Feasibility of Using Intraoperative Neuromonitoring in the Prophylaxis of Dysesthesia in Transforaminal Endoscopic Discectomies of the Lumbar Spine
Abstract
1. Introduction
2. Material and Method
2.1. Patients
2.2. Inclusion/Exclusion Criteria
2.3. Preoperative Radiographic Evaluation
2.4. Anesthesia and Neuromonitoring
2.5. Endoscopic Discectomy Procedure
2.6. Clinical Follow-Up and Primary Outcome Measures
2.7. Postoperative Rehabilitation
2.8. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Disclaimer
References
- Lewandrowski, K.U. Incidence, Management, and Cost of Complications After Transforaminal Endoscopic Decompression Surgery for Lumbar Foraminal and Lateral Recess Stenosis: A Value Proposition for Outpatient Ambulatory Surgery. Int. J. Spine Surg. 2019, 13, 53–67. [Google Scholar] [CrossRef]
- Zhou, C.; Zhang, G.; Panchal, R.R.; Ren, X.; Xiang, H.; Xuexiao, M.; Chen, X.; Tongtong, G.; Hong, W.; Dixson, A.D. Unique Complications of Percutaneous Endoscopic Lumbar Discectomy and Percutaneous Endoscopic Interlaminar Discectomy. Pain Physician 2018, 21, E105–E112. [Google Scholar]
- Li, Z.Z.; Hou, S.X.; Shang, W.L.; Cao, G.; Qin, J.; Cai, Y. The strategy and early clinical outcome of full-endoscopic L5/S1 discectomy through interlaminar approach. Clin. Neurol. Neurosurg. 2015, 133, 40–45. [Google Scholar] [CrossRef]
- Xu, H.; Liu, X.; Liu, G.; Zhao, J.; Fu, Q.; Xu, B. Learning curve of full-endoscopic technique through interlaminar approach for L5/S1 disk herniations. Cell Biochem. Biophys. 2014, 70, 1069–1074. [Google Scholar] [CrossRef]
- Butler, A.J.; Alam, M.; Wiley, K.; Ghasem, A.; Iii, A.J.R.; Wang, J.C. Endoscopic Lumbar Surgery: The State of the Art in 2019. Neurospine 2019, 16, 15–23. [Google Scholar] [CrossRef]
- Asch, H.L.; Lewis, P.J.; Moreland, D.B.; Egnatchik, J.G.; Yu, Y.J.; Clabeaux, D.E.; Hyland, A.H. Prospective multiple outcomes study of outpatient lumbar microdiscectomy: should 75 to 80% success rates be the norm? J. Neurosurg. Spine 2002, 96, 34–44. [Google Scholar] [CrossRef]
- Lewandrowski, K.-U.; Dowling, Á.; De Carvalho, P.; Calderaro, A.L.; Dos Santos, T.S.; Silva, M.S.D.L.E.; León, J.F.R.; Yeung, A. Indication And Contraindication Of Endoscopic Transforaminal Lumbar Decompression. World Neurosurg. 2020. [Google Scholar] [CrossRef]
- Lewandrowski, K.-U.; Ransom, N.A. Five-year clinical outcomes with endoscopic transforaminal outside-in foraminoplasty techniques for symptomatic degenerative conditions of the lumbar spine. J. Spine Surg. 2020, 6, S54–S65. [Google Scholar] [CrossRef]
- Tsou, P.M.; Yeung, C.A.; Yeung, A.T. Posterolateral transforaminal selective endoscopic discectomy and thermal annuloplasty for chronic lumbar discogenic pain: a minimal access visualized intradiscal surgical procedure. Spine J. 2004, 4, 564–573. [Google Scholar] [CrossRef]
- Yeung, A.; Lewandrowski, K.-U. Five-year clinical outcomes with endoscopic transforaminal foraminoplasty for symptomatic degenerative conditions of the lumbar spine: a comparative study of inside-out versus outside-in techniques. J. Spine Surg. 2020, 6, S66–S83. [Google Scholar] [CrossRef]
- Yeung, A.; Lewandrowski, K.-U. Early and staged endoscopic management of common pain generators in the spine. J. Spine Surg. 2020, 6, S1–S5. [Google Scholar] [CrossRef]
- Yeung, A.; Roberts, A.; Zhu, L.; Qi, L.; Zhang, J.; Lewandrowski, K.-U. Treatment of Soft Tissue and Bony Spinal Stenosis by a Visualized Endoscopic Transforaminal Technique Under Local Anesthesia. Neurospine 2019, 16, 52–62. [Google Scholar] [CrossRef]
- Yeung, A.T. Minimally Invasive Disc Surgery with the Yeung Endoscopic Spine System (YESS). Surg. Technol. Int. 1999, 8, 267–277. [Google Scholar]
- Yeung, A.T. The Evolution and Advancement of Endoscopic Foraminal Surgery: One Surgeon’s Experience Incorporating Adjunctive Techologies. SAS J. 2007, 1, 108–117. [Google Scholar] [CrossRef]
- Yeung, A.T.; Gore, S. In-vivo Endoscopic Visualization of Patho-anatomy in Symptomatic Degenerative Conditions of the Lumbar Spine II: Intradiscal, Foraminal, and Central Canal Decompression. Surg. Technol. Int. 2011, 21, 299–319. [Google Scholar]
- Yeung, A.T.; Tsou, P.M. Posterolateral endoscopic excision for lumbar disc herniation: Surgical technique, outcome, and complications in 307 consecutive cases. Spine 2002, 27. [Google Scholar] [CrossRef]
- Yeung, A.T.; Yeung, C.A. Advances in endoscopic disc and spine surgery: foraminal approach. Surg. Technol. Int. 2003, 11, 255–263. [Google Scholar]
- Yeung, A.T.; Yeung, C.A. In-vivo endoscopic visualization of patho-anatomy in painful degenerative conditions of the lumbar spine. Surg. Technol. Int. 2006, 15, 243–256. [Google Scholar]
- Dowling, Á.; Lewandrowski, K.-U.; Da Silva, F.H.P.; Parra, J.A.A.; Portillo, D.M.; Giménez, Y.C.P. Patient selection protocols for endoscopic transforaminal, interlaminar, and translaminar decompression of lumbar spinal stenosis. J. Spine Surg. 2020, 6, S120–S132. [Google Scholar] [CrossRef]
- Lewandrowski, K.-U. Readmissions After Outpatient Transforaminal Decompression for Lumbar Foraminal and Lateral Recess Stenosis. Int. J. Spine Surg. 2018, 12, 342–351. [Google Scholar] [CrossRef]
- Bechara, B.P.; Agarwal, V.; Boardman, J.; Perera, S.; Weiner, D.K.; Vo, N.; Kang, J.; Sowa, G.A. Correlation of Pain With Objective Quantification of Magnetic Resonance Images in Older Adults With Chronic Low Back Pain. Spine 2014, 39, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Lewandrowski, K.-U. The strategies behind "inside-out" and "outside-in" endoscopy of the lumbar spine: treating the pain generator. J. Spine Surg. 2020, 6, S35–S39. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.G.V.; Vijayaraghavan, G.; Ravikumar, N.; Ding, Y.; Yin, M.L.; Patel, R.S.; Naresh, N.; Hey, H.W.D.; Lau, L.L.; Liu, G. Intraoperative Neuromonitoring (IONM): Is There a Role in Metastatic Spine Tumor Surgery? Spine 2019, 44, E219–E240. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Lenina, S.; Mosley, G.; Meaike, J.; Tran, B.; Kim, J.S.; Cho, S.K. The Efficacy of Intraoperative Neurophysiological Monitoring to Detect Postoperative Neurological Deficits in Transforaminal Lumbar Interbody Fusion Surgery. Oper. Neurosurg. 2018, 16, 71–78. [Google Scholar] [CrossRef]
- Kahraman, S.; Göçmen, S.; Gokmen, M.H.A.; Acka, G.; Pusat, S. Intraoperative Neurophysiologic Monitoring for Lumbar Intradural Schwannomas: Does It Affect Clinical Outcome? World Neurosurg. 2019. [Google Scholar] [CrossRef]
- Riley, M.; Doan, A.T.; Vogel, R.; Aguirre, A.O.; Pieri, K.S.; Scheid, E.H. Use of motor evoked potentials during lateral lumbar interbody fusion reduces postoperative deficits. Spine J. 2018, 18, 1763–1778. [Google Scholar] [CrossRef]
- Barzilai, O.; Lidar, Z.; Constantini, S.; Salame, K.; Bitan-Talmor, Y.; Korn, A. Continuous mapping of the corticospinal tracts in intramedullary spinal cord tumor surgery using an electrified ultrasonic aspirator. J. Neurosurgery Spine 2017, 27, 161–168. [Google Scholar] [CrossRef]
- Acharya, S.; Palukuri, N.; Gupta, P.; Kohli, M. Transcranial Motor Evoked Potentials during Spinal Deformity Corrections—Safety, Efficacy, Limitations, and the Role of a Checklist. Front. Surg. 2017, 4. [Google Scholar] [CrossRef]
- Polly, D.; Rice, K.; Tamkus, A. What Is the Frequency of Intraoperative Alerts During Pediatric Spinal Deformity Surgery Using Current Neuromonitoring Methodology? A Retrospective Study of 218 Surgical Procedures. Neurodiagnostic J. 2016, 56, 17–31. [Google Scholar] [CrossRef]
- Lee, J.M.; Kim, N.H.; Kim, H.S.; Choi, B.K.; Han, I.H. The Applicability of Intraoperative Neuromonitoring in Patients with Preoperative Motor Weakness during Spine Surgery. Korean J. Spine 2016, 13, 9–12. [Google Scholar] [CrossRef]
- Zieliński, P.; Furtak, J. Influence of intraoperative neurophysiologic monitoring on the development of surgical dissection techniques. Expert Rev. Med. Devices 2012, 9, 571–575. [Google Scholar] [CrossRef] [PubMed]
- Eager, M.; Shimer, A.; Jahangiri, F.R.; Shen, F.; Arlet, V. Intraoperative neurophysiological monitoring (IONM): lessons learned from 32 case events in 2069 spine cases. Am. J. Electroneurodiagnostic Technol. 2011, 51, 247–263. [Google Scholar] [CrossRef] [PubMed]
- Jea, A. Editorial. Intraoperative neuromonitoring: gold standard or fool’s gold? Neurosurg. Focus 2017, 43, E9. [Google Scholar] [CrossRef] [PubMed]
- Cole, T.; Veeravagu, A.; Zhang, M.; Li, A.; Ratliff, J.K. Intraoperative Neuromonitoring in Single-Level Spinal Procedures. Spine 2014, 39, 1950–1959. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.Y.; Lee, H.-Y.; Lee, S.-H. Prevention of Development of Postoperative Dysesthesia in Transforaminal Percutaneous Endoscopic Lumbar Discectomy for Intracanalicular Lumbar Disc Herniation: Floating Retraction Technique. min - Minim. Invasive Neurosurg. 2011, 54, 214–218. [Google Scholar] [CrossRef]
- Hasegawa, T.; An, H.S.; Haughton, V.; Nowicki, B.H. Lumbar foraminal stenosis: critical heights of the intervertebral discs and foramina. A cryomicrotome study in cadavera. J. Bone Jt. Surgery-American Vol. 1995, 77, 32–38. [Google Scholar] [CrossRef]
- Botwin, K.; Brown, L.A.; Fishman, M.; Rao, S. Fluoroscopically guided caudal epidural steroid injections in degenerative lumbar spine stenosis. Pain Physician 2007, 10, 547–558. [Google Scholar]
- El-Khoury, G.Y.; Ehara, S.; Weinstein, J.N.; Montgomery, W.J.; Kathol, M.H. Epidural steroid injection: a procedure ideally performed with fluoroscopic control. Radiology 1988, 168, 554–557. [Google Scholar] [CrossRef]
- El-Khoury, G.Y.; Renfrew, D.L. Percutaneous procedures for the diagnosis and treatment of lower back pain: diskography, facet-joint injection, and epidural injection. Am. J. Roentgenol. 1991, 157, 685–691. [Google Scholar] [CrossRef]
- Erçalık, T.; Atalay, K.G.; Toprak, C.Ş.; Gündüz, O.H. Outcome measurement in patients with low back pain undergoing epidural steroid injection. Turk. J. Phys. Med. Rehabil. 2019, 65, 154–159. [Google Scholar] [CrossRef]
- Lee, I.S.; Kim, S.H.; Lee, J.W.; Hong, S.H.; Choi, J.-Y.; Kang, H.S.; Song, J.-W.; Kwon, A.K. Comparison of the temporary diagnostic relief of transforaminal epidural steroid injection approaches: conventional versus posterolateral technique. Am. J. Neuroradiol. 2007, 28, 204–208. [Google Scholar]
- Lee, J.W.; Kim, S.H.; Lee, I.S.; Choi, J.-A.; Choi, J.-Y.; Hong, S.H.; Kang, H.S. Therapeutic Effect and Outcome Predictors of Sciatica Treated Using Transforaminal Epidural Steroid Injection. Am. J. Roentgenol. 2006, 187, 1427–1431. [Google Scholar] [CrossRef] [PubMed]
- Lewandrowski, K.-U. Successful outcome after outpatient transforaminal decompression for lumbar foraminal and lateral recess stenosis: The positive predictive value of diagnostic epidural steroid injection. Clin. Neurol. Neurosurg. 2018, 173, 38–45. [Google Scholar] [CrossRef] [PubMed]
- MacVicar, J.; King, W.; Landers, M.H.; Bogduk, N. The Effectiveness of Lumbar Transforaminal Injection of Steroids: A Comprehensive Review with Systematic Analysis of the Published Data. Pain Med. 2013, 14, 14–28. [Google Scholar] [CrossRef] [PubMed]
- Lewandrowski, K.U. “Outside-in” technique, clinical results, and indications with transforaminal lumbar endoscopic surgery: a retrospective study on 220 patients on applied radiographic classification of foraminal spinal stenosis. Int. J. Spine Surg. 2014, 8, 26. [Google Scholar] [CrossRef]
- Beyaz, S.G.; Inanmaz, M.E.; Zengin, E.Ş.; Ülgen, A.M. Combined Use of High Radiofrequency Disk Ablation, Annulus Modulation, and Manual Nucleotomy in a Patient with Extruded Disk Herniation. Pain Pr. 2016, 16, E74–E80. [Google Scholar] [CrossRef] [PubMed]
- Huskisson, E.C.; Jones, J.; Scott, P.J. Application Of Visual-Analogue Scales To The Measurement Of Functional Capacity. Rheumatology 1976, 15, 185–187. [Google Scholar] [CrossRef]
- Fairbank, J.C.; Pynsent, P.B. The Oswestry Disability Index. Spine 2000, 25, 2940–2952. [Google Scholar] [CrossRef]
- Fairbank, J. Use of Oswestry Disability Index (ODI). Spine 1995, 20, 1535–1537. [Google Scholar] [CrossRef]
- Macnab, I. Negative disc exploration. An analysis of the causes of nerve-root involvement in sixty-eight patients. J. Bone Joint Surg. Am. 1971, 53, 891–903. [Google Scholar] [CrossRef]
- Macnab, I. The surgery of lumbar disc degeneration. Surg. Annu. 1976, 8, 447–480. [Google Scholar] [PubMed]
- Liberati, A.; Altman, U.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; A Ioannidis, J.P.; Clarke, M.; Devereaux, P.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef] [PubMed]
- Lewandrowski, K.-U.; Dowling, Á.; Calderaro, A.L.; Dos Santos, T.S.; Bergamaschi, J.P.M.; León, J.F.R.; Yeung, A. Dysethesia due to irritation of the dorsal root ganglion following lumbar transforaminal endoscopy: analysis of frequency and contributing factors. Clin. Neurol. Neurosurg. 2020, 197, 106073. [Google Scholar] [CrossRef] [PubMed]
- Nellensteijn, J.; Ostelo, R.; Bartels, R.; Peul, W.; Van Royen, B.; Van Tulder, M.W. Transforaminal endoscopic surgery for symptomatic lumbar disc herniations: a systematic review of the literature. Eur. Spine J. 2010, 19, 181–204. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-S.; Chu, L.; Chen, C.-M.; Wang, X.-F.; Xie, P.-G.; Deng, R.; Yu, K.; Shi, L.; Zhang, Z.-X.; Rong, L.; et al. Foraminoplasty at the Tip or Base of the Superior Articular Process for Lateral Recess Stenosis in Percutaneous Endoscopic Lumbar Discectomy: A Multicenter, Retrospective, Controlled Study with 2-Year Follow-Up. BioMed Res. Int. 2018, 2018, 1–9. [Google Scholar] [CrossRef]
- Laratta, J.L.; Shillingford, J.N.; Ha, A.; Lombardi, J.M.; Reddy, H.P.; Saifi, C.; Ludwig, S.C.; Lehman, R.A.; Lenke, L.G. Utilization of intraoperative neuromonitoring throughout the United States over a recent decade: an analysis of the nationwide inpatient sample. J. Spine Surg. 2018, 4, 211–219. [Google Scholar] [CrossRef]
- Sharan, A.; Groff, M.W.; Dailey, A.T.; Ghogawala, Z.; Resnick, D.K.; Watters, W.C.; Mummaneni, P.V.; Choudhri, T.F.; Eck, J.C.; Wang, J.C.; et al. Guideline update for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 15: Electrophysiological monitoring and lumbar fusion. J. Neurosurg. Spine 2014, 21, 102–105. [Google Scholar] [CrossRef]
- Krause, K.L.; Ii, B.C.; Obayashi, J.T.; Kawamoto, A.; Than, K.D. Intraoperative neuromonitoring for one-level lumbar discectomies is low yield and cost-ineffective. J. Clin. Neurosci. 2020, 71, 97–100. [Google Scholar] [CrossRef]
- Grosland, J.O.; Todd, M.M.; Goldstein, P.A. Neuromonitoring in the ambulatory anesthesia setting: a pro-con discussion. Curr. Opin. Anaesthesiol. 2018, 31, 667–672. [Google Scholar] [CrossRef]


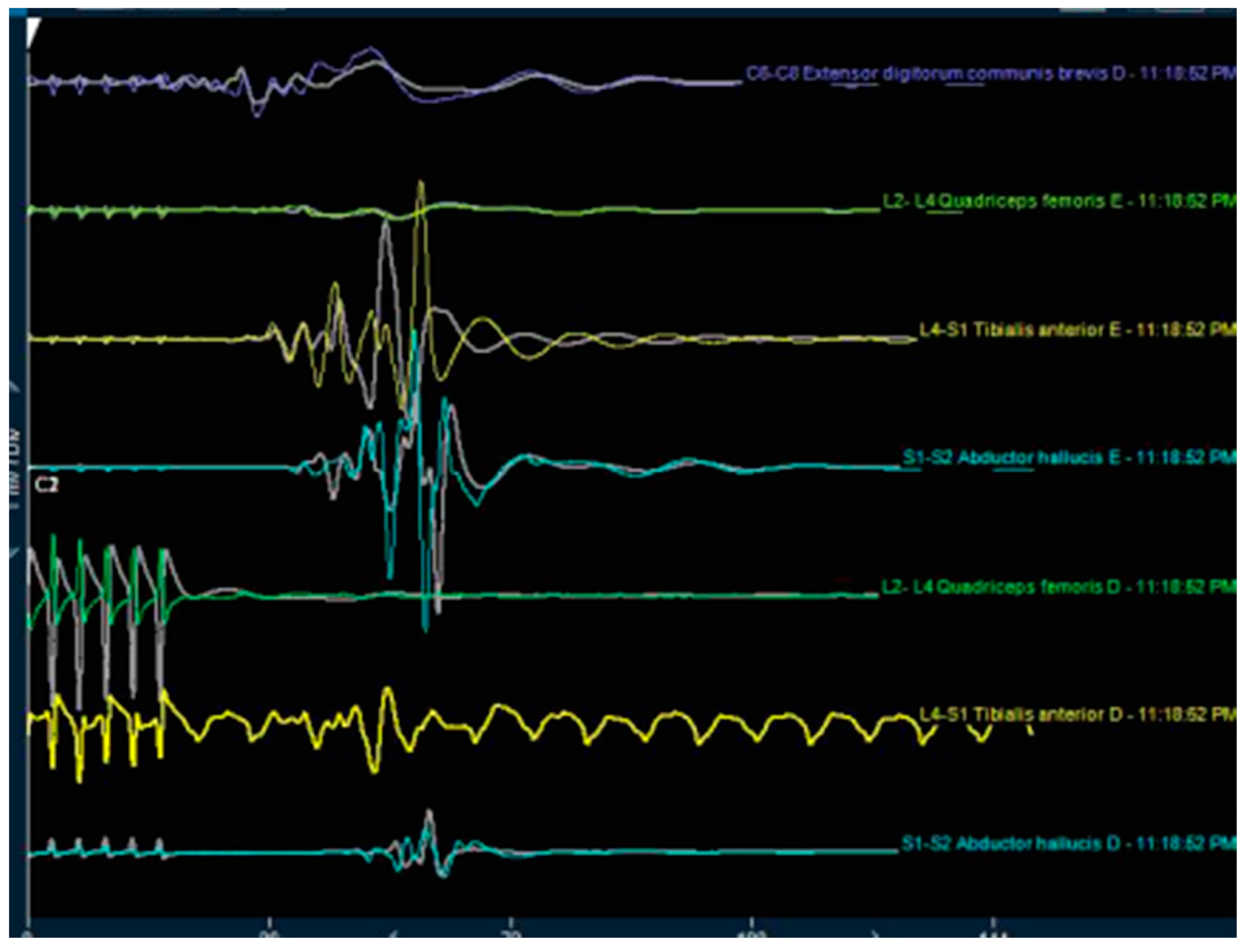
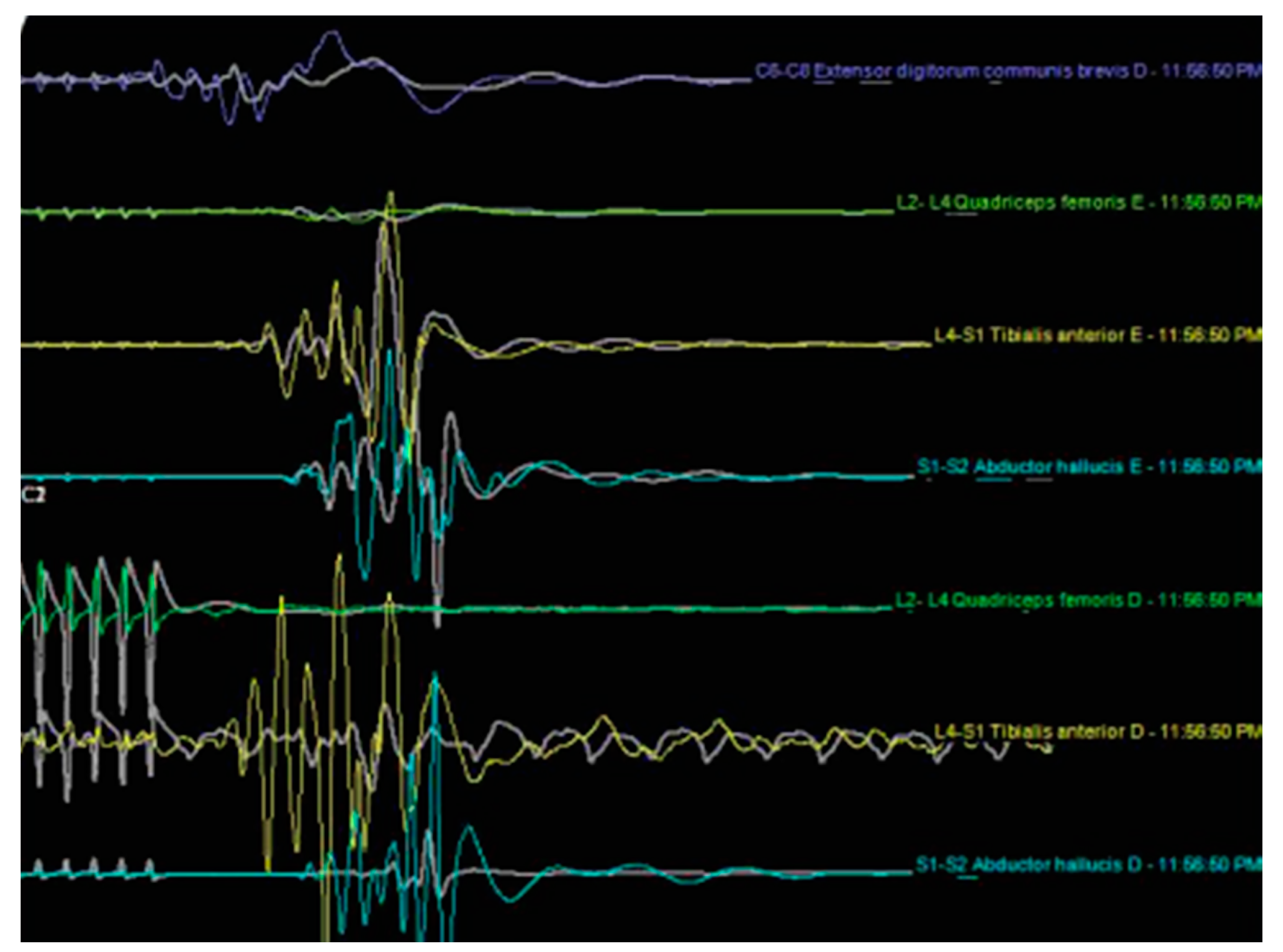
| Level | Number of Patients | Percent | Cumulative Percent |
|---|---|---|---|
| L2-L3 | 1 | 1.5 | 1.5 |
| L3-L4 | 6 | 9.2 | 10.8 |
| L3-L5 | 4 | 6.2 | 16.9 |
| L4-L5 | 29 | 44.6 | 61.5 |
| L4-S1 | 10 | 15.4 | 76.9 |
| L5-S1 | 15 | 23.1 | 100.0 |
| Total | 65 | 100.0 |
| Mean | Standard Deviation | Standard Error Mean | 95% Confidence Interval | t | Degree of Freedom | Significance (2-Tailed) | ||
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||
| ODI-Preop–ODI-Postop | 12.692 | 13.122 | 1.628 | 9.441 | 15.944 | 7.798 | 64 | <0.0001 |
| VAS-Back Preop–VAS-Back Postop | 1.723 | 1.206 | 0.150 | 1.424 | 2.022 | 11.523 | 64 | <0.0001 |
| VAS-Leg Preop–VAS-Leg Postop | 7.708 | 1.902 | 0.236 | 7.236 | 8.179 | 32.677 | 64 | <0.0001 |
| Neuromonitoring Modality | Mean Voltage (MV) | Number of Patients | Standard Deviation | Standard Error Mean | ||||
|---|---|---|---|---|---|---|---|---|
| SSEP Intraoperatively | 11.82 | 33 | 1.845 | 0.321 | ||||
| SSEP Postoperativly | 15.36 | 33 | 1.245 | 0.217 | ||||
| TCMEP Intraoperatively | 10.61 | 33 | 1.413 | 0.246 | ||||
| TCMEP Postoperatively | 17.64 | 33 | 0.962 | 0.168 | ||||
| Mean Voltage | Standard Deviation | Standard Error Mean | 95% Confidence Interval | t | df | Significance (2-tailed) | ||
| Lower | Upper | |||||||
| SSEP MV Intra–SSEP MV Postoperatively | −3.545 | 2.223 | 0.387 | −4.334 | −2.757 | −9.161 | 32 | <0.0001 |
| TCMEP MV intra–TCMEP MV Postoperatively | −7.030 | 1.311 | 0.228 | −7.495 | −6.566 | −30.814 | 32 | <0.0001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Carvalho, P.S.T.; Ramos, M.R.F.; da Silva Meireles, A.C.; Peixoto, A.; de Carvalho, P., Jr.; Ramírez León, J.F.; Yeung, A.; Lewandrowski, K.-U. Feasibility of Using Intraoperative Neuromonitoring in the Prophylaxis of Dysesthesia in Transforaminal Endoscopic Discectomies of the Lumbar Spine. Brain Sci. 2020, 10, 522. https://doi.org/10.3390/brainsci10080522
de Carvalho PST, Ramos MRF, da Silva Meireles AC, Peixoto A, de Carvalho P Jr., Ramírez León JF, Yeung A, Lewandrowski K-U. Feasibility of Using Intraoperative Neuromonitoring in the Prophylaxis of Dysesthesia in Transforaminal Endoscopic Discectomies of the Lumbar Spine. Brain Sciences. 2020; 10(8):522. https://doi.org/10.3390/brainsci10080522
Chicago/Turabian Stylede Carvalho, Paulo Sérgio Teixeira, Max Rogério Freitas Ramos, Alcy Caio da Silva Meireles, Alexandre Peixoto, Paulo de Carvalho, Jr., Jorge Felipe Ramírez León, Anthony Yeung, and Kai-Uwe Lewandrowski. 2020. "Feasibility of Using Intraoperative Neuromonitoring in the Prophylaxis of Dysesthesia in Transforaminal Endoscopic Discectomies of the Lumbar Spine" Brain Sciences 10, no. 8: 522. https://doi.org/10.3390/brainsci10080522
APA Stylede Carvalho, P. S. T., Ramos, M. R. F., da Silva Meireles, A. C., Peixoto, A., de Carvalho, P., Jr., Ramírez León, J. F., Yeung, A., & Lewandrowski, K.-U. (2020). Feasibility of Using Intraoperative Neuromonitoring in the Prophylaxis of Dysesthesia in Transforaminal Endoscopic Discectomies of the Lumbar Spine. Brain Sciences, 10(8), 522. https://doi.org/10.3390/brainsci10080522






