Transient Administration of Dopaminergic Precursor Causes Inheritable Overfeeding Behavior in Young Drosophila melanogaster Adults
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fly Strains and Maintenance
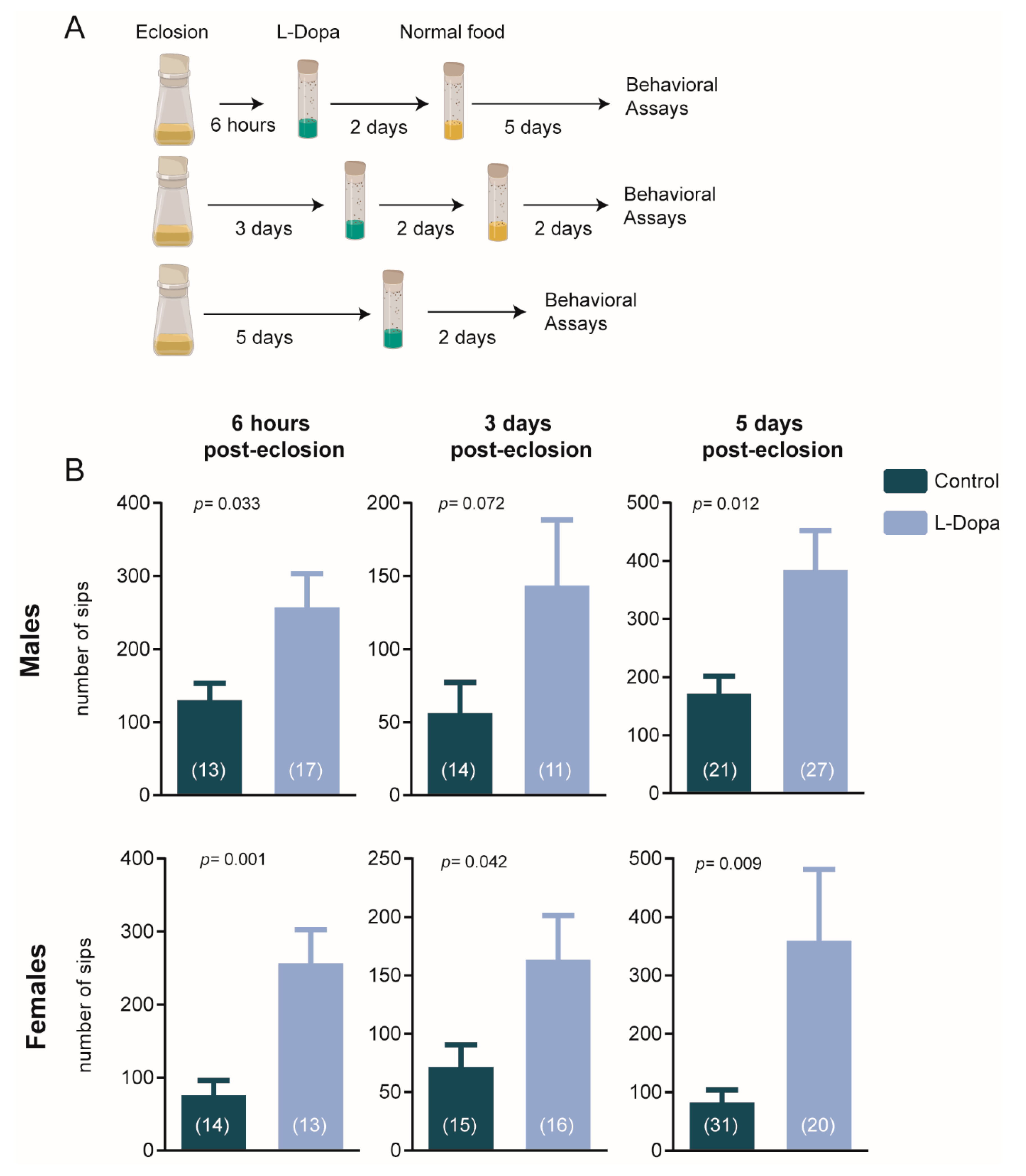
2.2. Pharmacological Treatment
2.3. Feeding Behavior
2.4. General Activity and Sleep
2.5. Statistical Analysis
3. Results
3.1. Transient Dopaminergic Disturbances Affect Feeding Behavior
3.2. Transient Dopaminergic Dysregulation Does Not Influence General Activity or Sleep
3.3. Early Dopaminergic Application Affects Transgenerational Feeding Behavior
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Eyles, D.; Feldon, J.; Meyer, U. Schizophrenia: Do all roads lead to dopamine or is this where they start? Evidence from two epidemiologically informed developmental rodent models. Transl. Psychiatry 2012, 2, e81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belujon, P.; Grace, A.A. Dopamine System Dysregulation in Major Depressive Disorders. Int. J. Neuropsychopharmacol. 2017, 20, 1036–1046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cousins, D.A.; Butts, K.; Young, A.H. The role of dopamine in bipolar disorder. Bipolar Disord. 2009, 11, 787–806. [Google Scholar] [CrossRef] [PubMed]
- Areal, L.B.; Blakely, R.D. Neurobehavioral changes arising from early life dopamine signaling perturbations. Neurochem. Int. 2020, 137, 104747. [Google Scholar] [CrossRef]
- Calcagno, B.; Eyles, D.; van Alphen, B.; van Swinderen, B. Transient activation of dopaminergic neurons during development modulates visual responsiveness, locomotion and brain activity in a dopamine ontogeny model of schizophrenia. Transl. Psychiatry 2013, 3, e206. [Google Scholar] [CrossRef] [Green Version]
- Ferguson, L.; Petty, A.; Rohrscheib, C.; Troup, M.; Kirszenblat, L.; Eyles, D.W.; van Swinderen, B. Transient Dysregulation of Dopamine Signaling in a Developing Drosophila Arousal Circuit Permanently Impairs Behavioral Responsiveness in Adults. Front. Psychiatry 2017, 8. [Google Scholar] [CrossRef] [Green Version]
- Alsiö, J.; Olszewski, P.K.; Levine, A.S.; Schiöth, H.B. Feed-forward mechanisms: Addiction-like behavioral and molecular adaptations in overeating. Front. Neuroendocrinol. 2012, 33, 127–139. [Google Scholar] [CrossRef]
- Kessler, R.M.; Hutson, P.H.; Herman, B.K.; Potenza, M.N. The neurobiological basis of binge-eating disorder. Neurosci. Biobehav. Rev. 2016, 63, 223–238. [Google Scholar] [CrossRef] [Green Version]
- Volkow, N.D.; Wise, R.A.; Baler, R. The dopamine motive system: Implications for drug and food addiction. Nat. Rev. Neurosci. 2017, 18, 741–752. [Google Scholar] [CrossRef]
- Williams, M.J.; Akram, M.; Barkauskaite, D.; Patil, S.; Kotsidou, E.; Kheder, S.; Vitale, G.; Filaferro, M.; Blemings, S.W.; Maestri, G.; et al. CCAP regulates feeding behavior via the NPF pathway in Drosophila adults. Proc. Natl. Acad. Sci. USA 2020, 117, 7401–7408. [Google Scholar] [CrossRef]
- Heijmans, B.T.; Tobi, E.W.; Stein, A.D.; Putter, H.; Blauw, G.J.; Susser, E.S.; Slagboom, P.E.; Lumey, L.H. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc. Natl. Acad. Sci. USA 2008, 105, 17046–17049. [Google Scholar] [CrossRef] [Green Version]
- Painter, R.; Osmond, C.; Gluckman, P.; Hanson, M.; Phillips, D.; Roseboom, T. Transgenerational effects of prenatal exposure to the Dutch famine on neonatal adiposity and health in later life. BJOG Int. J. Obstet. Gynaecol. 2008, 115, 1243–1249. [Google Scholar] [CrossRef]
- Yeshurun, S.; Hannan, A.J. Transgenerational epigenetic influences of paternal environmental exposures on brain function and predisposition to psychiatric disorders. Mol. Psychiatry 2019, 24, 536–548. [Google Scholar] [CrossRef] [PubMed]
- Bozler, J.; Kacsoh, B.Z.; Bosco, G. Transgenerational inheritance of ethanol preference is caused by maternal NPF repression. Elife 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Karunakar, P.; Bhalla, A.; Sharma, A. Transgenerational inheritance of cold temperature response in Drosophila. FEBS Lett. 2019, 593, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Ferdenache, M.; Bezzar-Bendjazia, R.; Marion-Poll, F.; Kilani-Morakchi, S. Transgenerational effects from single larval exposure to azadirachtin on life history and behavior traits of Drosophila melanogaster. Sci. Rep. 2019, 9, 17015. [Google Scholar] [CrossRef] [PubMed]
- Buescher, J.L.; Musselman, L.P.; Wilson, C.A.; Lang, T.; Keleher, M.; Baranski, T.J.; Duncan, J.G. Evidence for transgenerational metabolic programming in Drosophila. Dis. Model. Mech. 2013, 6, 1123–1132. [Google Scholar] [CrossRef] [Green Version]
- Cichewicz, K.; Garren, E.J.; Adiele, C.; Aso, Y.; Wang, Z.; Wu, M.; Birman, S.; Rubin, G.M.; Hirsh, J. A new brain dopamine-deficient Drosophila and its pharmacological and genetic rescue. Genes Brain Behav. 2017, 16, 394–403. [Google Scholar] [CrossRef] [Green Version]
- Riemensperger, T.; Isabel, G.; Coulom, H.; Neuser, K.; Seugnet, L.; Kume, K.; Iche-Torres, M.; Cassar, M.; Strauss, R.; Preat, T.; et al. Behavioral consequences of dopamine deficiency in the Drosophila central nervous system. Proc. Natl. Acad. Sci. USA 2011, 108, 834–839. [Google Scholar] [CrossRef] [Green Version]
- Bogomolova, E.V.; Rauschenbach, I.Y.; Alekseev, A.A.; Faddeeva, N.V.; Gruntenko, N.E. The effect of dopamine on alkaline phosphatase activity in Drosophila is mediated by D2-like receptors. Dokl. Biochem. Biophys. 2010, 431, 87–89. [Google Scholar] [CrossRef]
- Rauschenbach, I.Y.; Karpova, E.K.; Alekseev, A.A.; Adonyeva, N.V.; Shumnaya, L.V.; Gruntenko, N.E. Interplay of insulin and dopamine signaling pathways in the control of Drosophila melanogaster fitness. Dokl. Biochem. Biophys. 2015, 461, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.-D.; Zheng, Y.; Wang, J.-L.; Wang, Y.-F. Drug induces depression-like phenotypes and alters gene expression profiles in Drosophila. Brain Res. Bull. 2017, 132, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Itskov, P.M.; Moreira, J.-M.; Vinnik, E.; Lopes, G.; Safarik, S.; Dickinson, M.H.; Ribeiro, C. Automated monitoring and quantitative analysis of feeding behaviour in Drosophila. Nat. Commun. 2014, 5, 4560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, M.J.; Perland, E.; Eriksson, M.M.; Carlsson, J.; Erlandsson, D.; Laan, L.; Mahebali, T.; Potter, E.; Frediksson, R.; Benedict, C.; et al. Recurrent Sleep Fragmentation Induces Insulin and Neuroprotective Mechanisms in Middle-Aged Flies. Front. Aging Neurosci. 2016, 8. [Google Scholar] [CrossRef]
- Williams, M.J.; Klockars, A.; Eriksson, A.; Voisin, S.; Dnyansagar, R.; Wiemerslage, L.; Kasagiannis, A.; Akram, M.; Kheder, S.; Ambrosi, V.; et al. The Drosophila ETV5 Homologue Ets96B: Molecular Link between Obesity and Bipolar Disorder. PLoS Genet. 2016, 12, e1006104. [Google Scholar] [CrossRef]
- Cedernaes, J.; Schiöth, H.B.; Benedict, C. Determinants of Shortened, Disrupted, and Mistimed Sleep and Associated Metabolic Health Consequences in Healthy Humans: Figure 1. Diabetes 2015, 64, 1073–1080. [Google Scholar] [CrossRef] [Green Version]
- Turrigiano, G.G.; Nelson, S.B. Homeostatic plasticity in the developing nervous system. Nat. Rev. Neurosci. 2004, 5, 97–107. [Google Scholar] [CrossRef]
- Moulin, T.C.; Rayêe, D.; Williams, M.J.; Schiöth, H.B. The synaptic scaling literature: A systematic review of methodologies and quality of reporting. Front. Cell. Neurosci. 2020. [Google Scholar] [CrossRef]
- Friedman, A.K.; Walsh, J.J.; Juarez, B.; Ku, S.M.; Chaudhury, D.; Wang, J.; Li, X.; Dietz, D.M.; Pan, N.; Vialou, V.F.; et al. Enhancing Depression Mechanisms in Midbrain Dopamine Neurons Achieves Homeostatic Resilience. Science 2014, 344, 313–319. [Google Scholar] [CrossRef] [Green Version]
- Moulin, T.C.; Schiöth, H.B. Excitability, synaptic balance, and addiction: The homeostatic dynamics of ionotropic glutamatergic receptors in VTA after cocaine exposure. Behav. Brain Funct. 2020, 16, 6. [Google Scholar] [CrossRef]
- Hudson, J.I.; Hiripi, E.; Pope, H.G.; Kessler, R.C. The Prevalence and Correlates of Eating Disorders in the National Comorbidity Survey Replication. Biol. Psychiatry 2007, 61, 348–358. [Google Scholar] [CrossRef] [Green Version]
- Brookheart, R.T.; Duncan, J.G. Drosophila melanogaster: An emerging model of transgenerational effects of maternal obesity. Mol. Cell. Endocrinol. 2016, 435, 20–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aldrich, J.C.; Maggert, K.A. Transgenerational Inheritance of Diet-Induced Genome Rearrangements in Drosophila. PLoS Genet. 2015, 11, e1005148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Öst, A.; Lempradl, A.; Casas, E.; Weigert, M.; Tiko, T.; Deniz, M.; Pantano, L.; Boenisch, U.; Itskov, P.M.; Stoeckius, M.; et al. Paternal Diet Defines Offspring Chromatin State and Intergenerational Obesity. Cell 2014, 159, 1352–1364. [Google Scholar] [CrossRef] [Green Version]
- Sweatt, J.D. Dynamic DNA methylation controls glutamate receptor trafficking and synaptic scaling. J. Neurochem. 2016, 137, 312–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morgan, C.P.; Bale, T.L. Early Prenatal Stress Epigenetically Programs Dysmasculinization in Second-Generation Offspring via the Paternal Lineage. J. Neurosci. 2011, 31, 11748–11755. [Google Scholar] [CrossRef] [Green Version]

| Movement Counts (Daily Average ± SEM) | Sleep Counts (Daily Average ± SEM) | ||||
|---|---|---|---|---|---|
| Males | Females | Males | Females | ||
| 6 h p.e. | Control | 536.6 ± 45.88 | 457.9 ± 36.7 | 1053 ± 204.8 | 1083 ± 210.7 |
| Levodopa | 597.6 ± 45.3 | 407.2 ± 33.2 | 1031 ± 155.3 | 1050 ± 193.4 | |
| p-value | 0.3490 | 0.3077 | 0.5850 | 0.4629 | |
| 3 days p.e. | Control | 654.8 ± 41.5 | 522.5 ± 50.4 | 1030 ± 169.3 | 1047 ± 208.9 |
| Levodopa | 644.4 ± 49.0 | 542.5 ± 43.2 | 1030 ± 142.5 | 1031 ± 186.6 | |
| p-value | 0.8704 | 0.7635 | 0.9883 | 0.6927 | |
| 5 days p.e. | Control | 617.6 ± 50.1 | 493.1 ± 36.3 | 1037 ± 168.3 | 1017 ± 210.1 |
| Levodopa | 565.6 ± 52.9 | 507.7 ± 66.3 | 1036 ± 182.9 | 1076 ± 184.8 | |
| p-value | 0.5187 | 0.8516 | 0.9719 | 0.1871 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moulin, T.C.; Ferro, F.; Berkins, S.; Hoyer, A.; Williams, M.J.; Schiöth, H.B. Transient Administration of Dopaminergic Precursor Causes Inheritable Overfeeding Behavior in Young Drosophila melanogaster Adults. Brain Sci. 2020, 10, 487. https://doi.org/10.3390/brainsci10080487
Moulin TC, Ferro F, Berkins S, Hoyer A, Williams MJ, Schiöth HB. Transient Administration of Dopaminergic Precursor Causes Inheritable Overfeeding Behavior in Young Drosophila melanogaster Adults. Brain Sciences. 2020; 10(8):487. https://doi.org/10.3390/brainsci10080487
Chicago/Turabian StyleMoulin, Thiago C., Federico Ferro, Samuel Berkins, Angela Hoyer, Michael J. Williams, and Helgi B. Schiöth. 2020. "Transient Administration of Dopaminergic Precursor Causes Inheritable Overfeeding Behavior in Young Drosophila melanogaster Adults" Brain Sciences 10, no. 8: 487. https://doi.org/10.3390/brainsci10080487
APA StyleMoulin, T. C., Ferro, F., Berkins, S., Hoyer, A., Williams, M. J., & Schiöth, H. B. (2020). Transient Administration of Dopaminergic Precursor Causes Inheritable Overfeeding Behavior in Young Drosophila melanogaster Adults. Brain Sciences, 10(8), 487. https://doi.org/10.3390/brainsci10080487





