Amygdala: Neuroanatomical and Morphophysiological Features in Terms of Neurological and Neurodegenerative Diseases
Abstract
:1. Introduction
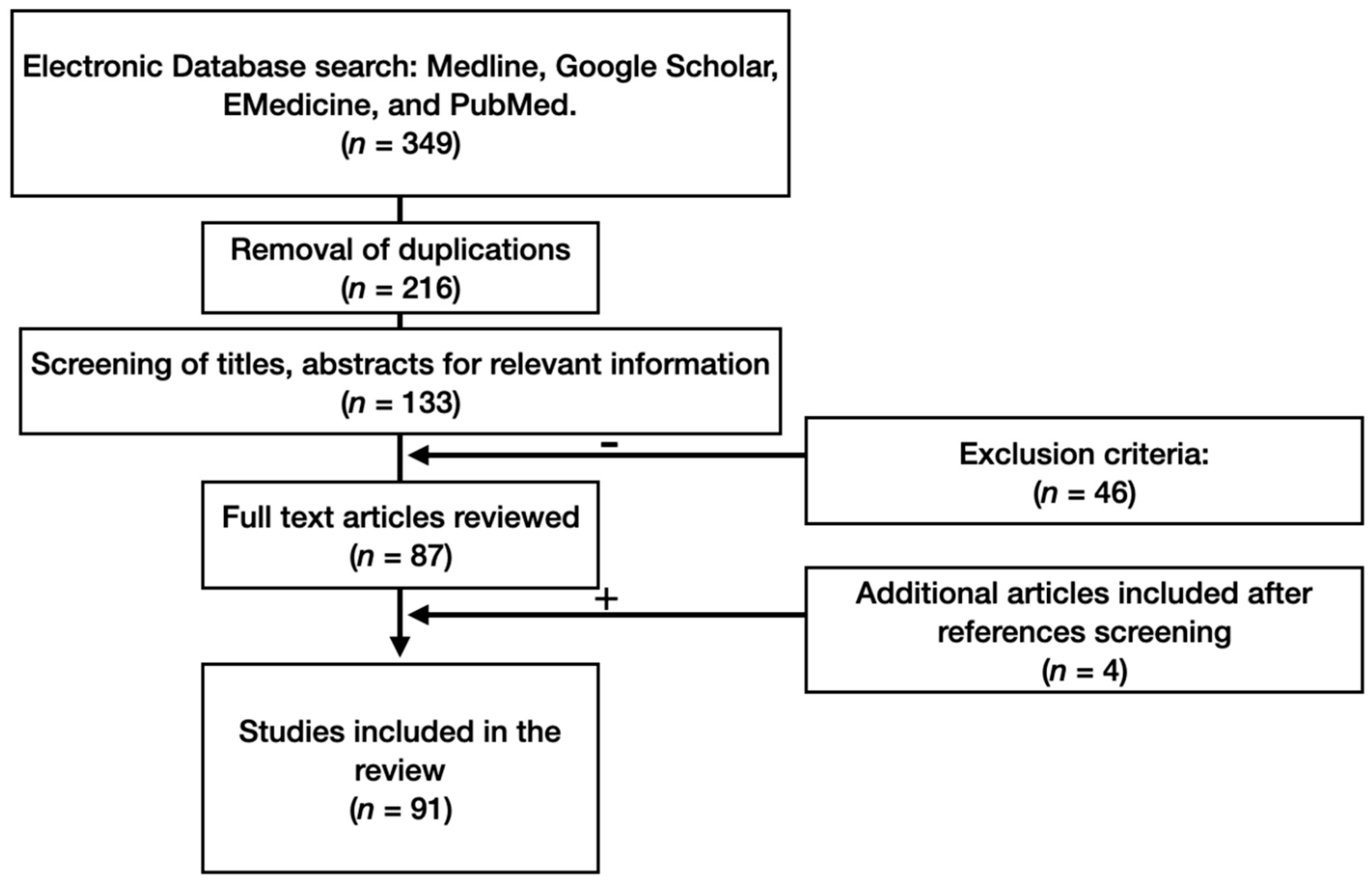
2. Materials and Methods
3. Results
3.1. Anatomy of the Amygdala
3.2. Neuronal Complexes of the Amygdala
3.2.1. Basolateral Neural Complex
3.2.2. The Central Nuclei
3.3. Intercalated Cell Masses
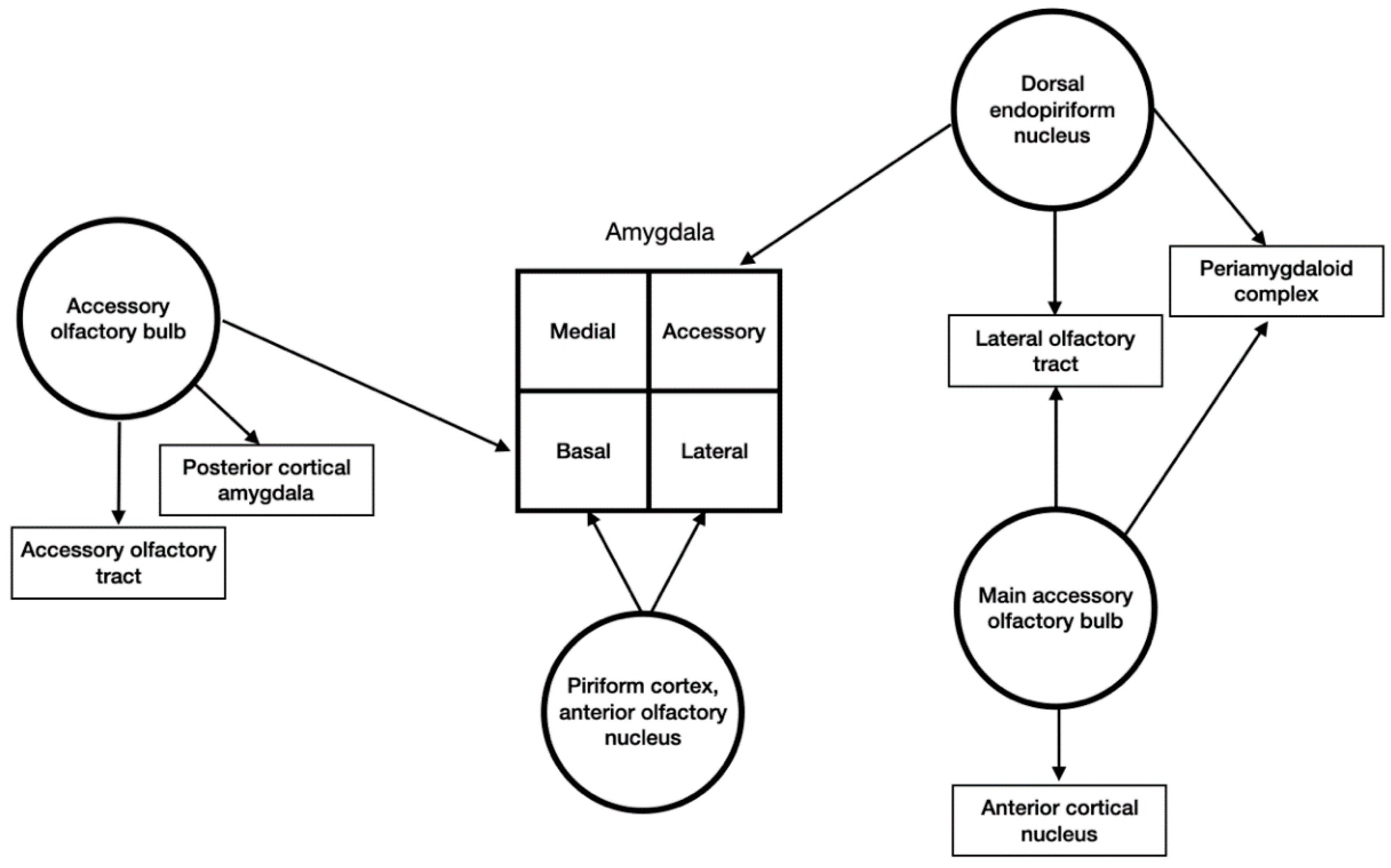
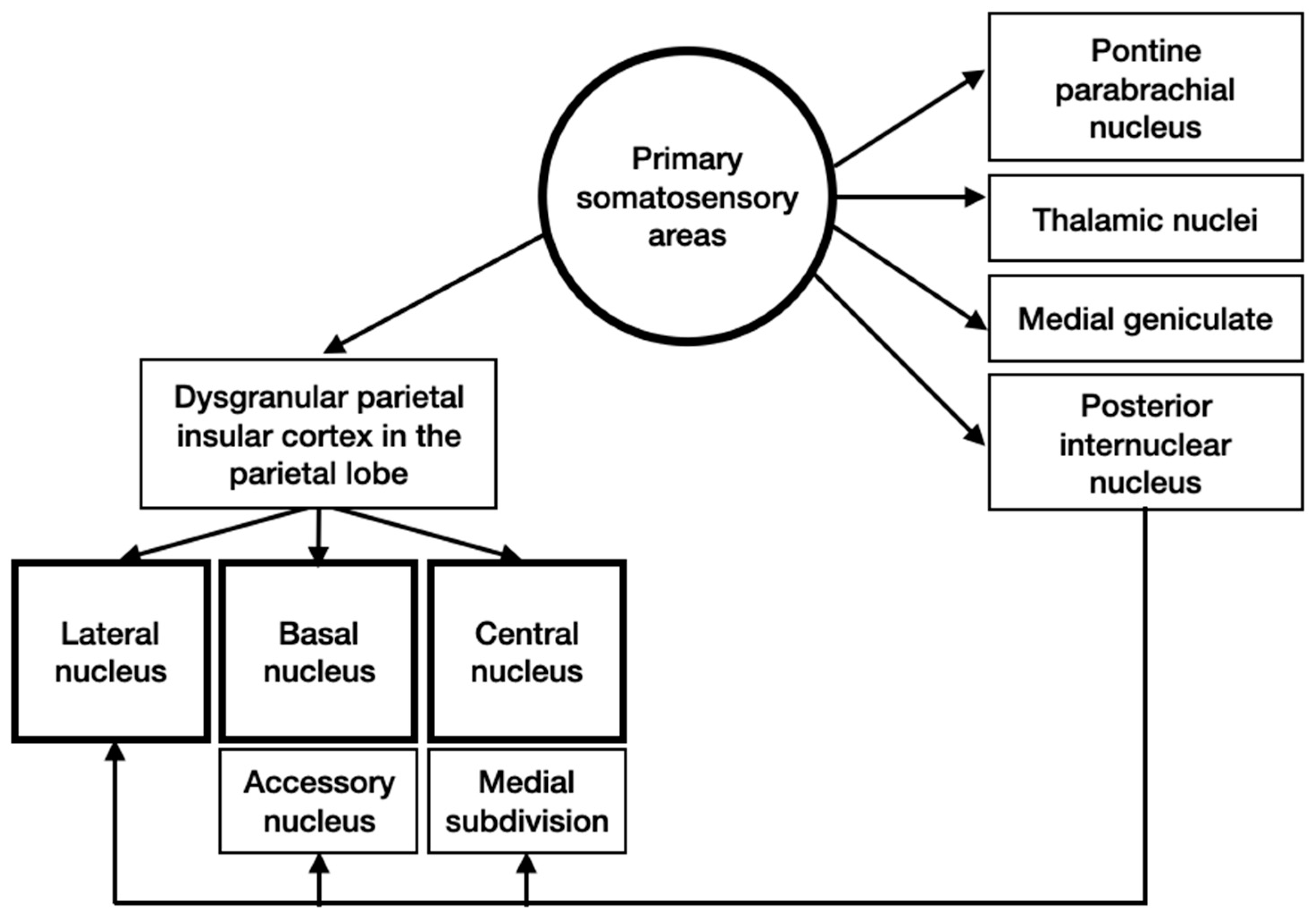
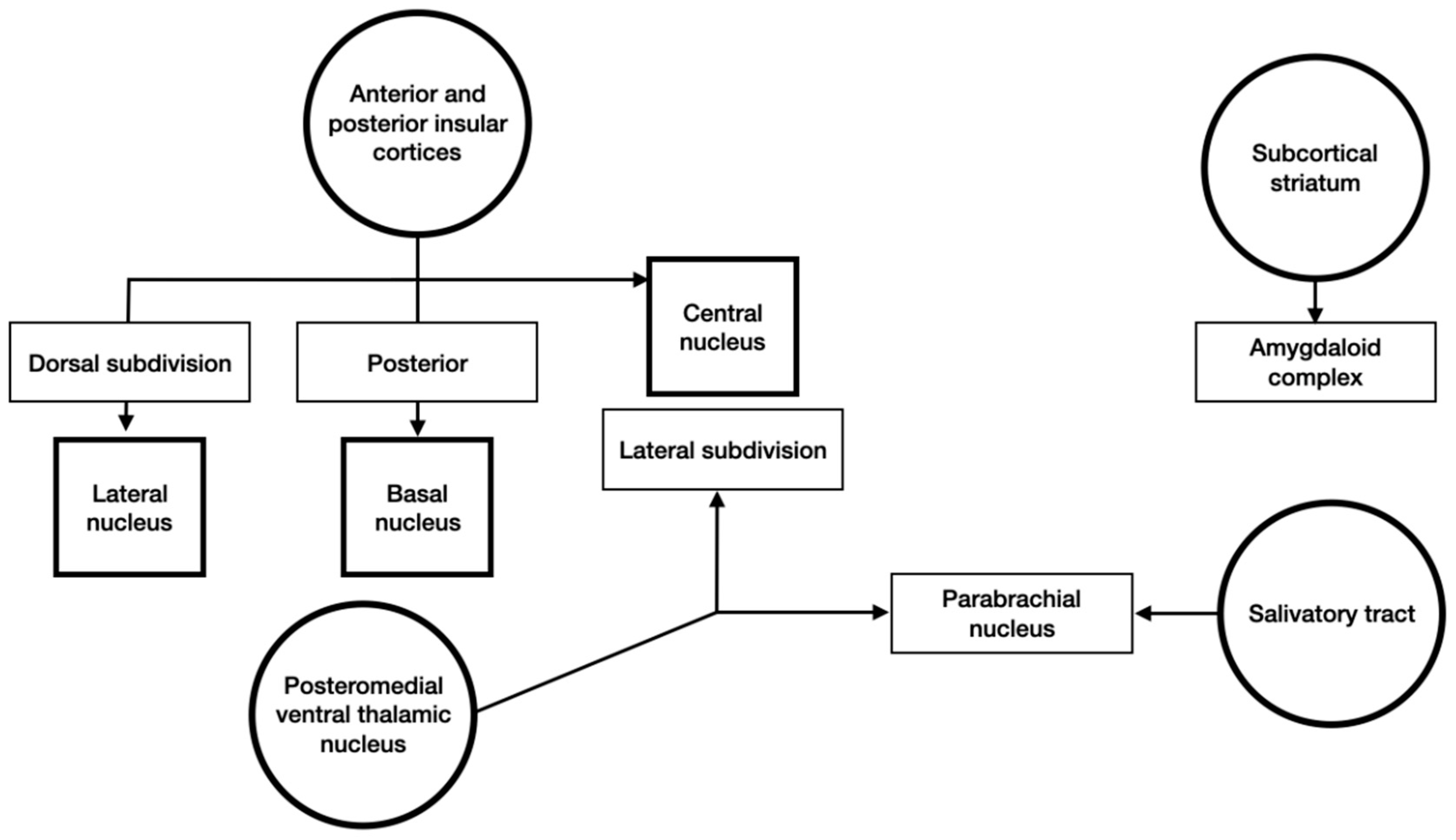
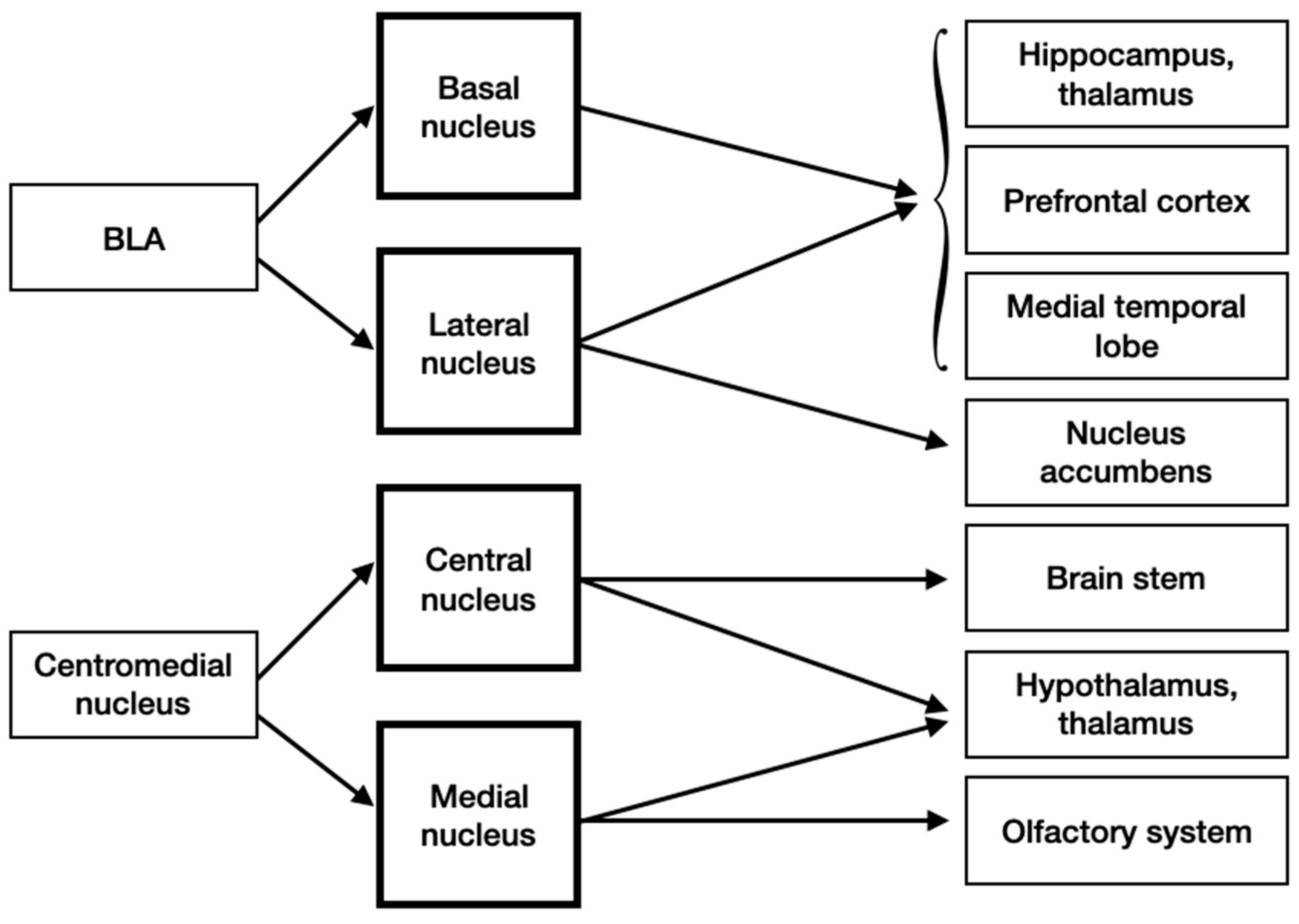
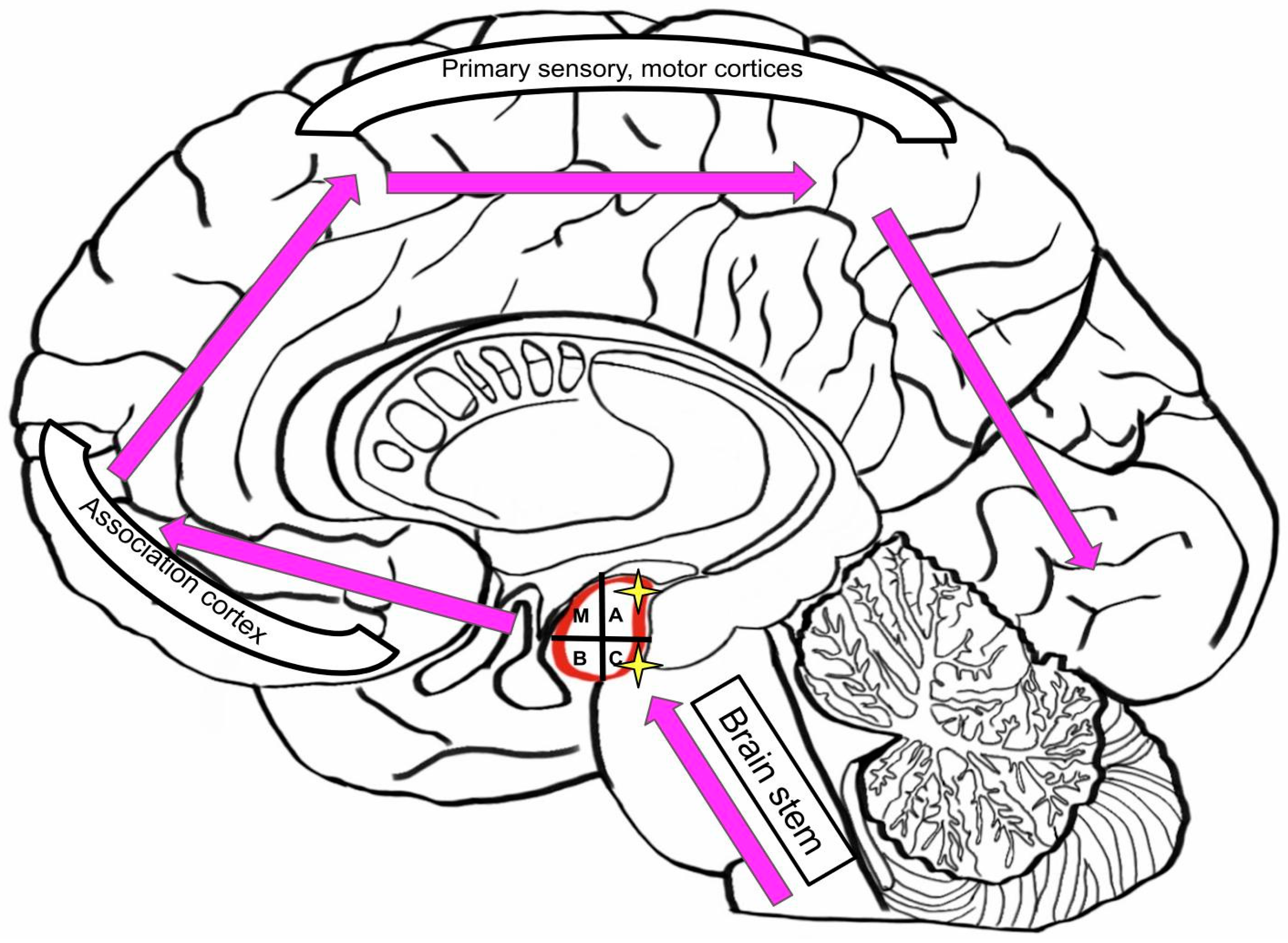
3.4. Input and Output Connections
3.5. Neurodegenerative Diseases: Hypothesis of Pathologic Synergy in the Amygdala
3.6. Role of Amygdala in Neurological and Neurodegenerative Diseases
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- LeDoux, J. The amygdala. Curr. Boil. 2007, 17, R868–R874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajmohan, V.; Mohandas, E. The limbic system. Indian J. Psychiatry 2007, 49, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Akhmadeev, A.V.; Kalimullina, L.B. What is the Amygdaloid Complex of the Brain? Adv. Physiol. Stud. 2017, 48, 56–71. [Google Scholar]
- Sah, P.; Faber, E.S.L.; De Armentia, M.L.; Power, J.M. The Amygdaloid Complex: Anatomy and Physiology. Physiol. Rev. 2003, 83, 803–834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nelson, P.T.; Abner, E.L.; Patel, E.; Anderson, S.; Wilcock, N.M.; Kryscio, R.J.; Van Eldik, L.J.; Jicha, G.; Gal, Z.; Nelson, R.S.; et al. The Amygdala as a Locus of Pathologic Misfolding in Neurodegenerative Diseases. J. Neuropathol. Exp. Neurol. 2017, 77, 2–20. [Google Scholar] [CrossRef]
- McDonald, A. Topographical organization of amygdaloid projections to the caudatoputamen, nucleus accumbens, and related striatal-like areas of the rat brain. Neuroscience 1991, 44, 15–33. [Google Scholar] [CrossRef]
- McDonald, A.J. Neurons of the lateral and basolateral amygdaloid nuclei: A golgi study in the rat. J. Comp. Neurol. 1982, 212, 293–312. [Google Scholar] [CrossRef]
- Faber, E.; Callister, R.J.; Sah, P. Morphological and electrophysiological properties of principal neurons in the rat lateral amygdala in vitro. J. Neurophysiol. 2001, 85, 714–723. [Google Scholar] [CrossRef] [Green Version]
- Millhouse, O.; DeOlmos, J. Neuronal configurations in lateral and basolateral amygdala. Neuroscience 1983, 10, 1269–1300. [Google Scholar] [CrossRef]
- Mason, A.; Larkman, A. Correlations between morphology and electrophysiology of pyramidal neurons in slices of rat visual cortex. II. Electrophysiology. J. Neurosci. 1990, 10, 1407–1414. [Google Scholar] [CrossRef] [Green Version]
- Paré, D.; Gaudreau, H. Projection Cells and Interneurons of the Lateral and Basolateral Amygdala: Distinct Firing Patterns and Differential Relation to Theta and Delta Rhythms in Conscious Cats. J. Neurosci. 1996, 16, 3334–3350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hall, E. The amygdala of the cat: A golgi study. Cell Tissue Res. 1972, 134, 439–458. [Google Scholar] [CrossRef] [PubMed]
- Lang, E.J.; Paré, D. Synaptic responsiveness of interneurons of the cat lateral amygdaloid nucleus. Neuroscience 1998, 83, 877–889. [Google Scholar] [CrossRef]
- Kemppainen, S.; Pitkänen, A. Distribution of parvalbumin, calretinin, and calbindin-D(28k) immunoreactivity in the rat amygdaloid complex and colocalization with gamma-aminobutyric acid. J. Comp. Neurol. 2000, 426, 441–467. [Google Scholar] [CrossRef]
- McDonald, A.J.; Mascagni, F. Colocalization of calcium-binding proteins and GABA in neurons of the rat basolateral amygdala. Neuroscience 2001, 105, 681–693. [Google Scholar] [CrossRef]
- McDonald, A.J. Calbindin-D28k immunoreactivity in the rat amygdala. J. Comp. Neurol. 1997, 383, 231–244. [Google Scholar] [CrossRef]
- Faulkner, B.; Brown, T.H. Morphology and physiology of neurons in the rat perirhinal-lateral amygdala area. J. Comp. Neurol. 1999, 411, 613–642. [Google Scholar] [CrossRef]
- Kamal, A.M.; Tömböl, T. Golgi studies on the amygdaloid nuclei of the cat. J. fur Hirnforsch. 1975, 16, 175–201. [Google Scholar]
- Mahanty, N.K.; Sah, P. Calcium-permeable AMPA receptors mediate long-term potentiation in interneurons in the amygdala. Nature 1998, 394, 683–687. [Google Scholar] [CrossRef]
- Paré, D.; Pape, H.C.; Dong, J. Bursting and oscillating neurons of the cat basolateral amygdaloid complex in vivo: Electrophysiological properties and morphological features. J. Neurophysiol. 1995, 74, 1179–1191. [Google Scholar] [CrossRef]
- Chapman, P.F.; Kairiss, E.W.; Keenan, C.L.; Brown, T.H. Long-term synaptic potentiation in the amygdala. Synapse 1990, 6, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Yajeya, J.; Juan, A.D.L.F.; A Merchan, M.; Riolobos, A.S.; Heredia, M.; Criado, J.M. Cholinergic responses of morphologically and electrophysiologically characterized neurons of the basolateral complex in rat amygdala slices. Neuroscience 1997, 78, 731–743. [Google Scholar] [CrossRef]
- Funahashi, M.; Matsuo, R.; Stewart, M. Propagation of synchronous burst discharges from entorhinal cortex to morphologically and electrophysiologically identified neurons of rat lateral amygdala. Brain Res. 2000, 884, 104–115. [Google Scholar] [CrossRef]
- Rainnie, D.G.; Asprodini, E.K.; Shinnick-Gallagher, P. Intracellular recordings from morphologically identified neurons of the basolateral amygdala. J. Neurophysiol. 1993, 69, 1350–1362. [Google Scholar] [CrossRef] [Green Version]
- Washburn, M.; Moises, H. Electrophysiological and morphological properties of rat basolateral amygdaloid neurons in vitro. J. Neurosci. 1992, 12, 4066–4079. [Google Scholar] [CrossRef]
- Pin, J.P.; Duvoisin, R. The metabotropic glutamate receptors: Structure and functions. Neuropharmacology 1995, 34, 1–26. [Google Scholar] [CrossRef]
- Farb, C.R.; Ledoux, J.E. NMDA and AMPA receptors in the lateral nucleus of the amygdala are postsynaptic to auditory thalamic afferents. Synapse 1997, 27, 106–121. [Google Scholar] [CrossRef]
- Farb, C.R.; Ledoux, J.E. Afferents from rat temporal cortex synapse on lateral amygdala neurons that express NMDA and AMPA receptors. Synapse 1999, 33, 218–229. [Google Scholar] [CrossRef]
- Jonas, P. Differences in Ca2+ permeability of AMPA-type glutamate receptor channels in neocortical neurons caused by differential GluR-B subunit expression. Neuron 1994, 12, 1281–1289. [Google Scholar] [CrossRef]
- Washburn, M.S.; Numberger, M.; Zhang, S.; Dingledine, R. Differential Dependence on GluR2 Expression of Three Characteristic Features of AMPA Receptors. J. Neurosci. 1997, 17, 9393–9406. [Google Scholar] [CrossRef] [Green Version]
- McBain, C.J.; Mayer, M.L. N-methyl-D-aspartic acid receptor structure and function. Physiol. Rev. 1994, 74, 723–760. [Google Scholar] [CrossRef] [PubMed]
- Cull-Candy, S.G.; Brickley, S.G.; Farrant, M. NMDA receptor subunits: Diversity, development and disease. Curr. Opin. Neurobiol. 2001, 11, 327–335. [Google Scholar] [CrossRef]
- Mayer, M.L.; Westbrook, G.L.; Guthrie, P.B. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature 1984, 309, 261–263. [Google Scholar] [CrossRef] [PubMed]
- Nowak, L.; Bregestovski, P.; Ascher, P.; Herbet, A.; Prochiantz, A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature 1984, 307, 462–465. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Phillips, R.; LeDoux, J. NMDA and non-NMDA receptors contribute to synaptic transmission between the medial geniculate body and the lateral nucleus of the amygdala. Exp. Brain Res. 1995, 105, 87–100. [Google Scholar] [CrossRef]
- Weisskopf, M.G.; LeDoux, J.E. Distinct populations of NMDA receptors at subcortical and cortical inputs to principal cells of the lateral amygdala. J. Neurophysiol. 1999, 81, 930–934. [Google Scholar] [CrossRef] [Green Version]
- Alger, B.E.; Nicoll, R.A. Feed-forward dendritic inhibition in rat hippocampal pyramidal cells studied In Vitro. J. Physiol. 1982, 328, 105–123. [Google Scholar] [CrossRef]
- Szinyei, C.; Heinbockel, T.; Montagne, J.; Pape, H.-C. Putative Cortical and Thalamic Inputs Elicit Convergent Excitation in a Population of GABAergic Interneurons of the Lateral Amygdala. J. Neurosci. 2000, 20, 8909–8915. [Google Scholar] [CrossRef] [Green Version]
- McDonald, A.J. Cytoarchitecture of the central amygdaloid nucleus of the rat. J. Comp. Neurol. 1982, 208, 401–418. [Google Scholar] [CrossRef]
- Cassell, M.; Gray, T.S. Morphology of peptide-immunoreactive neurons in the rat central nucleus of the amygdala. J. Comp. Neurol. 1989, 281, 320–333. [Google Scholar] [CrossRef]
- Schiess, M.C.; Callahan, P.M.; Zheng, H. Characterization of the electrophysiological and morphological properties of rat central amygdala neurons in vitro. J. Neurosci. Res. 1999, 58, 663–673. [Google Scholar] [CrossRef]
- Day, H.E.; Curran, E.J.; Watson, S.J.; Akil, H. Distinct neurochemical populations in the rat central nucleus of the amygdala and bed nucleus of the stria terminalis: Evidence for their selective activation by interleukin-1beta. J. Comp. Neurol. 1999, 413, 113–128. [Google Scholar] [CrossRef]
- Puelles, L. Thoughts on the development, structure and evolution of the mammalian and avian telencephalic pallium. Philos. Trans. R. Soc. B Boil. Sci. 2001, 356, 1583–1598. [Google Scholar] [CrossRef] [Green Version]
- Swanson, L.W.; Petrovich, G.D. What is the amygdala? Trends Neurosci. 1998, 21, 323–331. [Google Scholar] [CrossRef]
- Schiess, M.C.; Asprodini, E.K.; Rainnie, D.G.; Shinnick-Gallagher, P. The central nucleus of the rat amygdala: In Vitro intracellular recordings. Brain Res. 1993, 604, 283–297. [Google Scholar] [CrossRef]
- Martina, M.; Royer, S.; Paré, D. Physiological properties of central medial and central lateral amygdala neurons. J. Neurophysiol. 1999, 82, 1843–1854. [Google Scholar] [CrossRef] [PubMed]
- Dumont, E.C.; Martina, M.; Samson, R.D.; Drolet, G.; Paré, D. Physiological properties of central amygdala neurons: Species differences. Eur. J. Neurosci. 2002, 15, 545–552. [Google Scholar] [CrossRef]
- De Lopez, A.M.; Sah, P. Excitatory projections to the central lateral nucleus of the amygdala in the rat. Soc. Neurosci. Abstr. 2001, 713, 2. [Google Scholar]
- Royer, S.; Martina, M.; Paré, D. An Inhibitory Interface Gates Impulse Traffic between the Input and Output Stations of the Amygdala. J. Neurosci. 1999, 19, 10575–10583. [Google Scholar] [CrossRef] [Green Version]
- Neugebauer, V.; Zinebi, F.; Russell, R.; Gallagher, J.P.; Shinnick-Gallagher, P. Cocaine and kindling alter the sensitivity of group II and III metabotropic glutamate receptors in the central amygdala. J. Neurophysiol. 2000, 84, 759–770. [Google Scholar] [CrossRef]
- MacDermott, A.B.; Role, L.; Siegelbaum, S.A. Presynaptic ionotropic receptors and the control of transmitter release. Annu. Rev. Neurosci. 1999, 22, 443–485. [Google Scholar] [CrossRef] [PubMed]
- McGregor, A.; Roberts, D. Dopaminergic antagonism within the nucleus accumbens or the amygdala produces differential effects on intravenous cocaine self-administration under fixed and progressive ratio schedules of reinforcement. Brain Res. 1993, 624, 245–252. [Google Scholar] [CrossRef]
- Delaney, A.J.; Sah, P. GABA Receptors Inhibited by Benzodiazepines Mediate Fast Inhibitory Transmission in the Central Amygdala. J. Neurosci. 1999, 19, 9698–9704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whiting, P.J.; McKernan, R.M.; Wafford, K.A. Structure and Pharmacology of Vertebrate GABAA Receptor Subtypes. Int. Rev. Neurobiol. 1995, 38, 95–138. [Google Scholar] [CrossRef]
- Johnston, G. GABAA receptor pharmacology. Pharmacol. Ther. 1996, 69, 173–198. [Google Scholar] [CrossRef]
- Ragozzino, D.; Woodward, R.M.; Murata, Y.; Eusebi, F.; E Overman, L.; Miledi, R. Design and in vitro pharmacology of a selective gamma-aminobutyric acidC receptor antagonist. Mol. Pharmacol. 1996, 50, 1024–1030. [Google Scholar] [PubMed]
- Delaney, A.J.; Sah, P. Pathway-Specific Targeting of GABAA Receptor Subtypes to Somatic and Dendritic Synapses in the Central Amygdala. J. Neurophysiol. 2001, 86, 717–723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niehoff, D.; Kuhar, M. Benzodiazepine receptors: Localization in rat amygdala. J. Neurosci. 1983, 3, 2091–2097. [Google Scholar] [CrossRef] [Green Version]
- Nutt, D.J.; Malizia, A.L. New insights into the role of the GABAA–benzodiazepine receptor in psychiatric disorder. Br. J. Psychiatry 2001, 179, 390–396. [Google Scholar] [CrossRef] [Green Version]
- Paré, D.; Smith, Y. Distribution of GABA immunoreactivity in the amygdaloid complex of the cat. Neuroscience 1993, 57, 1061–1076. [Google Scholar] [CrossRef]
- Paré, D.; Smith, Y. The intercalated cell masses project to the central and medial nuclei of the amygdala in cats. Neuroscience 1993, 57, 1077–1090. [Google Scholar] [CrossRef]
- Millhouse, O.E. The intercalated cells of the amygdala. J. Comp. Neurol. 1986, 247, 246–271. [Google Scholar] [CrossRef] [PubMed]
- Royer, S.; Martina, M.; Paré, D. Bistable Behavior of Inhibitory Neurons Controlling Impulse Traffic through the Amygdala: Role of a Slowly Deinactivating K+ Current. J. Neurosci. 2000, 20, 9034–9039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Royer, S.; Martina, M.; Paré, D. Polarized synaptic interactions between intercalated neurons of the amygdala. J. Neurophysiol. 2000, 83, 3509–3518. [Google Scholar] [CrossRef] [PubMed]
- McDonald, A.J. Cell types and intrinsic connections of the amygdala. In The Amygdala: Neurobiological Aspects of Emotion, Memory and Mental Dysfunction; Wiley: Hoboken, NJ, USA, 1992; pp. 67–96. [Google Scholar]
- McDonald, A.J. Cortical pathways to the mammalian amygdala. Prog. Neurobiol. 1998, 55, 257–332. [Google Scholar] [CrossRef]
- Pitkanen, A. Connectivity of the rat amygdaloid complex. In The Amygdala: A Functional Analysis; Aggleton, J.P., Ed.; Oxford University Press: Oxford, UK, 2000; pp. 31–115. [Google Scholar]
- Price, J.L.; Russchen, F.T.; Amaral, D.G. The Limbic Region. In The Amygdaloid Complex; Elsevier Science: New York, NY, USA, 1987; pp. 279–388. [Google Scholar]
- Mascagni, F.; McDonald, A.J.; Coleman, J. Corticoamygdaloid and corticocortical projections of the rat temporal cortex: APhaseolus vulgaris leucoagglutinin study. Neuroscience 1993, 57, 697–715. [Google Scholar] [CrossRef]
- Nikolenko, V.N.; Oganesyan, M.V.; Vovkogon, A.D.; Nikitina, A.T.; Sozonova, E.A.; Kudryashova, V.A.; Rizaeva, N.A.; Cabezas, R.; Avila-Rodriguez, M.; Neganova, M.E.; et al. Current Understanding of Central Nervous System Drainage Systems: Implications in the Context of Neurodegenerative Diseases. Curr. Neuropharmacol. 2019, 17, 1. [Google Scholar] [CrossRef]
- Nelson, P.T.; Alafuzoff, I.; Bigio, E.H.; Bouras, C.; Braak, H.; Cairns, N.J.; Castellani, R.J.; Crain, B.J.; Davies, P.; Del Tredici, K.; et al. Correlation of Alzheimer Disease Neuropathologic Changes With Cognitive Status: A Review of the Literature. J. Neuropathol. Exp. Neurol. 2012, 71, 362–381. [Google Scholar] [CrossRef]
- Nelson, P.T.; Braak, H.; Markesbery, W.R. Neuropathology and Cognitive Impairment in Alzheimer Disease: A Complex but Coherent Relationship. J. Neuropathol. Exp. Neurol. 2009, 68, 1–14. [Google Scholar] [CrossRef]
- Johns, P. Parkinson’s disease. Clin. Neurosci. 2014, 163–179. [Google Scholar] [CrossRef]
- Boublay, N.; Schott, A.-M.; Krolak-Salmon, P. Neuroimaging correlates of neuropsychiatric symptoms in Alzheimer’s disease: A review of 20 years of research. Eur. J. Neurol. 2016, 23, 1500–1509. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, P.; Nowrangi, M.A.; Lyketsos, C.G. Neuropsychiatric symptoms in Alzheimer’s disease: What might be associated brain circuits? Mol. Asp. Med. 2015, 43, 25–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lozupone, M.; La Montagna, M.; D’Urso, F.; Piccininni, C.; Sardone, R.; Dibello, V.; Giannelli, G.; Solfrizzi, V.; Greco, A.; Daniele, A.; et al. Pharmacotherapy for the treatment of depression in patients with alzheimer’s disease: A treatment-resistant depressive disorder. Expert Opin. Pharmacother. 2018, 19, 823–842. [Google Scholar] [CrossRef] [PubMed]
- Orgeta, V.; Tabet, N.; Nilforooshan, R.; Howard, R. Efficacy of Antidepressants for Depression in Alzheimer’s Disease: Systematic Review and Meta-Analysis. J. Alzheimer’s Dis. 2017, 58, 725–733. [Google Scholar] [CrossRef] [Green Version]
- Son, J.H.; Han, D.H.; Min, K.J.; Kee, B.S. Correlation between gray matter volume in the temporal lobe and depressive symptoms in patients with Alzheimer’s disease. Neurosci. Lett. 2013, 548, 15–20. [Google Scholar] [CrossRef]
- Honda, H.; Terada, S.; Sato, S.; Oshima, E.; Ikeda, C.; Nagao, S.; Yokota, O.; Uchitomi, Y. Subjective depressive mood and regional cerebral blood flow in mild Alzheimer’s disease. Int. Psychogeriatr. 2014, 26, 817–823. [Google Scholar] [CrossRef]
- Tsai, C.F.; Hung, C.W.; Lirng, J.F.; Wang, S.J.; Fuh, J.L. Differences in brain metabolism associated with agitation and depression in Alzheimer’s disease. East. Asian Arch. Psychiatry 2013, 23, 86–90. [Google Scholar]
- Di Paola, M.; Phillips, O.R.; Orfei, M.D.; Piras, F.; Cacciari, C.; Caltagirone, C.; Spalletta, G. Corpus Callosum Structure is Topographically Correlated with the Early Course of Cognition and Depression in Alzheimer’s Disease. J. Alzheimer’s Dis. 2015, 45, 1097–1108. [Google Scholar] [CrossRef]
- Guo, Z.; Liu, X.; Jia, X.; Hou, H.; Cao, Y.; Wei, F.; Li, J.; Chen, X.; Zhang, Y.; Shen, Y.; et al. Regional Coherence Changes in Alzheimer’s Disease Patients with Depressive Symptoms: A Resting-State Functional MRI Study. J. Alzheimer’s Dis. 2015, 48, 603–611. [Google Scholar] [CrossRef]
- Dekens, D.W.; Naudé, P.J.W.; Engelborghs, S. Neutrophil gelatinase-associated lipocalin and its receptors in Alzheimer’s disease (AD) brain regions: Differential findings in AD with and without depression. J. Alzheimer’s Dis. 2015, 55, 763–776. [Google Scholar] [CrossRef] [Green Version]
- Pimontel, M.A.; Kanellopoulos, R.; Gunning, F. Neuroanatomical Abnormalities in Older Depressed Adults With Apathy: A Systematic Review. J. Geriatr. Psychiatry Neurol. 2019, 33. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Song, X.; Yuan, Y. Abnormal functional connectivity of the amygdala is associated with depression in Parkinson’s disease. Mov. Disord. 2015, 30, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Wassum, K.M.; Izquierdo, A. The basolateral amygdala in reward learning and addiction. Neurosci. Biobehav. Rev. 2015, 57, 271–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baidoo, N.; Wolter, M.; Leri, F. Opioid withdrawal and memory consolidation. Neurosci. Biobehav. Rev. 2020, 114, 16–24. [Google Scholar] [CrossRef] [PubMed]
- McDonald, R.J.; White, N.M. A triple dissociation of memory systems: Hippocampus, amygdala, and dorsal striatum. Behav. Neurosci. 1993, 107, 3–22. [Google Scholar] [CrossRef]
- Packard, M.G.; Chen, S.A. The basolateral amygdala is a cofactor in memory enhancement produced by intrahippocampal glutamate injections. Psychobiology 1999, 27, 377–385. [Google Scholar] [CrossRef]
- Santoro, G.; Carrion, J.; Patel, K.; Vilchez, C.; Veith, J.; Brodie, J.D.; Dewey, S.L. Sex Differences in Regional Brain Glucose Metabolism Following Opioid Withdrawal and Replacement. Neuropsychopharmacology 2017, 42, 1841–1849. [Google Scholar] [CrossRef] [Green Version]
- Duvarci, S.; Pare, D. Amygdala Microcircuits Controlling Learned Fear. Neuron 2014, 82, 966–980. [Google Scholar] [CrossRef] [Green Version]
- Morrison, S.E.; Salzman, C.D. Re-Valuing the amygdala. Curr. Opin. Neurobiol. 2010, 20, 221–230. [Google Scholar] [CrossRef]
- Janak, P.H.; Tye, K.M. From circuits to behaviour in the amygdala. Nature 2015, 517, 284–292. [Google Scholar] [CrossRef] [Green Version]
- Ehrlich, I.; Humeau, Y.; Grenier, F.; Ciocchi, S.; Herry, C.; Lüthi, A. Amygdala Inhibitory Circuits and the Control of Fear Memory. Neuron 2009, 62, 757–771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baczkowski, B.M.; Van Zutphen, L.; Siep, N.; Jacob, G.A.; Domes, G.; Maier, S.; Sprenger, A.; Senft, A.; Willenborg, B.; Tüscher, O.; et al. Deficient amygdala-prefrontal intrinsic connectivity after effortful emotion regulation in borderline personality disorder. Eur. Arch. Psychiatry Clin. Neurosci. 2016, 267, 551–565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villain, H.; Benkahoul, A.; Birmes, P.; Ferry, B.; Roullet, P. Influence of early stress on memory reconsolidation: Implications for post-traumatic stress disorder treatment. PLoS ONE 2018, 13, e0191563. [Google Scholar] [CrossRef] [Green Version]
- Maren, S.; Quirk, G.J. Neuronal signalling of fear memory. Nat. Rev. Neurosci. 2004, 5, 844–852. [Google Scholar] [CrossRef] [PubMed]
- Sah, P. Fear, Anxiety, and the Amygdala. Neuron 2017, 96, 1–2. [Google Scholar] [CrossRef] [Green Version]
- Heller, A.S. Cortical-Subcortical Interactions in Depression: From Animal Models to Human Psychopathology. Front. Syst. Neurosci. 2016, 10, 20. [Google Scholar] [CrossRef] [Green Version]
- Carballedo, A.; Scheuerecker, J.; Meisenzahl, E.; Schöpf, V.; Bokde, A.L.W.; Möller, H.-J.; Doyle, M.; Wiesmann, M.; Frodl, T. Functional connectivity of emotional processing in depression. J. Affect. Disord. 2011, 134, 272–279. [Google Scholar] [CrossRef]
- Tozzi, L.; Doolin, K.; Farrell, C.; Joseph, S.; O’Keane, V.; Frodl, T. Functional magnetic resonance imaging correlates of emotion recognition and voluntary attentional regulation in depression: A generalized psycho-physiological interaction study. J. Affect. Disord. 2017, 208, 535–544. [Google Scholar] [CrossRef]
- Lin, T.-W.; Shih, Y.-H.; Chen, S.-J.; Lien, C.-H.; Chang, C.-Y.; Huang, T.-Y.; Chen, S.-H.; Jen, C.J.; Kuo, Y.-M. Running exercise delays neurodegeneration in amygdala and hippocampus of Alzheimer’s disease (APP/PS1) transgenic mice. Neurobiol. Learn. Mem. 2015, 118, 189–197. [Google Scholar] [CrossRef]
- Popescu, A.; Lippa, C.F.; Lee, V.M.-Y.; Trojanowski, J.Q. Lewy Bodies in the Amygdala. Arch. Neurol. 2004, 61, 1915–1919. [Google Scholar] [CrossRef] [Green Version]
- Sorrentino, Z.A.; Goodwin, M.S.; Riffe, C.J.; Dhillon, J.-K.S.; Xia, Y.; Gorion, K.-M.; Vijayaraghavan, N.; McFarland, K.N.; Golbe, L.I.; Yachnis, A.T.; et al. Unique α-synuclein pathology within the amygdala in Lewy body dementia: Implications for disease initiation and progression. Acta Neuropathol. Commun. 2019, 7, 142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, J.P.; Hardy, J.; Fischbeck, K.H. Toxic proteins in neurodegenerative disease. Science 2002, 296, 1991–1995. [Google Scholar] [CrossRef] [PubMed]
- Poulin, S.P.; Dautoff, R.; Morris, J.C.; Barrett, L.F.; Dickerson, B.C. Alzheimer’s Disease Neuroimaging Initiative. Amygdala atrophy is prominent in early Alzheimer’s disease and relates to symptom severity. Psychiatry Res. Neuroimaging 2011, 194, 7–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del Val, L.P.; Cantero, J.L.; Atienza, M. Atrophy of amygdala and abnormal memory-related alpha oscillations over posterior cingulate predict conversion to Alzheimer’s disease. Sci. Rep. 2016, 6, 31859. [Google Scholar] [CrossRef] [Green Version]
- Unger, J.W.; Lapham, L.W.; McNeill, T.H.; Eskin, T.A.; Hamill, R.W. The amygdala in Alzheimer’s disease: Neuropathology and Alz 50 immunoreactivity. Neurobiol. Aging 1991, 12, 389–399. [Google Scholar] [CrossRef]
- Braak, H.; Braak, E.; Yilmazer, D.; de Vos, R.A.; Jansen, E.N.; Bohl, J.; Jellinger, K. Amygdala pathology in Parkinson’s disease. Acta Neuropathol. 1994, 88, 493–500. [Google Scholar] [CrossRef]
- Huang, P.; Xuan, M.; Gu, Q.; Yu, X.; Xu, X.; Luo, W.; Zhang, M. Abnormal amygdala function in Parkinson’s disease patients and its relationship to depression. J. Affect. Disord. 2015, 183, 263–268. [Google Scholar] [CrossRef]
- Vriend, C.; Boedhoe, P.S.; Rutten, S.; Berendse, H.W.; Van Der Werf, Y.D.; Heuvel, O.A.V.D. A smaller amygdala is associated with anxiety in Parkinson’s disease: A combined FreeSurfer—VBM study. J. Neurol. Neurosurg. Psychiatry 2015, 87, 493–500. [Google Scholar] [CrossRef]


| Nuclei | Location | Subdivision |
|---|---|---|
| Lateral | Dorsally in the amygdala, ventrally is the basal nucleus | Dorsolateral, Ventrolateral, Medial |
| Basal | Ventral to the lateral nucleus | Rostral magnocellular, Caudal intermediate, Parvicellular |
| Accessory | Ventral to the basal nucleus, near by the amygdala–hippocampus area | Magnocellular, Intermediate, Parvicellular |
| Nucleus | Location | Subdivision |
|---|---|---|
| Central | In the rostral part of the amygdala, medially to the basolateral nuclei, laterally to the stria terminalis, ventrally to the globus pallidus. | Capsular, Lateral, Intermediate, Medial |
| Medial | Laterally to the optic tract | Rostral, Central (Dorsal and Ventral), Caudal |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikolenko, V.N.; Oganesyan, M.V.; Rizaeva, N.A.; Kudryashova, V.A.; Nikitina, A.T.; Pavliv, M.P.; Shchedrina, M.A.; Giller, D.B.; Bulygin, K.V.; Sinelnikov, M.Y. Amygdala: Neuroanatomical and Morphophysiological Features in Terms of Neurological and Neurodegenerative Diseases. Brain Sci. 2020, 10, 502. https://doi.org/10.3390/brainsci10080502
Nikolenko VN, Oganesyan MV, Rizaeva NA, Kudryashova VA, Nikitina AT, Pavliv MP, Shchedrina MA, Giller DB, Bulygin KV, Sinelnikov MY. Amygdala: Neuroanatomical and Morphophysiological Features in Terms of Neurological and Neurodegenerative Diseases. Brain Sciences. 2020; 10(8):502. https://doi.org/10.3390/brainsci10080502
Chicago/Turabian StyleNikolenko, Vladimir N., Marine V. Oganesyan, Negoriya A. Rizaeva, Valentina A. Kudryashova, Arina T. Nikitina, Maria P. Pavliv, Marina A. Shchedrina, Dmitry B. Giller, Kirill V. Bulygin, and Mikhail Y. Sinelnikov. 2020. "Amygdala: Neuroanatomical and Morphophysiological Features in Terms of Neurological and Neurodegenerative Diseases" Brain Sciences 10, no. 8: 502. https://doi.org/10.3390/brainsci10080502
APA StyleNikolenko, V. N., Oganesyan, M. V., Rizaeva, N. A., Kudryashova, V. A., Nikitina, A. T., Pavliv, M. P., Shchedrina, M. A., Giller, D. B., Bulygin, K. V., & Sinelnikov, M. Y. (2020). Amygdala: Neuroanatomical and Morphophysiological Features in Terms of Neurological and Neurodegenerative Diseases. Brain Sciences, 10(8), 502. https://doi.org/10.3390/brainsci10080502





