Safety and Use of MLC601/MLC901 (NeuroAiDTM) in Primary Intracerebral Hemorrhage: A Cohort Study from the NeuroAiD Safe Treatment Registry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Inclusion Criteria
- Male or female;
- Any age;
- Any patient who is taking or has been prescribed NeuroAiD for any duration as judged by the physician and/or the participant;
- Agrees to be included in the registry and allows retrieval and analysis of data in accordance with local requirements.
2.2. Exclusion Criteria
- Unwillingness to participate;
- Contraindication to NeuroAiD.
- Demographics: date of birth, sex, ethnicity;
- The main diagnosis (indication) for taking NeuroAiD and date of onset;
- Other relevant medical conditions;
- NeuroAiD date started and dose;
- National Institutes of Health Stroke Scale (NIHSS) score;
- Glasgow Coma Scale (GCS) score;
- Modified Rankin Scale (mRS) score;
- Short Orientation-Memory-Concentration Test (SOMCT) score;
- Compliance with intake of NeuroAiD;
- Occurrence of any side effects related to NeuroAiD: date of onset, date of resolution, and severity.
3. Results
3.1. Baseline Characteristics
3.2. Safety and Compliance
3.3. Clinical Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Feigin, V.L.; Norrving, B.; Mensah, G.A. Global Burden of Stroke. Circ. Res. 2017, 120, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Hemphill, J.C.; Greenberg, S.M.; Anderson, C.S.; Becker, K.; Bendok, B.R.; Cushman, M.; Fung, G.L.; Goldstein, J.N.; Macdonald, R.L.; Mitchell, P.H.; et al. Guidelines for the Management of Spontaneous Intracerebral Hemorrhage: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2015, 46, 2032–2060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- An, S.J.; Kim, T.J.; Yoon, B.-W. Epidemiology, Risk Factors, and Clinical Features of Intracerebral Hemorrhage: An Update. J. Stroke 2017, 19, 3–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xi, G.; Keep, R.; Hoff, J.T. Mechanisms of Brain Injury after Intracerebral Haemorrhage. Lancet Neurol. 2006, 5, 53–63. [Google Scholar] [CrossRef]
- Garg, R.; Biller, J. Recent Advances in Spontaneous Intracerebral Hemorrhage. F1000Research 2019, 8, 302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- The Hemorrhagic Stroke Academia Industry (HEADS) Roundtable Participants; Selim, M.; Hanley, D.F.; Broderick, J.; Goldstein, J.N.; Gregson, B.; Falcione, G.; Gonzales, N.R.; Gurol, E.; Kersten, J.; et al. Unmet Needs and Challenges in Clinical Research of Intracerebral Hemorrhage. Stroke 2018, 49, 1299–1307. [Google Scholar] [CrossRef]
- Heurteaux, C.; Widmann, C.; Maati, H.M.O.; Quintard, H.; Gandin, C.; Borsotto, M.; Veyssiere, J.; Onténiente, B.; Lazdunski, M. NeuroAiD: Properties for Neuroprotection and Neurorepair. Cerebrovasc. Dis. 2013, 35, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Heurteaux, C.; Gandin, C.; Borsotto, M.; Widmann, C.; Brau, F.; Lhuillier, M.; Onténiente, B.; Lazdunski, M. Neuroprotective and Neuroproliferative Activities of NeuroAid (MLC601, MLC901), a Chinese Medicine, In Vitro and In Vivo. Neuropharmacology 2010, 58, 987–1001. [Google Scholar] [CrossRef] [PubMed]
- Quintard, H.; Borsotto, M.; Veyssiere, J.; Gandin, C.; Labbal, F.; Widmann, C.; Lazdunski, M.; Heurteaux, C. MLC901, a Traditional Chinese Medicine Protects the Brain Against Global Ischemia. Neuropharmacology 2011, 61, 622–631. [Google Scholar] [CrossRef] [PubMed]
- Quintard, H.; Lorivel, T.; Gandin, C.; Lazdunski, M.; Heurteaux, C. MLC901, a Traditional Chinese Medicine Induces Neuroprotective and Neuroregenerative Benefits after Traumatic Brain Injury in Rats. Neuroscience 2014, 277, 72–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Widmann, C.; Gandin, C.; Petit-Paitel, A.; Lazdunski, M.; Heurteaux, C. The Traditional Chinese Medicine MLC901 Inhibits Inflammation Processes after Focal Cerebral Ischemia. Sci. Rep. 2018, 8, 18062. [Google Scholar] [CrossRef] [PubMed]
- Venketasubramanian, N.; Young, S.; Tay, S.S.; Chang, H.M.; Umapathi, T.; Chan, B.; De Silva, A.; Wong, K.S.; Navarro, J.; Zhao, Y.-D.; et al. Chinese Medicine NeuroAiD Efficacy Stroke Recovery-Extension Study (CHIMES-E Study): An Observational Multicenter Study to Investigate the Longer-Term Efficacy of NeuroAiD in Stroke Recovery. Cerebrovasc. Dis. 2013, 35 (Suppl. S1), 18–22. [Google Scholar] [CrossRef] [PubMed]
- Suwanwela, N.C.; Chen, C.P.; Lee, C.F.; Young, S.H.; Tay, S.S.; Umapathi, T.; Lao, A.Y.; Gan, H.H.; Ii, A.C.B.; Navarro, J.C.; et al. Effect of Combined Treatment with MLC601 (NeuroAiDTM) and Rehabilitation on Post-Stroke Recovery: The CHIMES and CHIMES-E Studies. Cerebrovasc. Dis. 2018, 46, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Theadom, A.; Barker-Collo, S.; Jones, K.M.; Parmar, P.; Bhattacharjee, R.; Feigin, V.L. MLC901 (NeuroAiD II™) for Cognition after Traumatic Brain Injury: A Pilot Randomized Clinical Trial. Eur. J. Neurol. 2018, 25, 1055-e82. [Google Scholar] [CrossRef] [PubMed]
- Yeo, T.T.; Chou, N. Case Report on the Use of MLC601 (NeuroAiD) in Neurosurgical Pathologies. Int. J. Stroke 2010, 5 (Suppl. S2), 308. [Google Scholar] [CrossRef]
- Kumar, R.; Fadzil, F.; Soon, B.H. MLC601 (NeuroAiD) for brain injuries-open label use in a series of patients. In Proceedings of the Abstracts of the 14th Asian Australian Congress of Neurological Surgeons, AACNS 2015, Chongjin, Korea, 15–18 April 2015. [Google Scholar]
- Venketasubramanian, N.; Kumar, R.; Soertidewi, L.; Abu Bakar, A.; Laik, C.; Gan, R.N. The NeuroAiD Safe Treatment (NeST) Registry: A Protocol. BMJ Open 2015, 5, e009866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gliklich, R.E.; Dreyer, N.A.; Leavy, M.B. (Eds.) Registries for Evaluating Patient Outcomes: A User’s Guide, 3rd ed.; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2014. [Google Scholar]
- Venketasubramanian, N.; Lee, C.F.; Young, S.H.; Tay, S.S.; Umapathi, T.; Lao, A.Y.; Gan, H.H.; Ii, A.C.B.; Navarro, J.C.; Chang, H.M.; et al. Prognostic Factors and Pattern of Long-Term Recovery with MLC601 (NeuroAiD™) in the Chinese Medicine NeuroAiD Efficacy on Stroke Recovery-Extension Study. Cerebrovasc. Dis. 2016, 43, 36–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sia, S.F.; Tan, K.S.; Waran, V. Primary Intracerebral Haemorrhage in Malaysia: In-Hospital Mortality and Outcome in Patients from a Hospital Based Registry. Med. J. Malays. 2007, 62, 308–312. [Google Scholar]



| Variable | Result (n = 66) |
|---|---|
| Age in years (Median, Range) | 58.5 (28–87) |
| Male (n, %) | 44 (67%) |
| Female (n, %) | 22 (33%) |
| Most common comorbid conditions (n, %) | |
| Hypertension | 55 (83%) |
| Diabetes | 14 (21%) |
| Hyperlipidemia | 11 (17%) |
| Time from ICH onset to baseline assessment, in days (Median, Range) | 12 (0–238) |
| Time from ICH onset to first NeuroAiD dose, in days (Median, Range) | 11 (0–238) |
| Outcome measure (Median, Range) | |
| NIHSS score | 10.5 (0–33) |
| GCS score | 15 (3–15) |
| mRS score | 4 (0–5) |
| SOMCT score | 14 (0–28) |
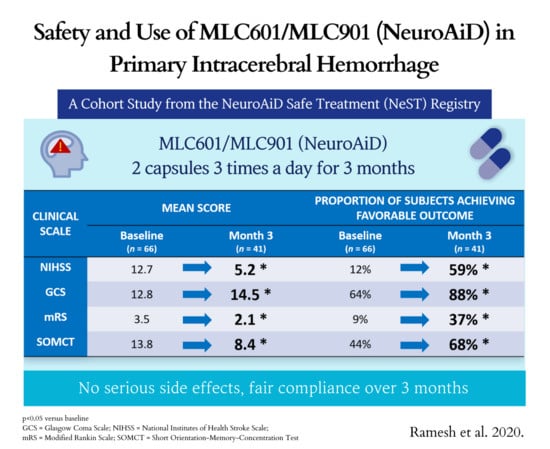
| Assessment | Baseline (n = 66) | Visit 1 (n = 50) | Visit 2 (n = 45) | Visit 3 (n = 41) | p-Value # |
|---|---|---|---|---|---|
| NIHSS | |||||
| Mean (SD) | 12.7 (8.3) | 8.1 (7.5) | 6.8 (7.6) | 5.2 (6.6) | <0.0001 * |
| Median (Q1, Q3) | 10.5 (6, 19) | 7 (2, 11) | 5 (2, 9) | 4 (0, 7) | |
| Min, Max | 0, 33 | 0, 33 | 0, 34 | 0, 31 | |
| Score 0–4, n (%) | 8 (12.1) | 16 (32) | 22 (48.9) | 24 (58.5) | <0.0001 $ |
| GCS | |||||
| Mean (SD) | 12.8 (3.1) | 13.9 (2.5) | 14.3 (2.1) | 14.5 (1.3) | 0.001 ** |
| Median (Q1, Q3) | 15 (11, 15) | 15 (15, 15) | 15 (15, 15) | 15 (15, 15) | |
| Min, Max | 3, 15 | 3, 15 | 3, 15 | 10, 15 | |
| Score 13–15, n (%) | 42 (63.6) | 41 (82.0) | 40 (88.9) | 36 (87.8) | 0.007 $ |
| mRS | |||||
| Mean (SD) | 3.5 (1.4) | 2.7 (1.9) | 2.6 (1.7) | 2.1 (1.6) | <0.0001 ** |
| Median (Q1, Q3) | 4 (2, 5) | 3 (1, 4) | 3 (1, 4) | 2 (0, 3) | |
| Min, Max | 0, 5 | 0, 5 | 0, 5 | 0, 5 | |
| Score 0–1, n (%) | 6 (9.1) | 15 (30.0) | 12 (26.7) | 15 (36.6) | 0.004 $ |
| SOMCT | |||||
| Mean (SD) | 13.8 (12.5) | 10.5 (11.2) | 8.6 (11.0) | 8.4 (11.6) | 0.032 ** |
| Median (Q1, Q3) | 14 (0, 28) | 6 (0, 22) | 2 (0, 16) | 0 (0, 16) | |
| Min, Max | 0, 28 | 0, 28 | 0, 28 | 0, 28 | |
| Score 0–8, n (%) | 29 (43.9) | 29 (59.2) | 29 (64.4) | 28 (68.3) | 0.029 $ |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, R.; Abu Bakar, A.; Thanabalan, J.; Paramasvaran, S.; Toh, C.J.; Jaffar, A.; Fadzil, F.; Kamalanathan, P.; Soon, B.H.; Venketasubramanian, N. Safety and Use of MLC601/MLC901 (NeuroAiDTM) in Primary Intracerebral Hemorrhage: A Cohort Study from the NeuroAiD Safe Treatment Registry. Brain Sci. 2020, 10, 499. https://doi.org/10.3390/brainsci10080499
Kumar R, Abu Bakar A, Thanabalan J, Paramasvaran S, Toh CJ, Jaffar A, Fadzil F, Kamalanathan P, Soon BH, Venketasubramanian N. Safety and Use of MLC601/MLC901 (NeuroAiDTM) in Primary Intracerebral Hemorrhage: A Cohort Study from the NeuroAiD Safe Treatment Registry. Brain Sciences. 2020; 10(8):499. https://doi.org/10.3390/brainsci10080499
Chicago/Turabian StyleKumar, Ramesh, Azizi Abu Bakar, Jegan Thanabalan, Sanmugarajah Paramasvaran, Charng Jeng Toh, Ainul Jaffar, Farizal Fadzil, Palaniandy Kamalanathan, Bee Hong Soon, and Narayanaswamy Venketasubramanian. 2020. "Safety and Use of MLC601/MLC901 (NeuroAiDTM) in Primary Intracerebral Hemorrhage: A Cohort Study from the NeuroAiD Safe Treatment Registry" Brain Sciences 10, no. 8: 499. https://doi.org/10.3390/brainsci10080499
APA StyleKumar, R., Abu Bakar, A., Thanabalan, J., Paramasvaran, S., Toh, C. J., Jaffar, A., Fadzil, F., Kamalanathan, P., Soon, B. H., & Venketasubramanian, N. (2020). Safety and Use of MLC601/MLC901 (NeuroAiDTM) in Primary Intracerebral Hemorrhage: A Cohort Study from the NeuroAiD Safe Treatment Registry. Brain Sciences, 10(8), 499. https://doi.org/10.3390/brainsci10080499