Brain Structural and Functional Alterations in Mice Prenatally Exposed to LPS Are Only Partially Rescued by Anti-Inflammatory Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. MIA Model
2.3. Drug Treatment
2.4. Offspring
2.5. Immunohistochemistry
2.6. Cortical Column Analysis
2.7. ELISA Test
2.8. Behavioral Testing
2.9. Pups Behavior
2.9.1. Ultrasonic Vocalizations in Pups
2.9.2. Homing Test
2.10. Juvenile Mice Behavior
Olfactory Habituation/Dishabituation Test
2.11. Adult Mice Behavior
2.11.1. Open Field Test
2.11.2. Reciprocal Social Interaction Test
2.11.3. Home Cage Observation Test
2.11.4. Marble Burying Test
2.12. Statistical Analysis
3. Results
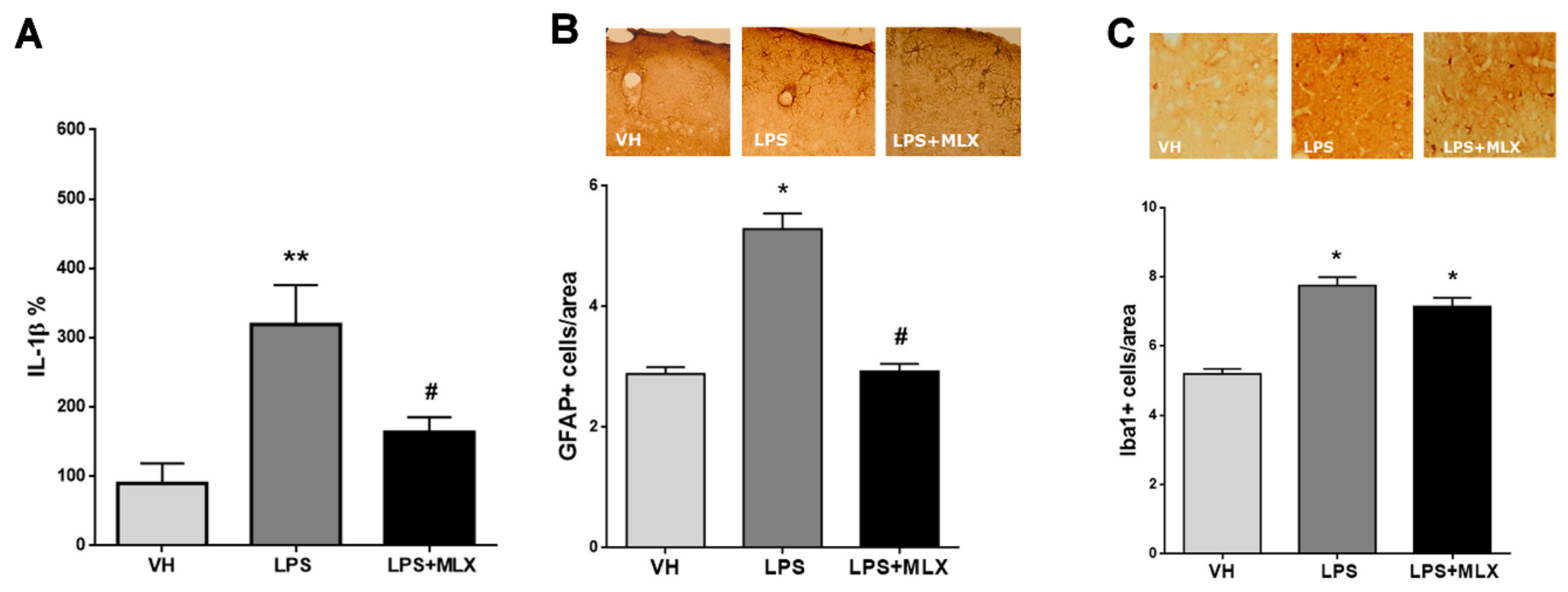
3.1. LPS Induced Both Peripheral and Central Inflammation, Partially Prevented by Meloxicam
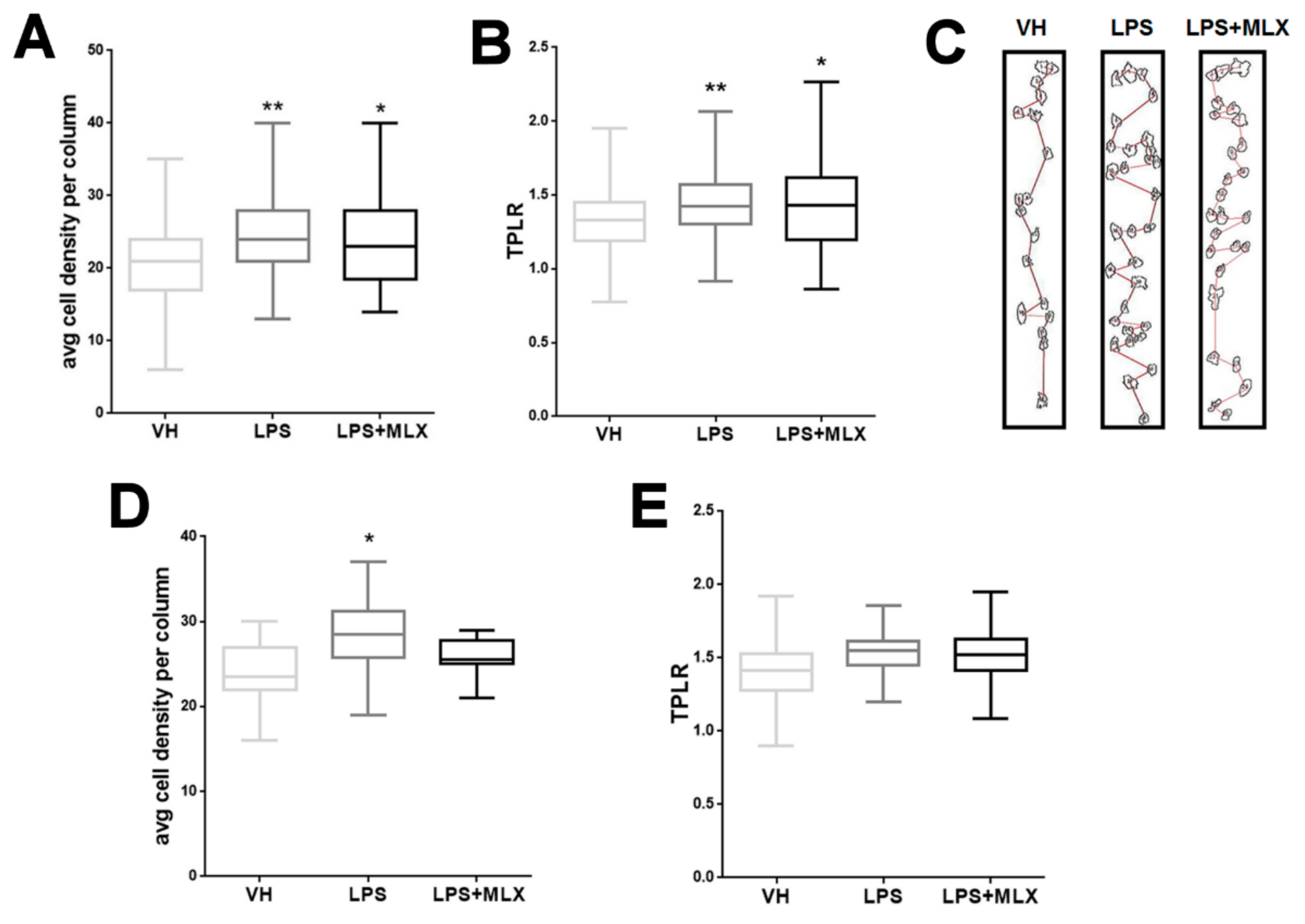
3.2. Abnormal Organization in the Cortex of Adult LPS-Mice
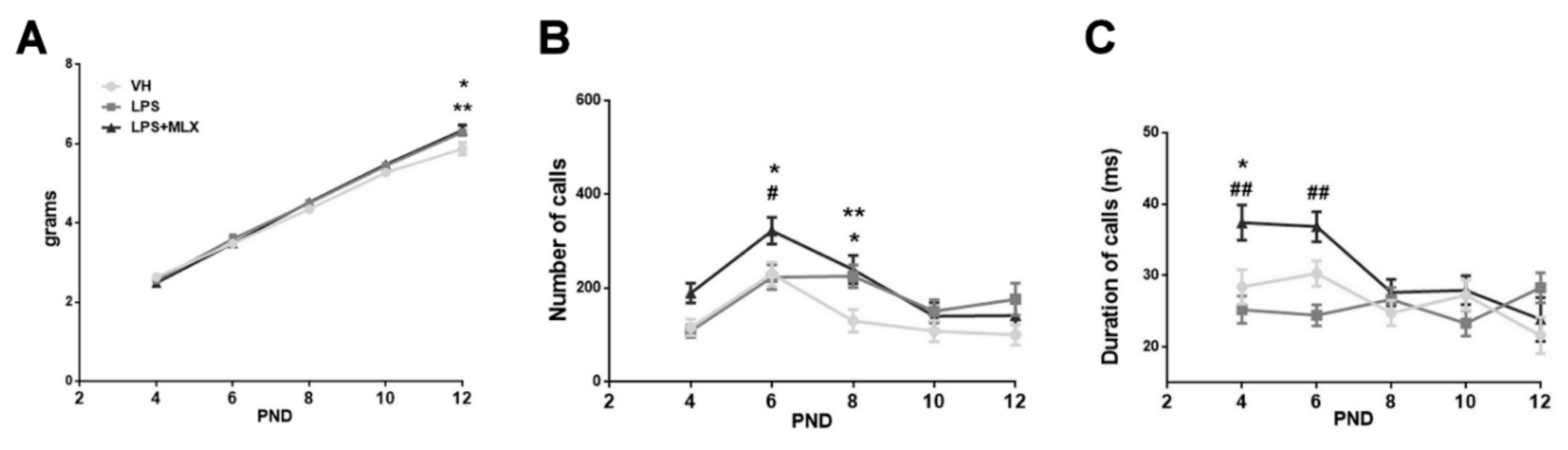
3.3. Effect of Prenatal LPS Exposure on Pups
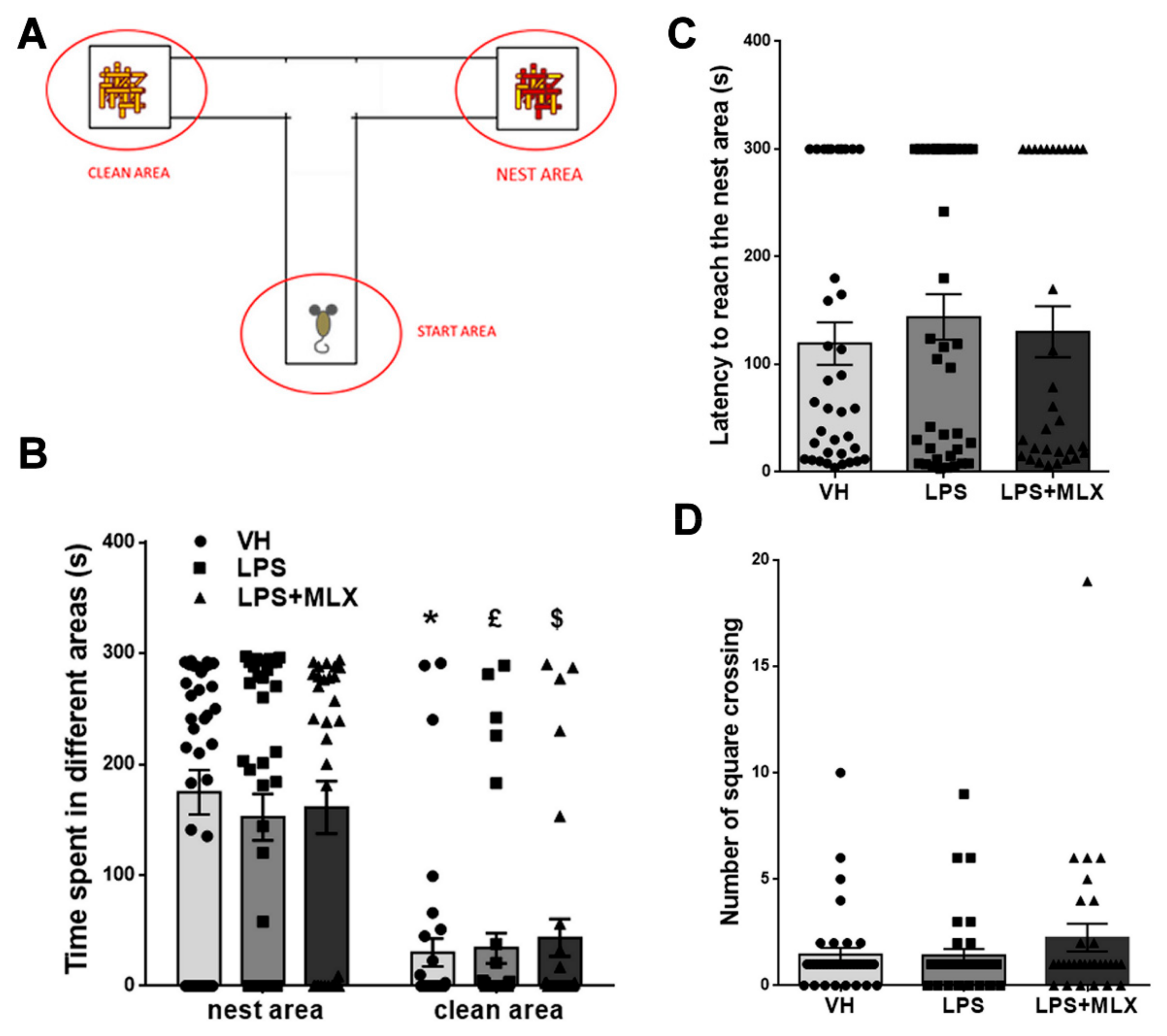
3.4. Prenatal LPS Exposure Does Not Affect Social Recognition and Motility in Pups
3.5. Olfactory Deficit in Juvenile LPS-Mice
3.6. Effect of Prenatal LPS Exposure on Adult Mice Behavior
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bonini, S.A.; Mastinu, A.; Ferrari-Toninelli, G.; Memo, M. Potential Role of Microtubule Stabilizing Agents in Neurodevelopmental Disorders. Int. J. Mol. Sci. 2017, 18, 1627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chow, J.; Jensen, M.; Amini, H.; Hormozdiari, F.; Penn, O.; Shifman, S.; Girirajan, S.; Hormozdiari, F. Dissecting the genetic basis of comorbid epilepsy phenotypes in neurodevelopmental disorders. Genome Med. 2019, 11, 65. [Google Scholar] [CrossRef] [Green Version]
- Thapar, A.; Cooper, M.; Rutter, M. Neurodevelopmental disorders. Lancet Psychiatry 2017, 4, 339–346. [Google Scholar] [CrossRef] [Green Version]
- Pletikos, M.; Sousa, A.M.; Sedmak, G.; Meyer, K.A.; Zhu, Y.; Cheng, F.; Li, M.; Kawasawa, Y.I.; Sestan, N. Temporal specification and bilaterality of human neocortical topographic gene expression. Neuron 2014, 81, 321–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberts, G.W.; Royston, M.C.; Gotz, M. Pathology of cortical development and neuropsychiatric disorders. Ciba Found. Symp. 1995, 193, 296–321. [Google Scholar]
- Stiles, J.; Jernigan, T.L. The basics of brain development. Neuropsychol. Rev. 2010, 20, 327–348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdallah, M.W.; Larsen, N.; Grove, J.; Norgaard-Pedersen, B.; Thorsen, P.; Mortensen, E.L.; Hougaard, D.M. Amniotic fluid inflammatory cytokines: Potential markers of immunologic dysfunction in autism spectrum disorders. World J. Biol. Psychiatry 2013, 14, 528–538. [Google Scholar] [CrossRef]
- Atladottir, H.O.; Thorsen, P.; Ostergaard, L.; Schendel, D.E.; Lemcke, S.; Abdallah, M.; Parner, E.T. Maternal infection requiring hospitalization during pregnancy and autism spectrum disorders. J. Autism Dev. Disord. 2010, 40, 1423–1430. [Google Scholar] [CrossRef]
- Brown, A.S. Epidemiologic studies of exposure to prenatal infection and risk of schizophrenia and autism. Dev. Neurobiol. 2012, 72, 1272–1276. [Google Scholar] [CrossRef] [Green Version]
- Clarke, M.C.; Tanskanen, A.; Huttunen, M.; Whittaker, J.C.; Cannon, M. Evidence for an interaction between familial liability and prenatal exposure to infection in the causation of schizophrenia. Am. J. Psychiatry 2009, 166, 1025–1030. [Google Scholar] [CrossRef]
- Dalton, P.; Deacon, R.; Blamire, A.; Pike, M.; McKinlay, I.; Stein, J.; Styles, P.; Vincent, A. Maternal neuronal antibodies associated with autism and a language disorder. Ann. Neurol. 2003, 53, 533–537. [Google Scholar] [CrossRef]
- Mortensen, P.B.; Pedersen, C.B.; Hougaard, D.M.; Norgaard-Petersen, B.; Mors, O.; Borglum, A.D.; Yolken, R.H. A Danish National Birth Cohort study of maternal HSV-2 antibodies as a risk factor for schizophrenia in their offspring. Schizophr. Res. 2010, 122, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Singer, H.S.; Morris, C.M.; Gause, C.D.; Gillin, P.K.; Crawford, S.; Zimmerman, A.W. Antibodies against fetal brain in sera of mothers with autistic children. J. Neuroimmunol. 2008, 194, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, H.J.; Mortensen, E.L.; Reinisch, J.M.; Mednick, S.A. Association between prenatal exposure to bacterial infection and risk of schizophrenia. Schizophr. Bull. 2009, 35, 631–637. [Google Scholar] [CrossRef] [Green Version]
- Bilbo, S.D.; Schwarz, J.M. Early-life programming of later-life brain and behavior: A critical role for the immune system. Front. Behav. Neurosci. 2009, 3, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boksa, P. Effects of prenatal infection on brain development and behavior: A review of findings from animal models. Brain Behav. Immun. 2010, 24, 881–897. [Google Scholar] [CrossRef]
- Harvey, L.; Boksa, P. Prenatal and postnatal animal models of immune activation: Relevance to a range of neurodevelopmental disorders. Dev. Neurobiol. 2012, 72, 1335–1348. [Google Scholar] [CrossRef]
- Meyer, U.; Feldon, J.; Dammann, O. Schizophrenia and autism: Both shared and disorder-specific pathogenesis via perinatal inflammation? Pediatr. Res. 2011, 69 Pt 2, 26R–33R. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef] [Green Version]
- Medzhitov, R. Inflammation 2010: New adventures of an old flame. Cell 2010, 140, 771–776. [Google Scholar] [CrossRef] [Green Version]
- Nathan, C.; Ding, A. Nonresolving inflammation. Cell 2010, 140, 871–882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Claycomb, K.I.; Johnson, K.M.; Winokur, P.N.; Sacino, A.V.; Crocker, S.J. Astrocyte regulation of CNS inflammation and remyelination. Brain Sci. 2013, 3, 1109–1127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deverman, B.E.; Patterson, P.H. Cytokines and CNS development. Neuron 2009, 64, 61–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garay, P.A.; McAllister, A.K. Novel roles for immune molecules in neural development: Implications for neurodevelopmental disorders. Front. Synaptic Neurosci. 2010, 2, 136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sloan, S.A.; Barres, B.A. Mechanisms of astrocyte development and their contributions to neurodevelopmental disorders. Curr. Opin. Neurobiol. 2014, 27, 75–81. [Google Scholar] [CrossRef] [Green Version]
- Cherry, J.D.; Olschowka, J.A.; O’Banion, M.K. Neuroinflammation and M2 microglia: The good, the bad, and the inflamed. J. Neuroinflamm. 2014, 11, 98. [Google Scholar] [CrossRef] [Green Version]
- Estes, M.L.; McAllister, A.K. Maternal immune activation: Implications for neuropsychiatric disorders. Science 2016, 353, 772–777. [Google Scholar] [CrossRef] [Green Version]
- Silverstein, A.M. The most elegant immunological experiment of the XIX century. Nat. Immunol. 2000, 1, 93–94. [Google Scholar] [CrossRef]
- Fatemi, S.H.; Earle, J.; Kanodia, R.; Kist, D.; Emamian, E.S.; Patterson, P.H.; Shi, L.; Sidwell, R. Prenatal viral infection leads to pyramidal cell atrophy and macrocephaly in adulthood: Implications for genesis of autism and schizophrenia. Cell. Mol. Neurobiol. 2002, 22, 25–33. [Google Scholar] [CrossRef]
- Fatemi, S.H.; Emamian, E.S.; Kist, D.; Sidwell, R.W.; Nakajima, K.; Akhter, P.; Shier, A.; Sheikh, S.; Bailey, K. Defective corticogenesis and reduction in Reelin immunoreactivity in cortex and hippocampus of prenatally infected neonatal mice. Mol. Psychiatry 1999, 4, 145–154. [Google Scholar] [CrossRef] [Green Version]
- Knuesel, I.; Chicha, L.; Britschgi, M.; Schobel, S.A.; Bodmer, M.; Hellings, J.A.; Toovey, S.; Prinssen, E.P. Maternal immune activation and abnormal brain development across CNS disorders. Nat. Rev. Neurol. 2014, 10, 643–660. [Google Scholar] [CrossRef] [PubMed]
- Urakubo, A.; Jarskog, L.F.; Lieberman, J.A.; Gilmore, J.H. Prenatal exposure to maternal infection alters cytokine expression in the placenta, amniotic fluid, and fetal brain. Schizophr. Res. 2001, 47, 27–36. [Google Scholar] [CrossRef]
- Kentner, A.C.; Bilbo, S.D.; Brown, A.S.; Hsiao, E.Y.; McAllister, A.K.; Meyer, U.; Pearce, B.D.; Pletnikov, M.V.; Yolken, R.H.; Bauman, M.D. Maternal immune activation: Reporting guidelines to improve the rigor, reproducibility, and transparency of the model. Neuropsychopharmacology 2019, 44, 245–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayer, U. Neurodevelopmental Resilience and Susceptibility to Maternal Immune Activation. Trends Neurosci. 2019, 42, 793–806. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.S.; Meyer, U. Maternal Immune Activation and Neuropsychiatric Illness: A Translational Research Perspective. Am. J. Psychiatry 2018, 175, 1073–1083. [Google Scholar] [CrossRef] [Green Version]
- Meyer, U.; Feldon, J. Neural basis of psychosis-related behaviour in the infection model of schizophrenia. Behav. Brain Res. 2009, 204, 322–334. [Google Scholar] [CrossRef]
- Richetto, J.; Calabrese, F.; Riva, M.A.; Meyer, U. Prenatal immune activation induces maturation-dependent alterations in the prefrontal GABAergic transcriptome. Schizophr. Bull. 2014, 40, 351–361. [Google Scholar] [CrossRef] [Green Version]
- Shallie, P.D.; Naicker, T. The placenta as a window to the brain: A review on the role of placental markers in prenatal programming of neurodevelopment. Int. J. Dev. Neurosci. 2019, 73, 41–49. [Google Scholar] [CrossRef]
- Cui, K.; Ashdown, H.; Luheshi, G.N.; Boksa, P. Effects of prenatal immune activation on hippocampal neurogenesis in the rat. Schizophr. Res. 2009, 113, 288–297. [Google Scholar] [CrossRef]
- Golan, H.M.; Lev, V.; Hallak, M.; Sorokin, Y.; Huleihel, M. Specific neurodevelopmental damage in mice offspring following maternal inflammation during pregnancy. Neuropharmacology 2005, 48, 903–917. [Google Scholar] [CrossRef]
- Mastinu, A.; Bonini, S.A.; Rungratanawanich, W.; Aria, F.; Marziano, M.; Maccarinelli, G.; Abate, G.; Premoli, M.; Memo, M.; Uberti, D. Gamma-oryzanol Prevents LPS-induced Brain Inflammation and Cognitive Impairment in Adult Mice. Nutrients 2019, 11, 728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ortega, A.; Jadeja, V.; Zhou, H. Postnatal development of lipopolysaccharide-induced inflammatory response in the brain. Inflamm. Res. 2011, 60, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Pan, Z.L.; Pang, Y.; Evans, O.B.; Rhodes, P.G. Cytokine induction in fetal rat brains and brain injury in neonatal rats after maternal lipopolysaccharide administration. Pediatr. Res. 2000, 47, 64–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, F.R.; Brogan, A.; Smith, R.; Hodgson, D.M. A profile of the immediate endocrine, metabolic and behavioural responses following a dual exposure to endotoxin in early life. Physiol. Behav. 2004, 83, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Hodyl, N.A.; Walker, F.R.; Krivanek, K.M.; Clifton, V.L.; Hodgson, D.M. Prenatal endotoxin exposure alters behavioural pain responses to lipopolysaccharide in adult offspring. Physiol. Behav. 2010, 100, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Wischhof, L.; Irrsack, E.; Osorio, C.; Koch, M. Prenatal LPS-exposure—A neurodevelopmental rat model of schizophrenia—Differentially affects cognitive functions, myelination and parvalbumin expression in male and female offspring. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2015, 57, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Vane, J.R.; Bakhle, Y.S.; Botting, R.M. Cyclooxygenases 1 and 2. Annu. Rev. Pharmacol. Toxicol. 1998, 38, 97–120. [Google Scholar] [CrossRef] [PubMed]
- O’Banion, M.K.; Olschowka, J.A. Localization and distribution of cyclooxygenase-2 in brain tissue by immunohistochemistry. Methods Mol. Biol. 1999, 120, 55–66. [Google Scholar]
- Burdan, F.; Pliszczynska-Steuden, M.; Rozylo-Kalinowska, I.; Chalas, A.; Rozylo, T.K.; Staroslawska, E.; Klepacz, R.; Szumilo, J. Developmental outcome after exposure to cyclooxygenase inhibitors during pregnancy and lactation. Reprod. Toxicol. 2011, 32, 407–417. [Google Scholar] [CrossRef]
- Cappon, G.D.; Cook, J.C.; Hurtt, M.E. Relationship between cyclooxygenase 1 and 2 selective inhibitors and fetal development when administered to rats and rabbits during the sensitive periods for heart development and midline closure. Birth Defects Res. Part B Dev. Reprod. Toxicol. 2003, 68, 47–56. [Google Scholar] [CrossRef]
- Cella, M.; Farina, M.G.; Dominguez Rubio, A.P.; Di Girolamo, G.; Ribeiro, M.L.; Franchi, A.M. Dual effect of nitric oxide on uterine prostaglandin synthesis in a murine model of preterm labour. Br. J. Pharmacol. 2010, 161, 844–855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cook, J.C.; Jacobson, C.F.; Gao, F.; Tassinari, M.S.; Hurtt, M.E.; DeSesso, J.M. Analysis of the nonsteroidal anti-inflammatory drug literature for potential developmental toxicity in rats and rabbits. Birth Defects Res. Part B Dev. Reprod. Toxicol. 2003, 68, 5–26. [Google Scholar] [CrossRef] [PubMed]
- Streck, R.D.; Kumpf, S.W.; Ozolins, T.R.; Stedman, D.B. Rat embryos express transcripts for cyclooxygenase-1 and carbonic anhydrase-4, but not for cyclooxygenase-2, during organogenesis. Birth Defects Res. Part B Dev. Reprod. Toxicol. 2003, 68, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Minghetti, L. Cyclooxygenase-2 (COX-2) in inflammatory and degenerative brain diseases. J. Neuropathol. Exp. Neurol. 2004, 63, 901–910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitchell, J.A.; Akarasereenont, P.; Thiemermann, C.; Flower, R.J.; Vane, J.R. Selectivity of nonsteroidal antiinflammatory drugs as inhibitors of constitutive and inducible cyclooxygenase. Proc. Natl. Acad. Sci. USA 1993, 90, 11693–11697. [Google Scholar] [CrossRef] [Green Version]
- Borrell, J.; Vela, J.M.; Arevalo-Martin, A.; Molina-Holgado, E.; Guaza, C. Prenatal immune challenge disrupts sensorimotor gating in adult rats. Implications for the etiopathogenesis of schizophrenia. Neuropsychopharmacology 2002, 26, 204–215. [Google Scholar] [CrossRef] [Green Version]
- Engelhardt, G.; Bogel, R.; Schnitzer, C.; Utzmann, R. Meloxicam: Influence on arachidonic acid metabolism. Part 1. In Vitro findings. Biochem. Pharmacol. 1996, 51, 21–28. [Google Scholar] [CrossRef]
- Engelhardt, G.; Bogel, R.; Schnitzler, C.; Utzmann, R. Meloxicam: Influence on arachidonic acid metabolism. Part II. In Vivo findings. Biochem. Pharmacol. 1996, 51, 29–38. [Google Scholar] [CrossRef]
- Lee, P.R.; Kim, S.R.; Jung, B.K.; Kim, K.R.; Chung, J.Y.; Won, H.S.; Kim, A. Therapeutic effect of cyclo-oxygenase inhibitors with different isoform selectivity in lipopolysaccharide-induced preterm birth in mice. Am. J. Obstet. Gynecol. 2003, 189, 261–266. [Google Scholar] [CrossRef]
- Bonini, S.A.; Mastinu, A.; Maccarinelli, G.; Mitola, S.; Premoli, M.; La Rosa, L.R.; Ferrari-Toninelli, G.; Grilli, M.; Memo, M. Cortical Structure Alterations and Social Behavior Impairment in p50-Deficient Mice. Cereb. Cortex 2016, 26, 2832–2849. [Google Scholar] [CrossRef]
- Gaudissard, J.; Ginger, M.; Premoli, M.; Memo, M.; Frick, A.; Pietropaolo, S. Behavioral abnormalities in the Fmr1-KO2 mouse model of fragile X syndrome: The relevance of early life phases. Autism Res. 2017, 10, 1584–1596. [Google Scholar] [CrossRef] [PubMed]
- Premoli, M.; Bonini, S.A.; Mastinu, A.; Maccarinelli, G.; Aria, F.; Paiardi, G.; Memo, M. Specific profile of ultrasonic communication in a mouse model of neurodevelopmental disorders. Sci. Rep. 2019, 9, 15912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scattoni, M.L.; Gandhy, S.U.; Ricceri, L.; Crawley, J.N. Unusual repertoire of vocalizations in the BTBR T+tf/J mouse model of autism. PLoS ONE 2008, 3, e3067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, M.; Crawley, J.N. Simple behavioral assessment of mouse olfaction. Curr. Protoc. Neurosci. 2009, 48, 8–24. [Google Scholar] [CrossRef] [PubMed]
- Mastinu, A.; Premoli, M.; Maccarinelli, G.; Grilli, M.; Memo, M.; Bonini, S.A. Melanocortin 4 receptor stimulation improves social deficits in mice through oxytocin pathway. Neuropharmacology 2018, 133, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Silverman, J.L.; Yang, M.; Lord, C.; Crawley, J.N. Behavioural phenotyping assays for mouse models of autism. Nat. Rev. Neurosci. 2010, 11, 490–502. [Google Scholar] [CrossRef] [Green Version]
- Kedia, S.; Chattarji, S. Marble burying as a test of the delayed anxiogenic effects of acute immobilisation stress in mice. J. Neurosci. Methods 2014, 233, 150–154. [Google Scholar] [CrossRef]
- Patterson, P.H. Immune involvement in schizophrenia and autism: Etiology, pathology and animal models. Behav. Brain Res. 2009, 204, 313–321. [Google Scholar] [CrossRef]
- Adams, R.; David, A.S. Patterns of anterior cingulate activation in schizophrenia: A selective review. Neuropsychiatr. Dis. Treat. 2007, 3, 87–101. [Google Scholar] [CrossRef] [Green Version]
- Casanova, M.F.; van Kooten, I.A.; Switala, A.E.; van Engeland, H.; Heinsen, H.; Steinbusch, H.W.; Hof, P.R.; Trippe, J.; Stone, J.; Schmitz, C. Minicolumnar abnormalities in autism. Acta Neuropathol. 2006, 112, 287–303. [Google Scholar] [CrossRef]
- Drevets, W.C.; Savitz, J.; Trimble, M. The subgenual anterior cingulate cortex in mood disorders. CNS Spectr. 2008, 13, 663–681. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, D.P.; Jones, K.A.; Woolfrey, K.M.; Burgdorf, J.; Russell, T.A.; Kalmbach, A.; Lee, H.; Yang, C.; Bradberry, M.M.; Wokosin, D.; et al. Social, communication, and cortical structural impairments in Epac2-deficient mice. J. Neurosci. 2012, 32, 11864–11878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ingvorsen, C.; Brix, S.; Ozanne, S.E.; Hellgren, L.I. The effect of maternal Inflammation on foetal programming of metabolic disease. Acta Physiol. 2015, 214, 440–449. [Google Scholar] [CrossRef] [PubMed]
- Labouesse, M.A.; Langhans, W.; Meyer, U. Long-term pathological consequences of prenatal infection: Beyond brain disorders. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 309, R1–R12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyer, U.; Yee, B.K.; Feldon, J. The neurodevelopmental impact of prenatal infections at different times of pregnancy: The earlier the worse? Neuroscientist 2007, 13, 241–256. [Google Scholar] [CrossRef]
- Meyer, U.; Nyffeler, M.; Engler, A.; Urwyler, A.; Schedlowski, M.; Knuesel, I.; Yee, B.K.; Feldon, J. The time of prenatal immune challenge determines the specificity of inflammation-mediated brain and behavioral pathology. J. Neurosci. 2006, 26, 4752–4762. [Google Scholar] [CrossRef] [Green Version]
- Patterson, P.H. Maternal infection and immune involvement in autism. Trends Mol. Med. 2011, 17, 389–394. [Google Scholar] [CrossRef] [Green Version]
- Hagberg, H.; Gressens, P.; Mallard, C. Inflammation during fetal and neonatal life: Implications for neurologic and neuropsychiatric disease in children and adults. Ann. Neurol. 2012, 71, 444–457. [Google Scholar] [CrossRef]
- Fatemi, S.H.; Reutiman, T.J.; Folsom, T.D.; Huang, H.; Oishi, K.; Mori, S.; Smee, D.F.; Pearce, D.A.; Winter, C.; Sohr, R.; et al. Maternal infection leads to abnormal gene regulation and brain atrophy in mouse offspring: Implications for genesis of neurodevelopmental disorders. Schizophr. Res. 2008, 99, 56–70. [Google Scholar] [CrossRef] [Green Version]
- Patrich, E.; Piontkewitz, Y.; Peretz, A.; Weiner, I.; Attali, B. Maturation- and sex sensitive depression of hippocampal excitatory transmission in a rat schizophrenia model. Brain Behav. Immun. 2016, 51, 240–251. [Google Scholar] [CrossRef]
- Piontkewitz, Y.; Arad, M.; Weiner, I. Risperidone administered during asymptomatic period of adolescence prevents the emergence of brain structural pathology and behavioral abnormalities in an animal model of schizophrenia. Schizophr. Bull. 2011, 37, 1257–1269. [Google Scholar] [CrossRef] [Green Version]
- Short, S.J.; Lubach, G.R.; Karasin, A.I.; Olsen, C.W.; Styner, M.; Knickmeyer, R.C.; Gilmore, J.H.; Coe, C.L. Maternal influenza infection during pregnancy impacts postnatal brain development in the rhesus monkey. Biol. Psychiatry 2010, 67, 965–973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crum, W.R.; Sawiak, S.J.; Chege, W.; Cooper, J.D.; Williams, S.C.R.; Vernon, A.C. Evolution of structural abnormalities in the rat brain following in utero exposure to maternal immune activation: A longitudinal in vivo MRI study. Brain Behav. Immun. 2017, 63, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Giovanoli, S.; Engler, H.; Engler, A.; Richetto, J.; Feldon, J.; Riva, M.A.; Schedlowski, M.; Meyer, U. Preventive effects of minocycline in a neurodevelopmental two-hit model with relevance to schizophrenia. Transl. Psychiatry 2016, 6, e772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giovanoli, S.; Notter, T.; Richetto, J.; Labouesse, M.A.; Vuillermot, S.; Riva, M.A.; Meyer, U. Late prenatal immune activation causes hippocampal deficits in the absence of persistent inflammation across aging. J. Neuroinflamm. 2015, 12, 221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garay, P.A.; Hsiao, E.Y.; Patterson, P.H.; McAllister, A.K. Maternal immune activation causes age- and region-specific changes in brain cytokines in offspring throughout development. Brain Behav. Immun. 2013, 31, 54–68. [Google Scholar] [CrossRef] [Green Version]
- Mattei, D.; Djodari-Irani, A.; Hadar, R.; Pelz, A.; Fernandez de Cossío, L.; Goetz, T.; Matyash, M.; Kettenmann, H.; Winter, C.; Wolf, S.A. Minocycline rescues decrease in neurogenesis, increase in microglia cytokines and deficits in sensorimotor gating in an animal model of schizophrenia. Brain Behav. Immun. 2014, 38, 175–184. [Google Scholar] [CrossRef] [Green Version]
- Choi, G.B.; Yim, Y.S.; Wong, H.; Kim, S.; Kim, H.; Kim, S.V.; Hoeffer, C.A.; Littman, D.R.; Huh, J.R. The maternal interleukin-17a pathway in mice promotes autism-like phenotypes in offspring. Science 2016, 351, 933–939. [Google Scholar] [CrossRef] [Green Version]
- Smith, S.E.; Li, J.; Garbett, K.; Mirnics, K. Patterson PH. Maternal immune activation alters fetal brain development through interleukin-6. J. Neurosci. 2007, 27, 10695–10702. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Yang, J.; Zhang, H.; Yu, J.; Yao, Z. Oral probiotic administration duringpregnancy prevents autism-related behaviors in offspring induced by maternal im-mune activation via anti-inflammation in mice. Autism Res. 2019, 12, 576–588. [Google Scholar] [CrossRef]
- Labrousse, V.F.; Leyrolle, Q.; Amadieu, C.; Aubert, A.; Sere, A.; Coutureau, E.; Gregoire, S.; Bretillon, L.; Pallet, V.; Gressens, P.; et al. Dietaryomega-3 deficiency exacerbates inflammation and reveals spatial memory deficits inmice exposed to lipopolysaccharide during gestation. Brain Behav. Immun. 2018, 73, 427–440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casanova, M.F.; van Kooten, I.; Switala, A.E.; van Engeland, H.; Heinsen, H.; Steinbusch, H.W.M.; Hof, P.R.; Schmitz, C. Abnormalities of cortical minicolumnar organization in the prefrontal lobes of autistic patients. Clin. Neurosci. Res. 2006, 6, 127–133. [Google Scholar] [CrossRef]
- Chelini, G.; Zerbi, V.; Cimino, L.; Grigoli, A.; Markicevic, M.; Libera, F.; Robbiati, S.; Gadler, M.; Bronzoni, S.; Miorelli, S.; et al. Aberrant Somatosensory Processing and Connectivity in Mice Lacking Engrailed-2. J. Neurosci. 2019, 39, 1525–1538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yizhar, O.; Fenno, L.E.; Prigge, M.; Schneider, F.; Davidson, T.J.; O’Shea, D.J.; Sohal, V.S.; Goshen, I.; Finkelstein, J.; Paz, J.T.; et al. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature 2011, 477, 171–178. [Google Scholar] [CrossRef]
- da Silveira, V.T.; Medeiros, D.C.; Ropke, J.; Guidine, P.A.; Rezende, G.H.; Moraes, M.F.; Mendes, E.M.; Macedo, D.; Moreira, F.A.; de Oliveira, A.C. Effects of early or late prenatal immune activation in mice on behavioral and neuroanatomical abnormalities relevant to schizophrenia in the adulthood. Int. J. Dev. Neurosci. 2017, 58, 1–8. [Google Scholar] [CrossRef]
- Richetto, J.; Chesters, R.; Cattaneo, A.; Labouesse, M.A.; Gutierrez, A.M.C.; Wood, T.C.; Luoni, A.; Meyer, U.; Vernon, A.; Riva, M.A. Genome-wide transcriptional profiling and structural magnetic resonance imaging in the maternal immune activation model of neurodevelopmental disorders. Cereb. Cortex 2017, 27, 3397–3413. [Google Scholar] [CrossRef]
- Naviaux, R.K.; Zolkipli, Z.; Wang, L.; Nakayama, T.; Naviaux, J.C.; Le, T.P.; Schuchbauer, M.A.; Rogac, M.; Tang, Q.; Dugan, L.L.; et al. Antipurinergic therapy corrects the autism-like features in the poly(IC) mouse model. PLoS ONE 2013, 8, e57380. [Google Scholar] [CrossRef]
- Shi, L.; Smith, S.E.; Malkova, N.; Tse, D.; Su, Y.; Patterson, P.H. Activation of the maternal immune system alters cerebellar development in the offspring. Brain Behav. Immun. 2009, 23, 116–123. [Google Scholar] [CrossRef] [Green Version]
- Donegan, J.J.; Boley, A.M.; Lodge, D.J. Embryonic stem cell transplants as a therapeutic strategy in a rodent model of autism. Neuropsychopharmacology 2018, 43, 1789–1798. [Google Scholar] [CrossRef] [Green Version]
- Matsuura, A.; Ishima, T.; Fujita, Y.; Iwayama, Y.; Hasegawa, S.; Kawahara-Miki, R.; Maekawa, M.; Toyoshima, M.; Ushida, Y.; Suganuma, H.; et al. Dietary glucoraphanin prevents the onset of psychosis in the adult offspring after maternal immune activation. Sci. Rep. 2018, 8, 2158. [Google Scholar] [CrossRef] [Green Version]
- Wischhof, L.; Irrsack, E.; Dietz, F.; Koch, M. Maternal lipopolysaccharide treatment differentially affects 5-HT2A and mGlu2/3 receptor function in the adult male and female rat offspring. Neuropharmacology 2015, 97, 275–288. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; van Praag, H. Maternal immune activation differentially impacts mature and adult-born hippocampal neurons in male mice. Brain Behav. Immun. 2015, 45, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Antonson, A.M.; Balakrishnan, B.; Radlowski, E.C.; Petr, G.; Johnson, R.W. Altered hippocampal gene expression and morphology in fetal piglets following maternal respiratory viral infection. Dev. Neurosci. 2018, 40, 104–119. [Google Scholar] [CrossRef] [PubMed]
- Duchatel, R.J.; Jobling, P.; Graham, B.A.; Harms, L.R.; Michie, P.T.; Hodgson, D.M.; Tooney, P.A. Increased white matter neuron density in a rat model of maternal immune activation-Implications for schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 2016, 65, 118–126. [Google Scholar] [CrossRef]
- Nouel, D.; Burt, M.; Zhang, Y.; Harvey, L.; Boksa, P. Prenatal exposure to bacterial endotoxin reduces the number of GAD67- and reelin-immunoreactive neurons in the hippocampus of rat offspring. Eur. Neuropsychopharmacol. 2012, 22, 300–307. [Google Scholar] [CrossRef]
- Meyer, U.; Nyffeler, M.; Yee, B.K.; Knuesel, I.; Feldon, J. Adult brain and behavioral pathological markers of prenatal immune challenge during early/middle and late fetal development in mice. Brain Behav. Immun. 2008, 22, 469–486. [Google Scholar] [CrossRef]
- Branchi, I.; Santucci, D.; Alleva, E. Ultrasonic vocalisation emitted by infant rodents: A tool for assessment of neurobehavioural development. Behav. Brain Res. 2001, 125, 49–56. [Google Scholar] [CrossRef]
- Scattoni, M.L.; Crawley, J.; Ricceri, L. Ultrasonic vocalizations: A tool for behavioural phenotyping of mouse models of neurodevelopmental disorders. Neurosci. Biobehav. Rev. 2009, 33, 508–515. [Google Scholar] [CrossRef] [Green Version]
- Wohr, M.; Scattoni, M.L. Behavioural methods used in rodent models of autism spectrum disorders: Current standards and new developments. Behav. Brain Res. 2013, 251, 5–17. [Google Scholar] [CrossRef]
- Baharnoori, M.; Bhardwaj, S.K.; Srivastava, L.K. Neonatal behavioral changes in rats with gestational exposure to lipopolysaccharide: A prenatal infection model for developmental neuropsychiatric disorders. Schizophr. Bull. 2012, 38, 444–456. [Google Scholar] [CrossRef] [Green Version]
- Fernandez de Cossio, L.; Guzman, A.; van der Veldt, S.; Luheshi, G.N. Prenatal infection leads to ASD-like behavior and altered synaptic pruning in the mouse offspring. Brain Behav. Immun. 2017, 63, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Malkova, N.V.; Yu, C.Z.; Hsiao, E.Y.; Moore, M.J.; Patterson, P.H. Maternal immune activation yields offspring displaying mouse versions of the three core symptoms of autism. Brain Behav. Immun. 2012, 26, 607–616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pendyala, G.; Chou, S.; Jung, Y.; Coiro, P.; Spartz, E.; Padmashri, R.; Li, M.; Dunaevsky, A. Maternal immune activation causes behavioral impairments and altered cerebellar cytokine and synaptic protein expression. Neuropsychopharmacology 2017, 42, 1435–1446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yim, Y.S.; Park, A.; Berrios, J.; Lafourcade, M.; Pascual, L.M.; Soares, N.; Yeon Kim, J.; Kim, S.; Kim, H.; Waisman, A.; et al. Reversing behavioural abnormalities in mice exposed to maternal inflammation. Nature 2017, 549, 482–487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirsten, T.B.; Chaves, G.P.; Taricano, M.; Martins, D.O.; Florio, J.C.; Britto, L.R.; Torrao, A.S.; Palermo-Neto, J.; Bernardi, M.M. Prenatal LPS exposure reduces olfactory perception in neonatal and adult rats. Physiol. Behav. 2011, 104, 417–422. [Google Scholar] [CrossRef]
- Labouesse, M.A.; Dong, E.; Grayson, D.R.; Guidotti, A.; Meyer, U. Maternal immune activation induces GAD1 and GAD2 promoter remodeling in the offspring prefrontal cortex. Epigenetics 2015, 10, 1143–1155. [Google Scholar] [CrossRef] [Green Version]
- Hava, G.; Vered, L.; Yael, M.; Mordechai, H.; Mahoud, H. Alterations in behavior in adult offspring mice following maternal inflammation during pregnancy. Dev. Psychobiol. 2006, 48, 162–168. [Google Scholar] [CrossRef]
- Zhu, F.; Zheng, Y.; Liu, Y.; Zhang, X.; Zhao, J. Minocycline alleviates behavioral deficits and inhibits microglial activation in the offspring of pregnant mice after administration of polyriboinosinic polyribocytidilic acid. Psychiatry Res. 2014, 219, 680–686. [Google Scholar] [CrossRef]
- Hsiao, E.; McBride, S.W.; Hsien, S.; Sharon, G.; Hyde, E.R.; McCue, T.; Codelli, J.A.; Chow, J.; Reisman, S.E.; Petrosino, J.F.; et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell 2013, 155, 1451–1463. [Google Scholar] [CrossRef] [Green Version]
- Hsiao, E.Y.; McBride, S.W.; Chow, J.; Mazmanian, S.K.; Patterson, P.H. Modeling an autism risk factor in mice leads to permanent immune dysregulation. Proc. Natl. Acad. Sci. USA 2012, 109, 12776–12781. [Google Scholar] [CrossRef] [Green Version]
- Schwartzer, J.J.; Careaga, M.; Onore, C.E.; Rushakoff, J.A.; Berman, R.F.; Ashwood, P. Maternal immune activation and strain specific interactions in the development of autism-like behaviors in mice. Transl. Psychiatry 2013, 3, e240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, L.; Fatemi, S.H.; Sidwell, R.W.; Patterson, P.H. Maternal influenza infection causes marked behavioral and pharmacological changes in the offspring. J. Neurosci. 2003, 23, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Faggioni, R.; Fantuzzi, G.; Villa, P.; Buurman, W.; van Tits, L.J.; Ghezzi, P. Independent down-regulation of central and peripheral tumor necrosis factor production as a result of lipopolysaccharide tolerance in mice. Infect. Immun. 1995, 63, 1473–1477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lasala, N.; Zhou, H. Effects of maternal exposure to LPS on the inflammatory response in the offspring. J. Neuroimmunol. 2007, 189, 95–101. [Google Scholar] [CrossRef] [Green Version]
- Laye, S.; Parnet, P.; Goujon, E.; Dantzer, R. Peripheral administration of lipopolysaccharide induces the expression of cytokine transcripts in the brain and pituitary of mice. Brain Res. Mol. Brain Res. 1994, 27, 157–162. [Google Scholar] [CrossRef]
- Thibeault, I.; Laflamme, N.; Rivest, S. Regulation of the gene encoding the monocyte chemoattractant protein 1 (MCP-1) in the mouse and rat brain in response to circulating LPS and proinflammatory cytokines. J. Comp. Neurol. 2001, 434, 461–477. [Google Scholar] [CrossRef]
- Whiteside, M.B.; Quan, N.; Herkenharn, M. Induction of pituitary cytokine transcripts by peripheral lipopolysaccharide. J. Neuroendocrinol. 1999, 11, 115–120. [Google Scholar] [CrossRef]
- Haider, S.; Knofler, M. Human tumour necrosis factor: Physiological and pathological roles in placenta and endometrium. Placenta 2009, 30, 111–123. [Google Scholar] [CrossRef] [Green Version]
- Connors, E.J.; Shaik, A.N.; Migliore, M.M.; Kentner, A.C. Environmental enrichment mitigates the sex-specific effects of gestational inflammation on social engagement and the hypothalamic pituitary adrenal axis-feedback system. Brain Behav. Immun. 2014, 42, 178–190. [Google Scholar] [CrossRef]
- Chua, J.S.; Cowley, C.J.; Manavis, J.; Rofe, A.M.; Coyle, P. Prenatal exposure to lipopolysaccharide results in neurodevelopmental damage that is ameliorated by zinc in mice. Brain Behav. Immun. 2012, 26, 326–336. [Google Scholar] [CrossRef]
- Coyle, P.; Tran, N.; Fung, J.N.; Summers, B.L.; Rofe, A.M. Maternal dietary zinc supplementation prevents aberrant behaviour in an object recognition task in mice offspring exposed to LPS in early pregnancy. Behav. Brain Res. 2009, 197, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Weiser, M.J.; Mucha, B.; Denheyer, H.; Atkinson, D.; Schanz, N.; Vassiliou, E.; Benno, R.B. Dietary docosahexaenoic acid alleviates autistic-like behaviors resulting from maternal immune activation in mice. Prostaglandins Leukot. Essent. Fat. Acids 2016, 106, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Leung, Y.O.; Zhou, I.; Ho, L.C.; Kong, W.; Basil, P.; Wei, R.; Lam, S.; Zhang, X.; Law, A.C.K.; et al. Dietary supplementation with n-3 fatty acids from weaning limits brain biochemistry and behavioural changes elicited by prenatal exposure to maternal inflammation in the mouse model. Transl. Psychiatry 2015, 5, e641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lante, F.; Meunier, J.; Guiramand, J.; De Jesus Ferreira, M.C.; Cambonie, G.; Aimar, R.; Cohen-Solal, C.; Maurice, T.; Vignes, M.; Barbanel, G. Late N-acetylcysteine treatment prevents the deficits induced in the offspring of dams exposed to an immune stress during gestation. Hippocampus 2008, 18, 602–609. [Google Scholar] [CrossRef]
- Zavitsanou, K.; Chai, K.L.; Purves-Tyson, T.; Karl, T.; Kassiou, M.; Banister, S.D.; Guillemin, G.J.; Weickert, C.S. Effect of maternal immune activation on the kynurenine pathway in preadolescent rat offspring and on MK801-nduced hyperlocomotion in adulthood: Amelioration by COX-2 inhibition. Brain Behav. Immun. 2014, 41, 173–181. [Google Scholar] [CrossRef]
- Ruskin, D.N.; Murphy, M.I.; Slade, S.L.; Masino, S.A. Ketogenic diet improves behaviors in a maternal immune activation model of autism spectrum disorder. PLoS ONE 2017, 12, e0171643. [Google Scholar] [CrossRef] [Green Version]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aria, F.; Bonini, S.A.; Cattaneo, V.; Premoli, M.; Mastinu, A.; Maccarinelli, G.; Memo, M. Brain Structural and Functional Alterations in Mice Prenatally Exposed to LPS Are Only Partially Rescued by Anti-Inflammatory Treatment. Brain Sci. 2020, 10, 620. https://doi.org/10.3390/brainsci10090620
Aria F, Bonini SA, Cattaneo V, Premoli M, Mastinu A, Maccarinelli G, Memo M. Brain Structural and Functional Alterations in Mice Prenatally Exposed to LPS Are Only Partially Rescued by Anti-Inflammatory Treatment. Brain Sciences. 2020; 10(9):620. https://doi.org/10.3390/brainsci10090620
Chicago/Turabian StyleAria, Francesca, Sara A. Bonini, Valentina Cattaneo, Marika Premoli, Andrea Mastinu, Giuseppina Maccarinelli, and Maurizio Memo. 2020. "Brain Structural and Functional Alterations in Mice Prenatally Exposed to LPS Are Only Partially Rescued by Anti-Inflammatory Treatment" Brain Sciences 10, no. 9: 620. https://doi.org/10.3390/brainsci10090620






