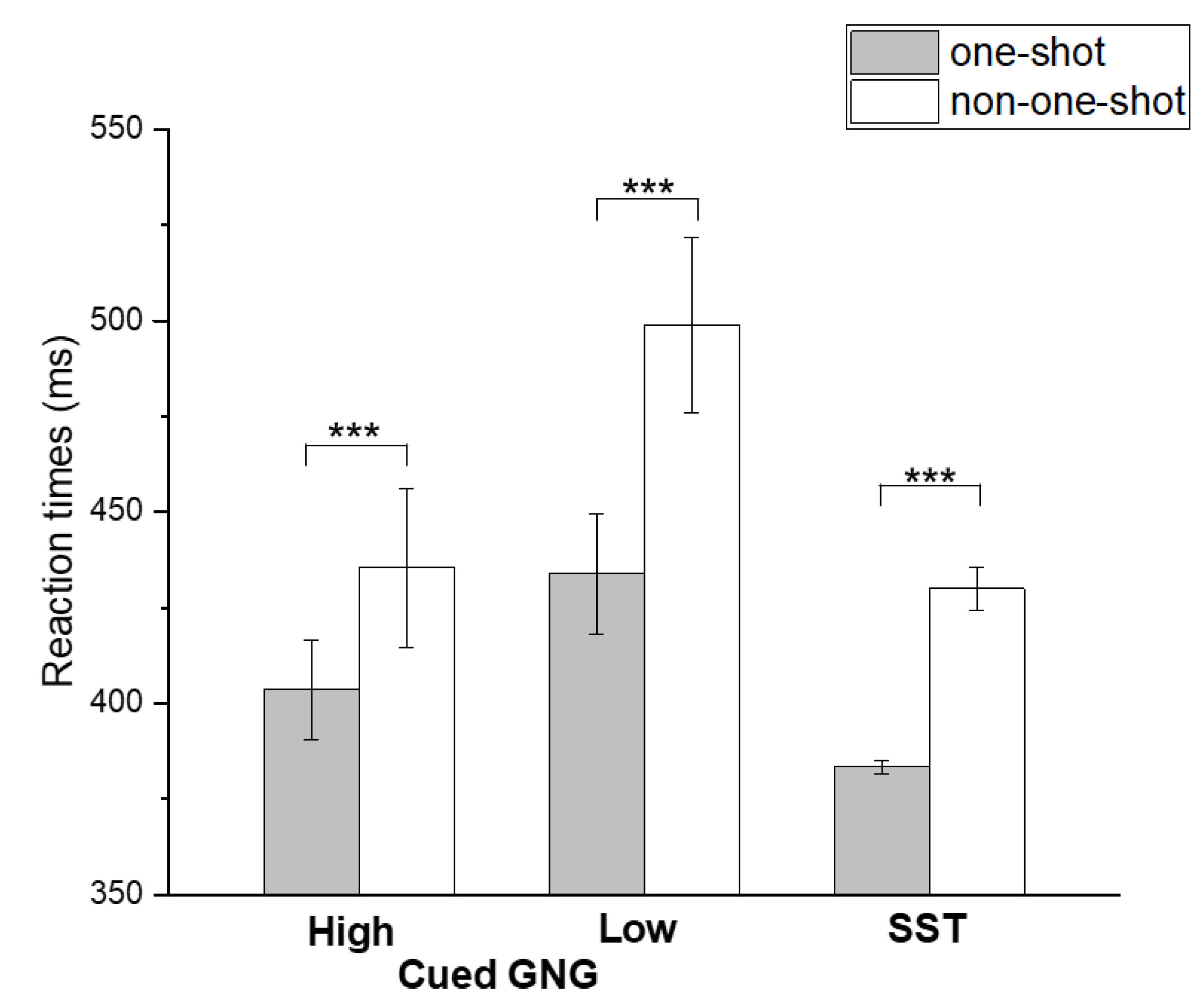
| No-go/Stop conditions (inhibition failures) | ||
| GNG (high GO-stimulus) | 403.4 ± 67.6 | 435.4 ± 150.2 |
| GNG (low GO-stimulus) | 433.8 ± 120.1 | 498.8 ± 182.5 |
| SST | 383.4 ± 65.1 | 430.6 ± 106.6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benedetti, V.; Gavazzi, G.; Giovannelli, F.; Bravi, R.; Giganti, F.; Minciacchi, D.; Mascalchi, M.; Cincotta, M.; Viggiano, M.P. Mouse Tracking to Explore Motor Inhibition Processes in Go/No-Go and Stop Signal Tasks. Brain Sci. 2020, 10, 464. https://doi.org/10.3390/brainsci10070464
Benedetti V, Gavazzi G, Giovannelli F, Bravi R, Giganti F, Minciacchi D, Mascalchi M, Cincotta M, Viggiano MP. Mouse Tracking to Explore Motor Inhibition Processes in Go/No-Go and Stop Signal Tasks. Brain Sciences. 2020; 10(7):464. https://doi.org/10.3390/brainsci10070464
Chicago/Turabian StyleBenedetti, Viola, Gioele Gavazzi, Fabio Giovannelli, Riccardo Bravi, Fiorenza Giganti, Diego Minciacchi, Mario Mascalchi, Massimo Cincotta, and Maria Pia Viggiano. 2020. "Mouse Tracking to Explore Motor Inhibition Processes in Go/No-Go and Stop Signal Tasks" Brain Sciences 10, no. 7: 464. https://doi.org/10.3390/brainsci10070464
APA StyleBenedetti, V., Gavazzi, G., Giovannelli, F., Bravi, R., Giganti, F., Minciacchi, D., Mascalchi, M., Cincotta, M., & Viggiano, M. P. (2020). Mouse Tracking to Explore Motor Inhibition Processes in Go/No-Go and Stop Signal Tasks. Brain Sciences, 10(7), 464. https://doi.org/10.3390/brainsci10070464






