Evidence for Attentional Phenotypes in Infancy and Their Role in Visual Cognitive Performance
Abstract
:1. Introduction
2. Experiment 1
2.1. Methods
2.1.1. Participants
2.1.2. Stimuli and Procedure
2.1.3. Measures
2.1.4. Data Reduction
2.2. Results
2.2.1. Assessing Attentional Phenotypes at 11-Months-of-Age
2.2.2. Assessing the Stability of Attentional Phenotypes
2.3. Discussion
3. Experiment 2
3.1. Methods
3.1.1. Participants
3.1.2. Stimuli and Procedure
3.1.3. Data Reduction
3.2. Results
3.3. Discussion
4. General Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ross-Sheehy, S.; Schneegans, S.; Spencer, J.P. The Infant Orienting With Attention task: Assessing the neural basis of spatial attention in infancy. Infancy 2015, 20, 467–506. [Google Scholar] [CrossRef]
- Johnson, M.H.; de Haan, M. Vision, Orienting, and Attention. In Developmental Cognitive Neuroscience, 4th ed.; Blackwell Publishers Ltd.: Hoboken, NJ, USA, 2015; pp. 83–109. [Google Scholar]
- Lewis, T.L.; Maurer, D. The development of the temporal and nasal visual fields during infancy. Vis. Res. 1992, 32, 903–911. [Google Scholar] [CrossRef]
- Johnson, S. Cortical Maturation and the Development of Visual Attention in Early Infancy. J. Cogn. Neurosci. 1990, 2, 81–95. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, J. Human visual development over the first 6 months of life. A review and a hypothesis. Hum. Neurobiol. 1984, 3, 61–74. [Google Scholar] [PubMed]
- Hainline, L.; Turkel, J.; Abramov, I.; Lemerise, E.; Harris, C.M. Characteristics of saccades in human infants. Vis. Res. 1984, 24, 1771–1780. [Google Scholar] [CrossRef]
- Aslin, R.N. Models of Oculomotor Variability in Infancy. Monogr. Soc. Res. Child Dev. 1997, 62, 146–149. [Google Scholar] [CrossRef]
- Ohnson, M.H. The inhibition of automatic saccades in early infancy. Dev. Psychobiol. 1995, 28, 281–291. [Google Scholar] [CrossRef]
- Canfield, R.L.; Kirkham, N.Z. Infant Cortical Development and the Prospective Control of Saccadic Eye Movements. Infancy 2001, 2, 197–211. [Google Scholar] [CrossRef]
- Canfield, R.L.; Haith, M.M. Young infants’ visual expectations for symmetric and asymmetric stimulus sequences. Dev. Psychol. 1991, 27, 198–208. [Google Scholar] [CrossRef]
- Haith, M.M.; Hazan, C.; Goodman, G.S. Expectation and anticipation of dynamic visual events by 3.5-month-old babies. Child Dev. 1988, 59, 467–479. [Google Scholar] [CrossRef]
- Van Der Stigchel, S.; Van Koningsbruggen, M.; Nijboer, T.C.; List, A.; Rafal, R. The role of the frontal eye fields in the oculomotor inhibition of reflexive saccades: Evidence from lesion patients. Neuropsychologia 2012, 50, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Markant, J.C.; Amso, D. The Development of Selective Attention Orienting is an Agent of Change in Learning and Memory Efficacy. Infancy 2015, 21, 154–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Markant, J.C.; Amso, D. Selective memories: Infants’ encoding is enhanced in selection via suppression. Dev. Sci. 2013, 16, 926–940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ross-Sheehy, S.; Oakes, L.M.; Luck, S.J. Exogenous attention influences visual short-term memory in infants. Dev. Sci. 2010, 14, 490–501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ellemberg, D.; Lewis, T.L.; Maurer, D.; Lui, C.H.; Brent, H.P. Spatial and temporal vision in patients treated for bilateral congenital cataracts. Vis. Res. 1999, 39, 3480–3489. [Google Scholar] [CrossRef] [Green Version]
- Le Grand, R.; Mondloch, C.J.; Maurer, D.; Brent, H.P.; Grand, R.L. Impairment in Holistic Face Processing Following Early Visual Deprivation. Psychol. Sci. 2004, 15, 762–768. [Google Scholar] [CrossRef]
- Geldart, S.; Brent, H.P.; Mondloch, C.J.; Maurer, D.; De Schonen, S. The effect of early visual deprivation on the development of face processing. Dev. Sci. 2002, 5, 490–501. [Google Scholar] [CrossRef]
- De Heering, A.; Dormal, G.; Pelland, M.; Lewis, T.; Maurer, D.; Collignon, O. A Brief Period of Postnatal Visual Deprivation Alters the Balance between Auditory and Visual Attention. Curr. Boil. 2016, 26, 3101–3105. [Google Scholar] [CrossRef] [Green Version]
- Ross-Sheehy, S.; Perone, S.; Macek, K.L.; Eschman, B. Visual orienting and attention deficits in 5- and 10-month-old preterm infants. Infant Behav. Dev. 2017, 46, 80–90. [Google Scholar] [CrossRef] [Green Version]
- Rose, S.A.; Feldman, J.F.; Jankowski, J.J.; Rossem, R. Pathways from Prematurity and Infant Abilities to Later Cognition. Child Dev. 2005, 76, 1172–1184. [Google Scholar] [CrossRef]
- Rose, S.A.; Feldman, J.F.; Jankowski, J.J. Attention and recognition memory in the 1st year of life: A longitudinal study of preterm and full-term infants. Dev. Psychol. 2001, 37, 135–151. [Google Scholar] [CrossRef] [PubMed]
- Dutton, G.N. The spectrum of cerebral visual impairment as a sequel to premature birth: An overview. Doc. Ophthalmol. 2013, 127, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Leung, M.; Thompson, B.; Black, J.; Dai, S.; Alsweiler, J.M. The effects of preterm birth on visual development. Clin. Exp. Optom. 2017, 101, 4–12. [Google Scholar] [CrossRef] [Green Version]
- Ricci, D.; Cesarini, L.; Gallini, F.; Serrao, F.; Leone, D.; Baranello, G.; Cota, F.; Pane, M.; Brogna, C.; De Rose, P.; et al. Cortical Visual Function in Preterm Infants in the First Year. J. Pediatr. 2010, 156, 550–555. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.H.; de Haan, M. Developmental Cognitive Neuroscience, 4th ed.; Blackwell Publishing: Hoboken, NJ, USA, 2015. [Google Scholar]
- Tau, G.; Peterson, B.S. Normal Development of Brain Circuits. Neuropsychopharmacology 2009, 35, 147–168. [Google Scholar] [CrossRef] [Green Version]
- Leigh, R.J.; Kennard, C. Using saccades as a research tool in the clinical neurosciences. Brain 2004, 127, 460–477. [Google Scholar] [CrossRef]
- Sparks, D.L. The brainstem control of saccadic eye movements. Nat. Rev. Neurosci. 2002, 3, 952–964. [Google Scholar] [CrossRef]
- Knickmeyer, R.C.; Gouttard, S.; Kang, C.; Evans, D.; Wilber, K.; Smith, J.K.; Hamer, R.M.; Lin, W.; Gerig, G.; Gilmore, J.H. A structural MRI study of human brain development from birth to 2 years. J. Neurosci. 2008, 28, 12176–12182. [Google Scholar] [CrossRef]
- Otero-Millan, J.; Optican, L.M.; Macknik, S.L.; Martinez-Conde, S. Modeling the Triggering of Saccades, Microsaccades, and Saccadic Intrusions. Front. Neurol. 2018, 9. [Google Scholar] [CrossRef] [Green Version]
- Hafed, Z.M.; Clark, J.J. Microsaccades as an overt measure of covert attention shifts. Vis. Res. 2002, 42, 2533–2545. [Google Scholar] [CrossRef] [Green Version]
- Koch, C.; Ullman, S. Shifts in selective visual attention: Towards the underlying neural circuitry. Hum. Neurobiol. 1985, 4, 219–227. [Google Scholar] [PubMed]
- Banks, M.S.; Salapatek, P. Infant pattern vision: A new approach based on the contrast sensitivity function. J. Exp. Child Psychol. 1981, 31, 1–45. [Google Scholar] [CrossRef]
- Canfield, R.L.; Smith, E.G.; Brezsnyak, M.P.; Snow, K.L.; Aslin, R.N.; Haith, M.M.; Wass, T.S.; Adler, S.A. Information Processing Through the First Year of Life: A Longitudinal Study Using the Visual Expectation Paradigm. Monogr. Soc. Res. Child Dev. 1997, 62. [Google Scholar] [CrossRef]
- Skoczenski, A.M.; Norcia, A.M. Neural noise limitations on infant visual sensitivity. Nature 1998, 391, 697–700. [Google Scholar] [CrossRef] [PubMed]
- Banks, M.S.; Bennett, P.J. Optical and photoreceptor immaturities limit the spatial and chromatic vision of human neonates. J. Opt. Soc. Am. A 1988, 5, 2059–2079. Available online: http://www.ncbi.nlm.nih.gov/pubmed/3068345 (accessed on 11 June 2019). [CrossRef] [Green Version]
- Gould, J.F.; Colombo, J.; Collins, C.T.; Makrides, M.; Hewawasam, E.; Smithers, L.G. Assessing whether early attention of very preterm infants can be improved by an omega-3 long-chain polyunsaturated fatty acid intervention: A follow-up of a randomised controlled trial. BMJ Open 2018, 8, e020043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hood, B.M.; Atkinson, J. Disengaging visual attention in the infant and adult. Infant Behav. Dev. 1993, 16, 405–422. [Google Scholar] [CrossRef]
- Matsuzawa, M.; Shimojo, S. Infants’ fast saccades in the gap paradigm and development of visual attention. Infant Behav. Dev. 1997, 20, 449–455. [Google Scholar] [CrossRef]
- Csibra, G.; Tucker, L.; Johnson, S. Neural correlates of saccade planning in infants: A high-density ERP study. Int. J. Psychophysiol. 1998, 29, 201–215. [Google Scholar] [CrossRef]
- Kulke, L.V.; Atkinson, J.; Braddick, O. Neural Differences between Covert and Overt Attention Studied using EEG with Simultaneous Remote Eye Tracking. Front. Hum. Neurosci. 2016, 10. [Google Scholar] [CrossRef]
- Prado, E.L.; Maleta, K.; Caswell, B.L.; George, M.; Oakes, L.M.; DeBolt, M.C.; Bragg, M.G.; Arnold, C.D.; Iannotti, L.L.; Lutter, C.K.; et al. Early Child Development Outcomes of a Randomized Trial Providing 1 Egg Per Day to Children Age 6 to 15 Months in Malawi. J. Nutr. 2020. [Google Scholar] [CrossRef]
- Kulke, L.V.; Atkinson, J.; Braddick, O. Neural mechanisms of attention become more specialised during infancy: Insights from combined eye tracking and EEG. Dev. Psychobiol. 2016, 59, 250–260. [Google Scholar] [CrossRef] [PubMed]
- Colombo, J.; Mitchell, D.W.; Horowitz, F.D. Infant visual attention in the paired-comparison paradigm: Test-retest and attention-performance relations. Child Dev. 1988, 59, 1198–1210. [Google Scholar] [CrossRef] [PubMed]
- Bornstein, M.H.; Brown, E.; Slater, A. Patterns of stability and continuity in attention across early infancy. J. Reprod. Infant Psychol. 1996, 14, 195–206. [Google Scholar] [CrossRef]
- Colombo, J.; Fagen, J.W. Individual Differences in Infancy: Reliability, Stability, Prediction; Lawrence Erlbaum Associates, Inc.: Hillsdale, NJ, USA, 1990. [Google Scholar]
- Abelkop, B.S.; Frick, J.E. Cross-Task Stability in Infant Attention: New Perspectives Using the Still-Face Procedure. Infancy 2003, 4, 567–588. [Google Scholar] [CrossRef]
- Wass, S.; Forssman, L.; Leppänen, J. Robustness and Precision: How Data Quality May Influence Key Dependent Variables in Infant Eye-Tracker Analyses. Infancy 2014, 19, 427–460. [Google Scholar] [CrossRef]
- Olsen, A. The tobii i-vt fixation filter. Tobii Technol. 2012. [Google Scholar]
- Muthén, L.K.; Muthén, B.O. Mplus User’s Guide (1998-2017), 8th ed.; Muthén & Muthén: Los Angeles, CA, USA, 2017. [Google Scholar]
- Hallquist, M.N.; Wiley, J.F. MplusAutomation: An R Package for Facilitating Large-Scale Latent Variable Analyses in Mplus. Struct. Equ. Model. A Multidiscip. J. 2018, 25, 621–638. [Google Scholar] [CrossRef]
- Little, T.D.; Jorgensen, T.D.; Lang, K.M.; Moore, E.W.G. On the Joys of Missing Data. J. Pediatr. Psychol. 2013, 39, 151–162. [Google Scholar] [CrossRef] [Green Version]
- Ferguson, S.L.; Moore, E.W.G.; Hull, D.M. Finding latent groups in observed data: A primer on latent profile analysis in Mplus for applied researchers. Int. J. Behav. Dev. 2019. [Google Scholar] [CrossRef]
- Berlin, K.S.; Williams, N.A.; Parra, G.R. An Introduction to Latent Variable Mixture Modeling (Part 1): Overview and Cross-Sectional Latent Class and Latent Profile Analyses. J. Pediatr. Psychol. 2013, 39, 174–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tein, J.-Y.; Coxe, S.; Cham, H. Statistical Power to Detect the Correct Number of Classes in Latent Profile Analysis. Struct. Equ. Model. A Multidiscip. J. 2013, 20, 640–657. [Google Scholar] [CrossRef] [PubMed]
- Nylund, K.L.; Asparouhov, T.; Muthén, B.O. Deciding on the Number of Classes in Latent Class Analysis and Growth Mixture Modeling: A Monte Carlo Simulation Study. Struct. Equ. Model. A Multidiscip. J. 2007, 14, 535–569. [Google Scholar] [CrossRef]
- Ross-Sheehy, S.; Eschman, B. Assessing visual STM in infants and adults: Eye movements and pupil dynamics reflect memory maintenance. Vis. Cogn. 2019, 27, 78–92. [Google Scholar] [CrossRef]
- Schmidt, B.K.; Vogel, E.K.; Woodman, G.F.; Luck, S.J. Voluntary and automatic attentional control of visual working memory. Percept. Psychophys. 2002, 64, 754–763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robison, M.K.; Unsworth, N. Pupillometry tracks fluctuations in working memory performance. Attention, Perception, Psychophys. 2018, 81, 407–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atkinson, J.; Braddick, O.; Anker, S.; Nardini, M.; Birtles, D.; Rutherford, M.; Mercuri, E.; Dyet, L.E.; Edwards, A.D.; Cowan, F. Cortical vision, MRI and developmental outcome in preterm infants. Arch. Dis. Child Fetal Neonatal Ed. 2007, 93, F292–F297. [Google Scholar] [CrossRef] [Green Version]
- Holmboe, K.; Fearon, R.P.; Csibra, G.; Tucker, L.; Johnson, S. Freeze-Frame: A new infant inhibition task and its relation to frontal cortex tasks during infancy and early childhood. J. Exp. Child Psychol. 2008, 100, 89–114. [Google Scholar] [CrossRef]
- Fanari, R.; Meloni, C.; Massidda, D. Visual and Spatial Working Memory Abilities Predict Early Math Skills: A Longitudinal Study. Front. Psychol. 2019, 10. [Google Scholar] [CrossRef] [Green Version]
- Bull, R.; Espy, K.A.; Wiebe, S.A. Short-Term Memory, Working Memory, and Executive Functioning in Preschoolers: Longitudinal Predictors of Mathematical Achievement at Age 7 Years. Dev. Neuropsychol. 2008, 33, 205–228. [Google Scholar] [CrossRef] [Green Version]
- Kail, R.V. Longitudinal Evidence That Increases in Processing Speed and Working Memory Enhance Children’s Reasoning. Psychol. Sci. 2007, 18, 312–313. [Google Scholar] [CrossRef] [PubMed]
- Oakes, L.M.; Ross-Sheehy, S.; Luck, S.J. Rapid Development of Feature Binding in Visual Short-Term Memory. Psychol. Sci. 2006, 17, 781–787. [Google Scholar] [CrossRef] [PubMed]
- Ross-Sheehy, S.; Oakes, L.M.; Luck, S.J. The Development of Visual Short-Term Memory Capacity in Infants. Child Dev. 2003, 74, 1807–1822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raghubar, K.P.; Barnes, M.A.; Hecht, S.A. Working memory and mathematics: A review of developmental, individual difference, and cognitive approaches. Learn. Individ. Differ. 2010, 20, 110–122. [Google Scholar] [CrossRef]
- Kibbe, M.M. Varieties of Visual Working Memory Representation in Infancy and Beyond. Curr. Dir. Psychol. Sci. 2015, 24, 433–439. [Google Scholar] [CrossRef] [Green Version]
- Wass, S. Applying cognitive training to target executive functions during early development. Child Neuropsychol. 2015, 21, 150–166. [Google Scholar] [CrossRef]
- Rose, S.A.; Feldman, J.F.; Jankowski, J.J. Implications of Infant Cognition for Executive Functions at Age 11. Psychol. Sci. 2012, 23, 1345–1355. [Google Scholar] [CrossRef]
- Ross-Sheehy, S.; Eschman, B.; Reynolds, E. Looking within a look: Bottom-up and top-down influences on infant saccade and fixation dynamics during naturalistic viewing. Unpublished work. 2020. [Google Scholar]
- Johnson, K.A.; Robertson, I.H.; Barry, E.; Mulligan, A.; Dáibhis, A.; Daly, M.; Watchorn, A.; Gill, M.; Bellgrove, M.A. Impaired conflict resolution and alerting in children with ADHD: Evidence from the Attention Network Task (ANT). J. Child Psychol. Psychiatry 2008, 49, 1339–1347. [Google Scholar] [CrossRef]
- Visser, S.N.; Danielson, M.L.; Bitsko, R.H.; Holbrook, J.R.; Kogan, M.D.; Ghandour, R.M.; Perou, R.; Blumberg, S.J. Trends in the parent-report of health care provider-diagnosed and medicated attention-deficit/hyperactivity disorder: United States, 2003–2011. J. Am. Acad. Child Adolesc. Psychiatry 2013, 53, 34–46. [Google Scholar] [CrossRef] [Green Version]


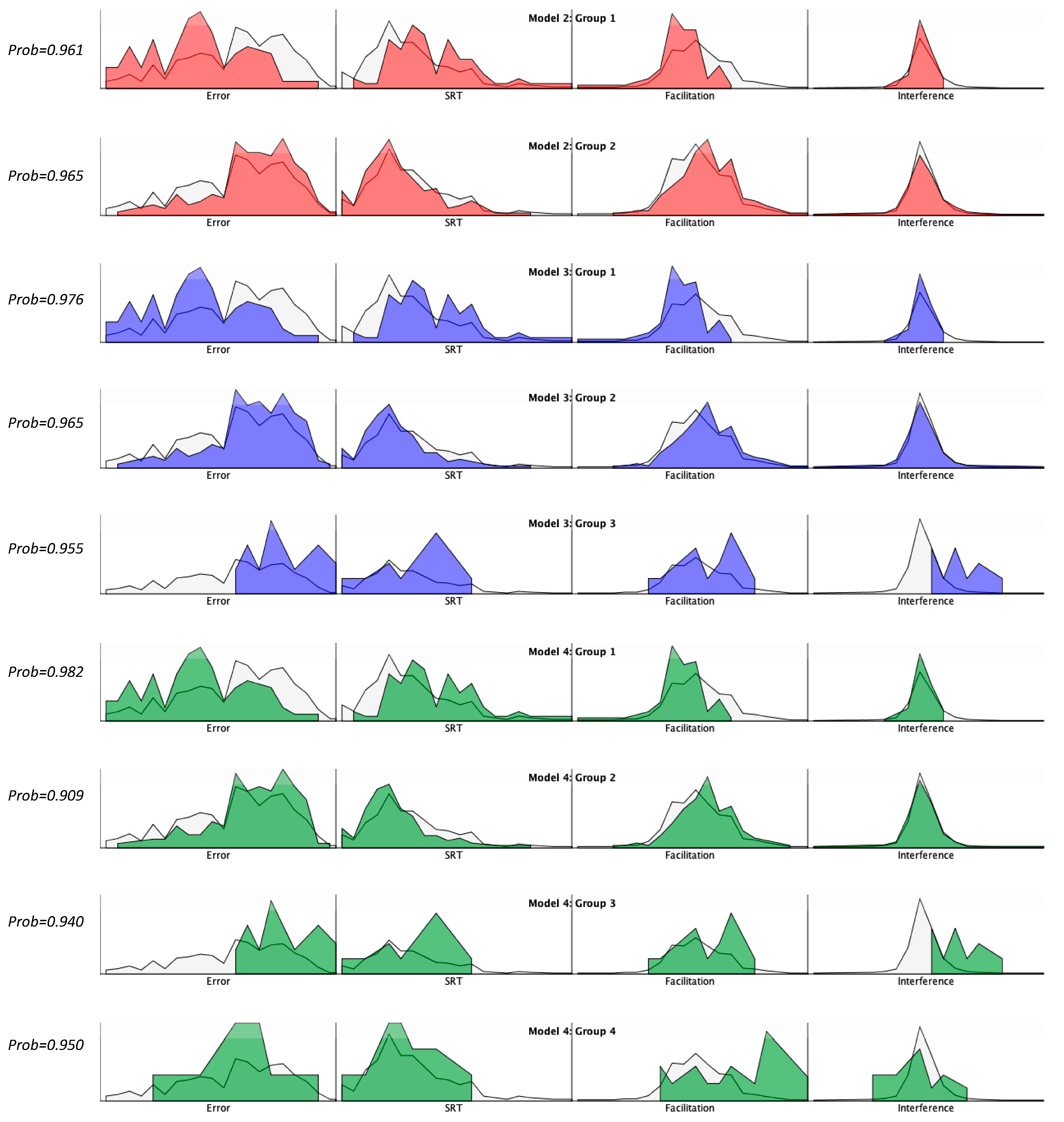




| Model | Profile Count (k) | logLik | AIC | BIC | Entropy | Smallest Profile (%) | LMR (p-Value) |
|---|---|---|---|---|---|---|---|
| m1 | 1 | −31.19 | 87.38 | 119.53 | |||
| m2 | 2 | 16.44 | 4913 | 56.94 | 0.854 | 38.7/61.3 | <0.000 |
| m3 | 3 | 42.62 | −32.24 | 38.24 | 0.892 | 5.4/55 | 0.306 |
| m4 | 4 | 65.52 | −64.04 | 24.11 | 0.909 | 5.4/49.6 | 0.358 |
| m5 | 5 | 80.93 | −80.87 | 27.94 | 0.896 | 4.5/46.9 | 0.223 |
| High Flexible | High Reactive | Low Reactive | |||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | ||
| 5 mos | Cue Facilitation | 0.356 | (0.307) | 0.449 | (0.502) | 0.248 | (0.252) |
| Cue Interference | 0.068 | (0.699) | 0.618 | (0.326) | 0.175 | (0.291) | |
| Cue Competition | 0.135 | (0.350) | −0.060 | (0.410) | 0.196 | (0.168) | |
| Baseline Error | 0.065 | (0.070) | 0.091 | (0.053) | 0.062 | (0.066) | |
| Task Error | 0.411 | (0.157) | 0.611 | (0.152) | 0.283 | (0.201) | |
| Mean SRT | 283.787 | (77.912) | 259.137 | (89.947) | 310.570 | (69.786) | |
| 8 mos | Cue Facilitation | 0.581 | (0.321) | 0.563 | (0.187) | 0.315 | (0.365) |
| Cue Interference | 0.328 | (0.865) | 0.705 | (0.599) | 0.223 | (0.308) | |
| Cue Competition | 0.283 | (0.208) | 0.730 | (0.782) | 0.202 | (0.239) | |
| Baseline Error | 0.062 | (0.067) | 0.030 | (0.026) | 0.047 | (0.058) | |
| Task Error | 0.544 | (0.118) | 0.586 | (0.143) | 0.361 | (0.213) | |
| Mean SRT | 243.302 | (59.068) | 267.673 | (110.289) | 314.393 | (106.944) | |
| 11 mos | Cue Facilitation | 0.725 | (0.297) | 0.756 | (0.256) | 0.318 | (0.160) |
| Cue Interference | −0.013 | (0.386) | 1.725 | (0.568) | 0.160 | (0.327) | |
| Cue Competition | 0.328 | (0.292) | 0.267 | (0.250) | 0.254 | (0.200) | |
| Baseline Error | 0.066 | (0.053) | 0.059 | (0.075) | 0.042 | (0.043) | |
| Task Error | 0.593 | (0.087) | 0.641 | (0.062) | 0.321 | (0.128) | |
| Mean SRT | 204.490 | (38.350) | 283.817 | (32.826) | 310.912 | (72.211) | |
| Cue Facilitation | Cue Interference | Cue Competition | Baseline Error | Task Error | Mean SRT | |
|---|---|---|---|---|---|---|
| Age | 0.029 | −0.016 | 0.03 | −0.01 | 0.01 | 0.677 |
| (−0.039) | (−0.078) | (−0.038) | (−0.008) | (−0.017) | (−8.752) | |
| High Flexible | −0.055 | 0.016 | −0.1 | −0.004 | 0.037 | 13.147 |
| (−0.117) | (−0.235) | (−0.115) | (−0.024) | (−0.055) | (−26.936) | |
| High Reactive | 0.034 | −0.309 | −0.179 | 0.017 | 0.267 * | −63.875 |
| (−0.231) | (−0.516) | (−0.227) | (−0.047) | (−0.107) | (−52.898) | |
| Age*High Flex. | 0.155 ** | −0.039 | 0.063 | 0.01 | 0.078 *** | −40.032 *** |
| (−0.048) | (−0.098) | (−0.048) | (−0.01) | (−0.022) | (−10.835) | |
| Age*High React. | 0.13 | 0.601 ** | 0.104 | −0.003 | 0.011 | 12.373 |
| (−0.097) | (−0.212) | (−0.097) | (−0.019) | (−0.043) | (−21.812) | |
| Constant | 0.238 * | 0.219 | 0.164 | 0.070 *** | 0.301 *** | 309.477 *** |
| (−0.097) | (−0.192) | (−0.094) | (−0.02) | (−0.045) | (−22.173) | |
| Observations | 240 | 224 | 240 | 240 | 240 | 240 |
| logLik | −38.277 | −186.163 | −29.951 | 344.025 | 141.288 | −1344.52 |
| AIC | 92.553 | 388.327 | 75.901 | −672.051 | −266.576 | 2705.04 |
| BIC | 120.398 | 415.62 | 103.746 | −644.206 | −238.731 | 2732.89 |
| Attention Score | Model | df | AIC | BIC | logLik | Test | L. Ratio | p-Value |
|---|---|---|---|---|---|---|---|---|
| Cue Facilitation | m1 | 4 | 139.56 | 153.48 | −65.78 | |||
| m2 | 8 | 92.55 | 120.40 | −38.28 | 1 vs. 2 | 55.01 | <0.001 | |
| Cue Interference | m1 | 4 | 426.39 | 440.04 | −209.20 | |||
| m2 | 8 | 388.33 | 415.62 | −186.16 | 1 vs. 2 | 46.07 | <0.001 | |
| Task Error | m1 | 4 | −181.52 | −167.60 | 94.76 | |||
| m2 | 8 | −266.58 | −238.73 | 141.29 | 1 vs. 2 | 93.06 | <0.001 | |
| Mean SRT | m1 | 4 | 2762.47 | 2776.40 | −1377.24 | |||
| m2 | 8 | 2705.04 | 2732.89 | −1344.52 | 1 vs. 2 | 65.43 | <0.001 | |
| Cue Competition | m1 | 4 | 71.47 | 85.39 | −31.73 | |||
| m2 | 8 | 75.90 | 103.75 | −29.95 | 1 vs. 2 | 3.57 | 0.468 | |
| Baseline Error | m1 | 4 | −674.80 | −660.88 | 341.40 | |||
| m2 | 8 | −672.05 | −644.21 | 344.03 | 1 vs. 2 | 5.25 | 0.263 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ross-Sheehy, S.; Reynolds, E.; Eschman, B. Evidence for Attentional Phenotypes in Infancy and Their Role in Visual Cognitive Performance. Brain Sci. 2020, 10, 605. https://doi.org/10.3390/brainsci10090605
Ross-Sheehy S, Reynolds E, Eschman B. Evidence for Attentional Phenotypes in Infancy and Their Role in Visual Cognitive Performance. Brain Sciences. 2020; 10(9):605. https://doi.org/10.3390/brainsci10090605
Chicago/Turabian StyleRoss-Sheehy, Shannon, Esther Reynolds, and Bret Eschman. 2020. "Evidence for Attentional Phenotypes in Infancy and Their Role in Visual Cognitive Performance" Brain Sciences 10, no. 9: 605. https://doi.org/10.3390/brainsci10090605
APA StyleRoss-Sheehy, S., Reynolds, E., & Eschman, B. (2020). Evidence for Attentional Phenotypes in Infancy and Their Role in Visual Cognitive Performance. Brain Sciences, 10(9), 605. https://doi.org/10.3390/brainsci10090605






