Serum BDNF’s Role as a Biomarker for Motor Training in the Context of AR-Based Rehabilitation after Ischemic Stroke
Abstract
:1. Introduction
2. Experimental Section
2.1. Studied Patients
2.2. Healthy Individuals
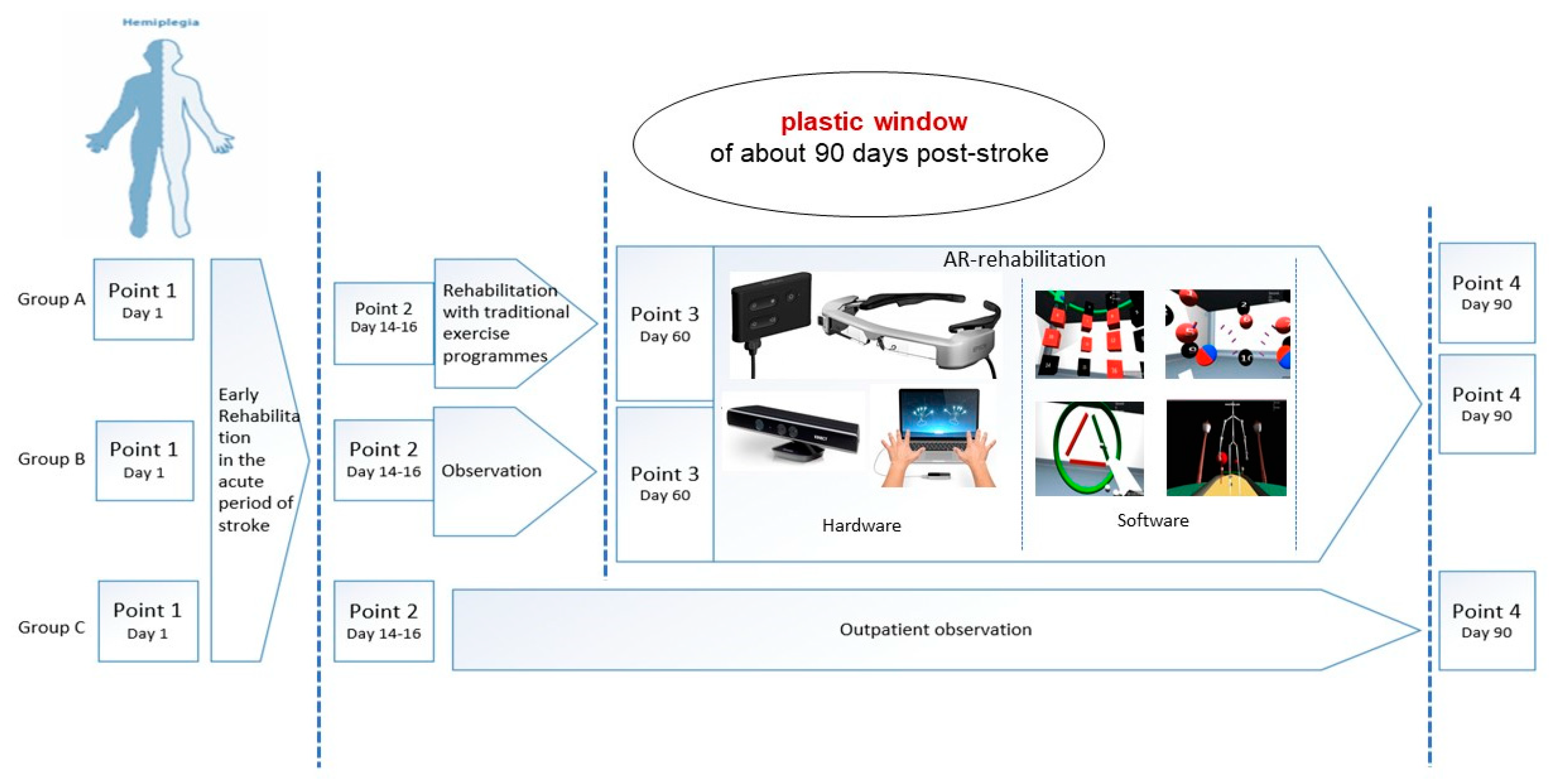
2.3. Study Design
- Point 1—first day of stroke.
- Point 2—discharge from the Tomsk RSC (median, 14th day; range, 14th–16th day).
- Point 3—after traditional rehabilitation/before AR-based rehabilitation (median, 45th day; range, 16th–60th day) (not in the group C-OPO).
- Point 4—after AR-based rehabilitation (median, 82nd day; range, 60th–90th day).
2.4. Exercise Training and Experimental Procedure
2.5. BDNF Assessment
2.6. Clinical Assessment of Motor Functions
- Variability of movements when following a given trajectory;
- Total number of completed movements (completed task) during one motor session;
- Maximum duration of a tasks series in one approach without a rest;
- Variance of the displacement for the central point of the body during the walk before crossing the obstacles;
- Height of raising the leg on the affected side when stepping through virtual barrier.
2.7. Statistics
3. Results
3.1. BDNF Levels
3.2. Clinical Assessment of Motor Recovery
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
Appendix B
Appendix C
- Air cryotherapy (the compact FRIGOSTREAM cryotherapy unit, Germany): airflow speed, 1200–1500 l/min; airflow temperature, −32 °C; distance to the surface of the body, 10–15 cm; speed of movement of the air jet, 1–2 cm/s. The labile technique was performed using a standard nozzle for 5–7 min on a zone with an area of no more than four palms of the patient. The procedures were performed daily, with 10–12 procedures per course.
- Functional electrical stimulation (FES) of muscles antagonistic to spastic ones (two-channel device, IONOSON-Expert, Germany). For FES was applied a pulse current “surge high voltage” (HVS) in bi-phasic mode with a frequency of 30–60 Hz, an increase and decrease in the current strength for 1 s, and a plateau of the maximum current strength for 2 s, with durations of sending and pausing of 4 and 6 s, respectively. The current strength was selected individually within 20–50 mA, according to the amplitude of visible muscle contractions, and should not have provoked an increase in muscle strength of more than 20–25% over the original strength. The procedure took no more than 20 min. The course consisted of 10–12 daily procedures.
- Manual classical massage of paretic limbs: duration—30–40 min; course—10–12 procedures.
- Mechanotherapy (ARTROMOT SIMULATORS): one session lasted 30 min. The course included 10–12 sessions.
- Therapeutic physical culture (Bobath Neuro-Developmental Treatment): active gymnastics, walking on a treadmill at a speed of 0.2–0.6 km/h for 3–10 min, and walking on rehabilitation bars and stairs with adjustable step height.
Appendix D
- RehabDT, a desktop application that implements a number of functions:
- (a)
- The collection of the coordinates of body and limb positions obtained from video capture sensors (Leap Motion, Microsoft Kinect).
- (b)
- The visualization of a kinetic model of the patient.
- (c)
- The transfer of the coordinates of body and limb position to augmented reality glasses.
- (d)
- The collection of the information about the interaction of the patient with virtual objects.
- (e)
- The recording of a file with the coordinates of the body and limb position.
- RehabAR, software visualizing the obtained data for the patient, which works under the Android 5.1 operating system with the augmented reality glasses Epson Moverio bt-300. The software generates biofeedback stimuli and visualizes a virtual scene and a patient kinetic model.The RehabAR software processes the execution of four different motor tasks for the neurorehabilitation program and quantifies their quality.
- Accuracy domain: the exercise is aimed at developing the coordination and muscle strength of the upper limbs. The patient sees red and black cubes in front of them. The task is to alternately press the red and black cubes, repainting them in green. The exercise is performed while sitting.
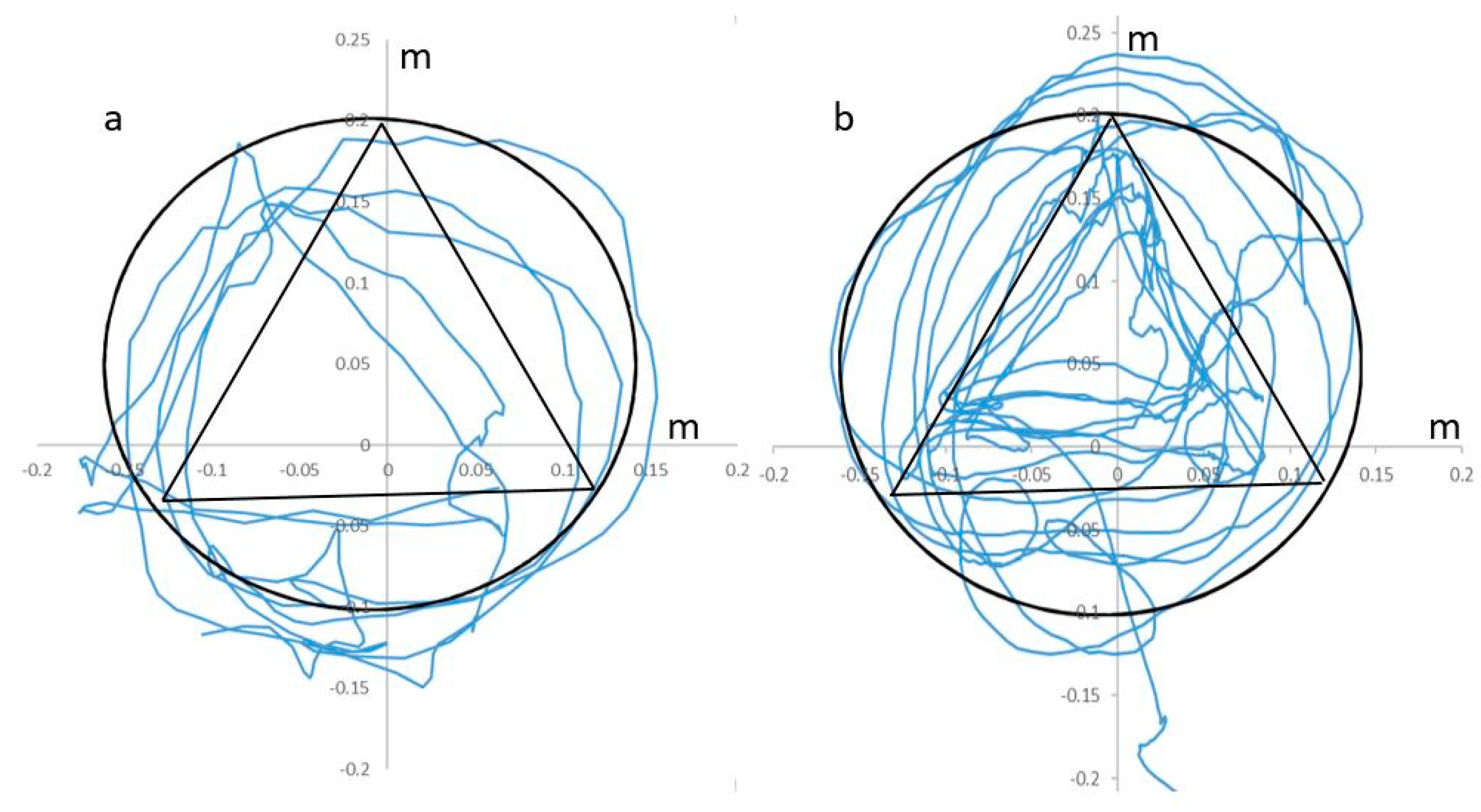
- Statics domain: the exercise is aimed at developing the coordination of the reciprocal muscles of the upper limb in a static position. The patient places the straightened index and middle fingers on the perimeter of the circle and triangle. The task is to outline geometric shapes as accurately as possible. When executed correctly, the shapes turn green. The exercise is performed while sitting.
- Capture domain: the exercise is aimed at developing fine grasp motor skills. The patient sees red and black spheres in front of them. Their task is to alternately squeeze red and black cubes into a fist. With proper execution and sufficient clenching of the fist, the spheres disappear. The exercise is performed while sitting.
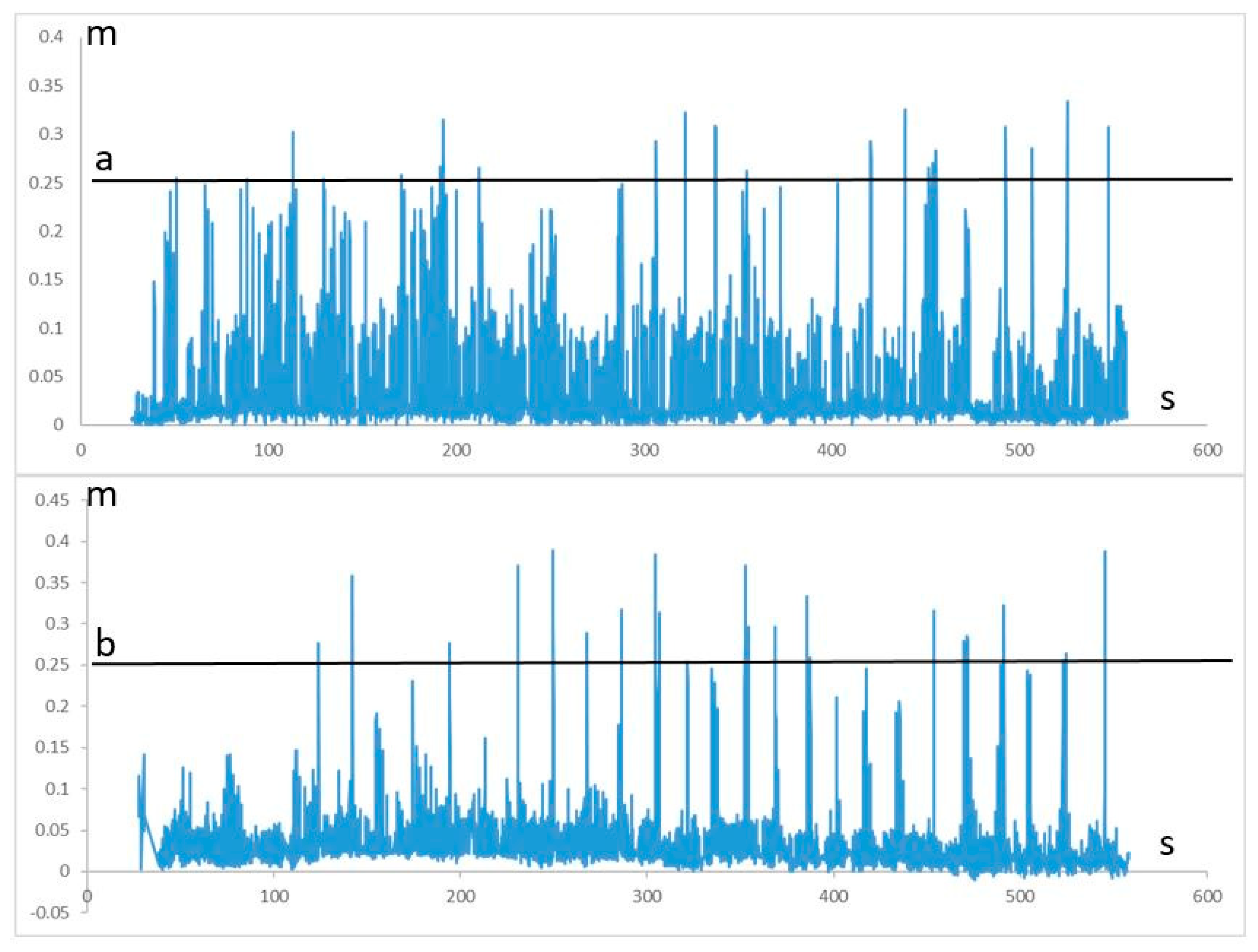
- Balance domain: the exercise aims to develop gait coordination and balance. The patient steps in place along the virtual road; every 20 s, a virtual barrier approaches them. The patient’s task is to choose the right moment and cross the barrier without hitting their foot. The exercise is accomplished while standing.
- RehabCS, software for storing, preparing, and processing motion capture data—a web-based application located on the local network. We used it for the structured storage and automated processing of video capture data.
Appendix E
| Ischemic stroke n = 50 | Controls n = 50 | PA-B | PB-C | PA-C | PA-Ctrl | PB-Ctrl | PC-Ctrl | |||
|---|---|---|---|---|---|---|---|---|---|---|
| A-Rehab-Traditional-AR n = 21 | B-Rehab- AR n = 14 | C-OPO n = 15 | ||||||||
| Median age m (IQR), y | 62 (57; 67) | 65.5 (60; 68) | 66 (60.5; 68) | 63 (56; 65) | 0.43 | 0.65 | 0.14 | 0.51 | 0.26 | 0.46 |
| Sex | 0.51 | 0.99 | 0.74 | 0.75 | 0.59 | 0.74 | ||||
| Male, n (%) | 13 (61.9%) | 7 (50.0%) | 8 (53.3%) | 29 (58%) | ||||||
| Female, n (%) | 8(38.1%) | 7 (50.0%) | 7 (46.7%) | 21 (42%) | ||||||
| Median height m (IQR), cm | 170 (162; 176.5) | 170 (161; 172) | 166 (163; 172) | 166 (158; 171) | 0.42 | 0.95 | 0.44 | 0.45 | 0.54 | 0.78 |
| Median weight m (IQR), kg | 78 (71; 85) | 75 (61; 90) | 82 (74; 90) | 79 (75; 87) | 0.42 | 0.13 | 0.33 | 0.68 | 0.34 | 0.56 |
| Duration of Hypertension m (IQR), y | 10 (10; 15) | 14 (10; 15) | 10 (9; 18) | 6 (5; 9) | 0.94 | 0.86 | 0.83 | 0.06 | 0.05 | 0.06 |
| Coronary heart disease, n (%) | 5 (23.8%) | 3 (21.4%) | 7 (46.7%) | 11 (22%) | 0.99 | 0.25 | 0.18 | 0.86 | 0.85 | 0.32 |
| Heart attack, n (%) | 3 (14.3%) | 1 (7.1%) | 4 (26.7%) | 0 (0.0%) | 0.64 | 0.33 | 0.42 | |||
| Atrial fibrillation, n (%) | 3 (14.3%) | 4 (28.6%) | 4 (26.7%) | 0 (0.0%) | 0.40 | 0.99 | 0.42 | |||
| Heart valve prosthesis, n (%) | 0 (0.0%) | 0 (0.0%) | 2 (13.3%) | 0 (0.0%) | ||||||
| Dyslipidemia, n (%) | 19 (90.5%) | 9 (64.3%) | 10 (66.7%) | 43 (86%) | 0.90 | 0.99 | 0.10 | 0.60 | 0.07 | 0.20 |
| Diabetes, n (%) | 6 (28.6%) | 2 (14.3%) | 2 (13.3%) | 14 (28%) | 0.43 | 0.99 | 0.42 | 0.96 | 0.30 | 0.25 |
| Duration of Diabetes m (IQR), y | 4 (4; 5) | 8 (5; 10) | 12 (5; 20) | 7 (4; 10) | 0.14 | 0.66 | 0.14 | 0.12 | 0.44 | 0.56 |
References
- GBD 2015 Neurological Disorders Collaborator Group. Global, regional, and national burden of neurological disorders during 1990-2015: A systematic analysis for the global burden of disease study 2015. Lancet Neurol. 2017, 16, 877–897. [Google Scholar] [CrossRef] [Green Version]
- GBD 2016 Neurology Collaborators. Global, regional, and national burden of neurological disorders, 1990-2016: A systematic analysis for the global burden of disease study 2016. Lancet Neurol. 2019, 18, 459–480. [Google Scholar] [CrossRef] [Green Version]
- GBD 2016 Lifetime Risk of Stroke Collaborators; Feigin, V.L.; Nguyen, G.; Cercy, K.; Johnson, C.O.; Alam, T.; Parmar, P.G.; Abajobir, A.A.; Abate, K.H.; Abd-Allah, F.; et al. Global, regional, and country-specific lifetime risks of stroke, 1990 and 2016. N. Engl. J. Med. 2018, 379, 2429–2437. [Google Scholar] [CrossRef]
- Krishnamurthi, R.V.; Ikeda, T.; Feigin, V.L. Global, regional and country-specific burden of ischaemic stroke, intracerebral haemorrhage and subarachnoid haemorrhage: A systematic analysis of the global burden of disease study 2017. Neuroepidemiology 2020, 54, 171–179. [Google Scholar] [CrossRef]
- Hatem, S.M.; Saussez, G.; Della Faille, M.; Prist, V.; Zhang, X.; Dispa, D.; Bleyenheuft, Y. Rehabilitation of motor function after stroke: A multiple systematic review focused on techniques to stimulate upper extremity recovery. Front. Hum. Neurosci. 2016, 10, 442. [Google Scholar] [CrossRef] [Green Version]
- De Rooij, I.J.; van de Port, I.G.; Meijer, J.G. Effect of virtual reality training on balance and gait ability in patients with stroke: Systematic review and meta-analysis. Phys. Ther. 2016, 96, 1905–1918. [Google Scholar] [CrossRef]
- Laver, K.E.; Lange, B.; George, S.; Deutsch, J.E.; Saposnik, G.; Crotty, M. Virtual reality for stroke rehabilitation. Cochrane Database Syst. Rev. 2017, 11, CD008349. [Google Scholar] [CrossRef] [Green Version]
- Iruthayarajah, J.; McIntyre, A.; Cotoi, A.; Macaluso, S.; Teasell, R. The use of virtual reality for balance among individuals with chronic stroke: A systematic review and meta-analysis. Top. Stroke Rehabil 2017, 24, 68–79. [Google Scholar] [CrossRef]
- Tinga, A.M.; Visser-Meily, J.M.; van der Smagt, M.J.; Van der Stigchel, S.; van Ee, R.; Nijboer, T.C. Multisensory stimulation to improve low- and higher-level sensory deficits after stroke: A systematic review. Neuropsychol. Rev. 2016, 26, 73–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.S.; Park, Y.J.; Park, S.W. The effects of virtual reality training on function in chronic stroke patients: A systematic review and meta-analysis. Bio. Res. Int. 2019, 2019, 7595639. [Google Scholar] [CrossRef] [Green Version]
- Langhorne, P.; Coupar, F.; Pollock, A. Motor recovery after stroke: A systematic review. Lancet Neurol. 2009, 8, 741–754. [Google Scholar] [CrossRef]
- Vourvopoulos, A.; Pardo, O.M.; Lefebvre, S.; Neureither, M.; Saldana, D.; Jahng, E.; Liew, S.-L. Effects of a brain-computer interface with virtual reality (VR) neurofeedback: A pilot study in chronic stroke patients. Front. Hum. Neurosci. 2019, 13, 210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barde, Y.A.; Edgar, D.; Thoenen, H. Purification of a new neurotrophic factor from mammalian brain. EMBO J. 1982, 1, 549–553. [Google Scholar] [CrossRef]
- Ibanez, C.F. Neurotrophic factors: From structure-function studies to designing effective therapeutics. Trends. Biotechnol. 1995, 13, 217–227. [Google Scholar] [CrossRef]
- Kowianski, P.; Lietzau, G.; Czuba, E.; Waskow, M.; Steliga, A.; Morys, J. BDNF: A key factor with multipotent impact on brain signaling and synaptic plasticity. Cell Mol. Neurobiol. 2018, 38, 579–593. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Nagappan, G.; Lu, Y. BDNF and synaptic plasticity, cognitive function, and dysfunction. Handb. Exp. Pharmacol. 2014, 220, 223–250. [Google Scholar] [CrossRef] [PubMed]
- Bathina, S.; Das, U.N. Brain-derived neurotrophic factor and its clinical implications. Arch. Med. Sci. 2015, 11, 1164–1178. [Google Scholar] [CrossRef] [PubMed]
- Benarroch, E.E. Brain derived neurotrophic factor: Regulation, effects, and potential clinical relevance. Neurology 2015, 84, 1693–1704. [Google Scholar] [CrossRef]
- Chen, S.D.; Wu, C.L.; Hwang, W.C.; Yang, D.I. More insight into BDNF against neurodegeneration: Anti-apoptosis, anti-oxidation, and suppression of autophagy. Int. J. Mol. Sci. 2017, 18, 545. [Google Scholar] [CrossRef] [Green Version]
- Leal, G.; Comprido, D.; Duarte, C.B. BDNF-induced local protein synthesis and synaptic plasticity. Neuropharmacology 2014, 76 Pt C, 639–656. [Google Scholar] [CrossRef] [Green Version]
- Leal, G.; Afonso, P.M.; Salazar, I.L.; Duarte, C.B. Regulation of hippocampal synaptic plasticity by BDNF. Brain Res. 2015, 1621, 82–101. [Google Scholar] [CrossRef] [PubMed]
- Feldman, D.E. Synaptic mechanisms for plasticity in neocortex. Annu. Rev. Neurosci. 2009, 32, 33–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kida, H.; Mitsushima, D. Mechanisms of motor learning mediated by synaptic plasticity in rat primary motor cortex. Neurosci. Res. 2018, 128, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Kwah, L.K.; Diong, J. National institutes of health stroke scale (NIHSS). J. Physiother. 2014, 60, 61. [Google Scholar] [CrossRef] [Green Version]
- Rankin, J. Cerebral vascular accidents in patients over the age of 60. II. prognosis. Scott. Med. J. 1957, 2, 200–215. [Google Scholar] [CrossRef]
- Ashworth, B. Preliminary trial of carisoprodol in multiple sclerosis. Practitioner 1964, 192, 540–542. [Google Scholar]
- Bohannon, R.W.; Smith, M.B. Interrater reliability of a modified ashworth scale of muscle spasticity. Phys. Ther. 1987, 67, 206–207. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Gaffary, Y.; Le Gouis, B.; Marchal, M.; Argelaguet, F.; Arnaldi, B.; Lecuyer, A. AR feels “softer” than VR: Haptic perception of stiffness in augmented versus virtual reality. IEEE Trans. Vis. Comput. Graph. 2017, 23, 2372–2377. [Google Scholar] [CrossRef] [Green Version]
- Bergmann, J.; Krewer, C.; Bauer, P.; Koenig, A.; Riener, R.; Müller, F. Virtual reality to augment robot-assisted gait training in non-ambulatory patients with a subacute stroke: A pilot randomized controlled trial. Eur. J. Phys. Rehabil. Med. 2018, 54, 397–407. [Google Scholar] [CrossRef]
- Fugl-Meyer, A.R.; Jaasko, L.; Leyman, I.; Olsson, S.; Steglind, S. The post-stroke hemiplegic patient. 1. a method for evaluation of physical performance. Scand. J. Rehabil. Med. 1975, 7, 13–31. [Google Scholar] [PubMed]
- Gladstone, D.J.; Danells, C.J.; Black, S.E. The fugl-meyer assessment of motor recovery after stroke: A critical review of its measurement properties. Neurorehabil. Neural Repair. 2002, 16, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Koroleva, E.S.; Kazakov, S.D.; Tolmachev, I.V.; Loonen, A.J.M.; Ivanova, S.A.; Alifirova, V.M. Clinical evaluation of different treatment strategies for motor recovery in stroke rehabilitation. 2020. (to be submitted for publication). [Google Scholar]
- Adams, H.P., Jr.; Bendixen, B.H.; Kappelle, L.J.; Biller, J.; Love, B.B.; Gordon, D.L.; Marsh, E.E., III. Classification of subtype of acute ischemic stroke. definitions for use in a multicenter clinical trial. TOAST. trial of org 10172 in acute stroke treatment. Stroke 1993, 24, 35–41. [Google Scholar] [CrossRef] [Green Version]
- Zeiler, S.R. Should we care about early post-stroke rehabilitation? not yet, but soon. Curr. Neurol. Neurosci. Rep. 2019, 19, 13. [Google Scholar] [CrossRef]
- Duncan, P.W.; Goldstein, L.B.; Matchar, D.; Divine, G.W.; Feussner, J. Measurement of motor recovery after stroke. outcome assessment and sample size requirements. Stroke 1992, 23, 1084–1089. [Google Scholar] [CrossRef] [Green Version]
- Fujimura, H.; Altar, C.A.; Chen, R.; Altar, A.; Chen, R.; Nakamura, T.; Nakahashi, T.; Kambayashi, J.; Sun, B.; Tandon, N.N. Brain-derived neurotrophic factor is stored in human platelets and released by agonist stimulation. Thromb. Haemost. 2002, 87, 728–734. [Google Scholar] [CrossRef] [Green Version]
- Radka, S.F.; Holst, P.A.; Fritsche, M.; Altar, C.A. Presence of brain-derived neurotrophic factor in brain and human and rat but not mouse serum detected by a sensitive and specific immunoassay. Brain Res. 1996, 709, 122–301. [Google Scholar] [CrossRef]
- Wang, J.; Gao, L.; Yang, Y.-L.; Li, Y.-Q.; Chang, T.; Man, M.-H.; Zhang, X.-Y.; Guo, S.-C.; Li, L.-H. Low serum levels of brain-derived neurotrophic factor were associated with poor short-term functional outcome and mortality in acute ischemic stroke. Mol. Neurobiol. 2017, 54, 7335–7342. [Google Scholar] [CrossRef]
- Rodier, M.; Quirie, A.; Prigent-Tessier, A.; Béjot, Y.; Jacquin, A.; Mossia, C.; Marie, C.; Garnier, P. Relevance of post-stroke circulating BDNF levels as a prognostic biomarker of stroke outcome. Impact of rt-PA treatment. PLoS ONE 2015, 10, e0140668. [Google Scholar] [CrossRef] [Green Version]
- Stanne, T.M.; Aberg, N.D.; Nilsson, S.; Jood, K.; Blomstrand, C.; Andreasso, U.; Blennow, K.; Zetterberg, H.; Isgaard, J.; Svensson, J.; et al. Low circulating acute brain-derived neurotrophic factor levels are associated with poor long-term functional outcome after ischemic stroke. Stroke 2016, 47, 1943–1945. [Google Scholar] [CrossRef] [PubMed]
- Sobrino, T.; Rodriguez-Yanez, M.; Campos, F.; Iglesias-Rey, R.; Millán, M.; de la Ossa, N.P.; Dávalos, A.; Delgado-Mederos, R.; Martínez-Domeño, A.; Martí-Fábregas, J.; et al. Association of high serum levels of growth factors with good outcome in ischemic stroke: A multicenter study. Transl. Stroke Res. 2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dancause, N.; Nudo, R.J. Shaping plasticity to enhance recovery after injury. Prog. Brain Res. 2011, 192, 273–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silasi, G.; Murphy, T.H. Stroke and the connectome: How connectivity guides therapeutic intervention. Neuron 2014, 83, 1354–1368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panja, D.; Bramham, C.R. BDNF mechanisms in late LTP formation: A synthesis and breakdown. Neuropharmacology 2014, 76 Pt C, 664–676. [Google Scholar] [CrossRef]
- Bramham, C.R.; Messaoudil, E. BDNF function in adult synaptic plasticity: The synaptic consolidation hypothesis. Prog. Neurobiol. 2005, 76, 99–125. [Google Scholar] [CrossRef]
- Bamji, S.X.; Rico, B.; Kimes, N.; Reichardt, L.F. BDNF mobilizes synaptic vesicles and enhances synapse formation by disrupting cadherin-beta-catenin interactions. J. Cell Biol. 2006, 174, 289–299. [Google Scholar] [CrossRef]
- Qin, L.; Jing, D.; Parauda, S.; Carmel, J.; Ratan, R.R.; Lee, F.S.; Cho, S. An adaptive role for BDNF Val66Met polymorphism in motor recovery in chronic stroke. J. Neurosci. 2014, 12, 2493–2502. [Google Scholar] [CrossRef]
- Sheikh, I.S.; Smith, G.M. Targeting neurotrophins to specific populations of neurons: NGF, BDNF, and NT-3 and their relevance for treatment of spinal cord injury. Int. J. Mol. Sci. 2017, 18, 548. [Google Scholar] [CrossRef]
- Lu, P.; Blesch, A.; Tuszynski, M.H. Neurotrophism without neurotropism: BDNF promotes survival but not growth of lesioned corticospinal neurons. J. Comp. Neurol. 2001, 436, 456–470. [Google Scholar] [CrossRef]
- Sandvig, I.; Augestad, I.L.; Håberg, A.K.; Sandvig, A. Neuroplasticity in stroke recovery. The role of microglia in engaging and modifying synapses and networks. Eur. J. Neurosci. 2018, 47, 1414–1428. [Google Scholar] [CrossRef] [PubMed]
- Horch, H.W.; Krüttgen, A.; Portbury, S.D.; Katz, L.C. Destabilization of cortical dendrites and spines by BDNF. Neuron 1999, 23, 353–364. [Google Scholar] [CrossRef] [Green Version]
- Alia, C.; Spalletti, C.; Lai, S.; Panarese, A.; Lamola, G.; Bertolucci, F.; Vallone, F.; Garbo, A.D.; Chisari, C.; Micera, S.; et al. Neuroplastic changes following brain ischemia and their contribution to stroke recovery: Novel approaches in neurorehabilitation. Front. Cell Neurosci. 2017, 11, 76. [Google Scholar] [CrossRef] [PubMed]
- Berretta, A.; Tzeng, Y.C.; Clarkson, A.N. Post-stroke recovery: The role of activity-dependent release of brain-derived neurotrophic factor. Expert Rev. Neurother. 2014, 14, 1335–1344. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Ninuma, S.; Hayashi, M.; Okuda, A.; Asaka, T.; Maejima, H. Effects of long-term exercise and low-level inhibition of GABAergic synapses on motor control and the expression of BDNF in the motor related cortex. Neurol. Res. 2018, 40, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Quirie, A.; Hervieu, M.; Garnier, P.; Demougeot, C.; Mossiat, C.; Bertrand, N.; Martin, A.; Marie, C.; Prigent-Tessier, A. Comparative effect of treadmill exercise on mature BDNF production in control versus stroke rats. PLoS ONE 2012, 7, e44218. [Google Scholar] [CrossRef] [Green Version]
- Tsai, S.F.; Liu, Y.W.; Kuo, Y.M. Acute and long-term treadmill running differentially induce c-fos expression in region- and time-dependent manners in mouse brain. Brain Struct. Funct. 2019, 224, 2677–2689. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.Y.; Wang, E.H.; Woodson, W.J.; Wang, S.; Sun, G.; Lee, A.G.; Arac, A.; Fenno, L.E.; Deisseroth, K.; Steinber, G.K. Optogenetic neuronal stimulation promotes functional recovery after stroke. Proc. Natl. Acad. Sci. USA 2014, 111, 12913–12918. [Google Scholar] [CrossRef] [Green Version]
- Blank, A.A.; French, J.A.; Pehlivan, A.U.; O’Malley, M.K. Current trends in robot-assisted upper-limb stroke rehabilitation: Promoting patient engagement in therapy. Curr. Phys. Med. Rehabil. Rep. 2014, 2, 184–195. [Google Scholar] [CrossRef]
- Turner-Stokes, L.; Sykes, N.; Silber, E. Guideline Development Group. Long-term neurological conditions: Management at the interface between neurology, rehabilitation and palliative care. Clin. Med. (Lond.) 2008, 8, 186–191. [Google Scholar] [CrossRef]
- Di Pino, G.; Pellegrino, G.; Assenza, G.; Capon, F.; Ferreri, F.; Formica, D.; Ranieri, F.; Tombini, M.; Ziemann, U.; Rothwell, J.C.; et al. Modulation of brain plasticity in stroke: A novel model for neurorehabilitation. Nat. Rev. Neurol. 2014, 10, 597–608. [Google Scholar] [CrossRef] [PubMed]
- Nahmani, M.; Turrigiano, G.G. Adult cortical plasticity following injury: Recapitulation of critical period mechanisms? Neuroscience 2014, 283, 4–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radak, Z.; Suzuki, K.; Higuchi, M.; Balogh, L.; Boldogh, I.; Koltai, E. Physical exercise, reactive oxygen species and neuroprotection. Free Radic. Biol. Med. 2016, 98, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Liu, W. A narrative review of gait training after stroke and a proposal for developing a novel gait training device that provides minimal assistance. Top. Stroke Rehabil. 2018, 25, 375–383. [Google Scholar] [CrossRef]
- Koroleva, E.S.; Alifirova, V.M.; Latypova, A.; Ivanova, S.A.; Levchuk, L.A.; Kazakov, S.D. Correlation between neurological deficit and serum BDNF in patients with ischemic stroke after early rehabilitation. Eur. J. Neurol. 2019, 26 (Suppl. 1), 394. [Google Scholar]
- Koroleva, E.S.; Alifirova, V.M.; Brazovskaya, N.G.; Plotnikov, D.M.; Levchuk, L.A.; Boyko, A.S.; Zapekin, S.G.; Semenenko, A.S.; Kataeva, N.G.; Ivanova, S.A. Clinical and laboratory assessment of the effectiveness of early rehabilitation of patients with stroke in the regional vascular center in tomsk with the use of auxiliary robotic mechanisms. Bull. Sib. Med. 2019, 18, 55. [Google Scholar] [CrossRef]
- Larpthaveesarp, A.; Ferriero, D.M.; Gonzalez, F.F. Growth factors for the treatment of ischemic brain injury (growth factor treatment). Brain Sci. 2015, 5, 165–177. [Google Scholar] [CrossRef] [Green Version]
- Ruan, L.; Wang, B.; ZhuGe, Q.; Jin, K. Coupling of neurogenesis and angiogenesis after ischemic stroke. Brain Res. 2015, 1623, 166–173. [Google Scholar] [CrossRef] [Green Version]
- Kotlęga, D.; Peda, B.; Zembroń-Łacny, A.; Gołąb-Janowska, M.; Nowacki, P. The role of brain-derived neurotrophic factor and its single nucleotide polymorphisms in stroke patients. Neurol. Neurochir. Pol. 2017, 51, 240–246. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.; Alam, A.; San, C.Y.; Eguchi, S.; Chen, Q.; Lian, Q.; Ma, D. Molecular mechanisms of brain-derived neurotrophic factor in neuro-protection: Recent developments. Brain Res. 2017, 1665, 1–21. [Google Scholar] [CrossRef]
- Wrann, C.D.; White, J.P.; Salogiannnis, J.; Laznik-Bogoslavski, D.; Wu, J.; Ma, D.; Lin, J.D.; Greenberg, M.E.; Spiegelman, B.M. Exercise induces hippocampal BDNF through a PGC-1alpha/FNDC5 pathway. Cell Metab. 2013, 18, 649–659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, B. BDNF (I)rising from exercise. Cell Metab. 2013, 18, 612–614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loonen, A.J.M.; Ivanova, S.A. New insights into the mechanism of drug-induced dyskinesia. CNS Spectr. 2013, 18, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Loonen, A.J.M.; Ivanova, S.A. Neurobiological mechanisms associated with antipsychotic drug-induced dystonia. J. Psychopharmacol. 2020, (in press). [Google Scholar]
- Conner, J.M.; Chiba, A.A.; Tuszynski, M.H. The basal forebrain cholinergic system is essential for cortical plasticity and functional recovery following brain injury. Neuron 2005, 46, 173–179. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.B.; Xu, Y.H.; He, Y.; Xue, F.; Wei, J.; Zhang, H.; Wu, J. Decreased serum brain-derived neurotrophic factor may indicate the development of poststroke depression in patients with acute ischemic stroke: A meta-analysis. J. Stroke Cerebrovasc. Dis. 2018, 27, 709–715. [Google Scholar] [CrossRef]
- Pikula, A.; Beiser, A.S.; Chen, T.C.; Preis, S.R.; Vorgias, D.; DeCarli, C.; Au, R.; Kelly-Hayes, M.; Kase, C.S.; Wolf, P.A.; et al. Serum brain-derived neurotrophic factor and vascular endothelial growth factor levels are associated with risk of stroke and vascular brain injury: Framingham study. Stroke 2013, 44, 2768–2775. [Google Scholar] [CrossRef] [Green Version]


| ΔBDNF (pg/mL) | Ischemic Stroke n = 50 | PA-B | PB-C | PA-C | ||
|---|---|---|---|---|---|---|
| A-Rehab-Traditional-AR n = 21 | B-Rehab-AR n = 14 | C-OPO n = 15 | ||||
| ΔBDNF day82−day14 | −525 (−1073; 698) | −1231 (−1178; 2120) | −2415 (−3117; −760) | 0.476 | 0.021 * | 0.049 * |
| ΔBDNF day82−day45 | 513 (281; 1565) | 2327 (924; 2529) | n/a | 0.049 * | n/a | n/a |
| ΔBDNF day14−initial | 572 (−307; 1521) | 751 (182; 1020) | 493 (147; 1093) | 0.689 | 0.557 | 0.778 |
| Points | Serum BDNF (pg/mL) | PA-Ctrl | PB-Ctrl | PC–Ctrl | |||
|---|---|---|---|---|---|---|---|
| A-Rehab-Traditional-AR n = 21 | B-Rehab-AR n = 14 | C-OPO n = 15 | Control Group n = 50 | ||||
| Point 1 | 2190 (1218; 2829) | 2537 (1968; 4777) | 2906.5 (1855; 4043) | 4250 (2215; 5152) | 0.001 * | 0.295 | 0.086 |
| Point 2 | 2525 (2050; 3144) | 4460 (2317; 4958) | 3164 (2002; 4686) | 4250 (2215; 5152) | 0.045 * | 0.886 | 0.391 |
| Point 3 | 1917 (1158; 2973) | 1489 (877; 2366) | n/a | 4250 (2215; 5152) | 0.022 * | <0.001 * | n/a |
| Point 4 | 1923 (1149; 3488) | 3719 (3485; 4929) | 1131 (679; 1484) | 4250 (2215; 5152) | 0.012 * | 0.693 | <0.001 * |
| A-Rehab-Traditional-AR n = 21 | Point 1 | Point 2 | Point 3 | Point 4 | P1-2 | P2-3 | P3-4 |
|---|---|---|---|---|---|---|---|
| FMA-Upper extremity | 35 (31; 40) | 42 (38; 50) | 49 (43; 57) | 61 (56; 64) | <0.001 * | <0.001 * | <0.001 * |
| FMA-Low extremity | 24 (21; 27) | 28 (24; 30) | 29 (27; 33) | 33 (29; 34) | 0.002 * | 0.001 * | 0.001 * |
| FMA-Balance | 10 (9; 12) | 12 (10; 13) | 12 (12; 14) | 13 (12; 14) | 0.003 * | 0.06 | 0.002 * |
| B-Rehab-AR n = 14 | Point 1 | Point 2 | Point 3 | Point 4 | P1-2 | P2-3 | P3-4 |
| FMA-Upper extremity | 39 (28; 45) | 53 (49; 54) | 53 (50; 57) | 63 (58; 64) | <0.001 * | 0.110 | <0.001 * |
| FMA-Low extremity | 26 (22; 28) | 31 (26; 34) | 32 (29; 34) | 33 (29; 34) | 0.010 * | 0.070 | 0.080 |
| FMA-Balance | 12 (11; 12) | 13 (12; 14) | 13 (12; 14) | 14 (13; 14) | 0.010 * | 0.320 | 0.040 * |
| C-OPO n = 15 | Point 1 | Point 2 | Point 3 | Point 4 | P1-2 | P2-3 | P3-4 |
| FMA-Upper extremity | 39 (15; 45) | 54 (47; 58) | N/A | 54 (47; 59) | <0.001 * | N/A | N/A |
| FMA-Low extremity | 24 (20; 29) | 28 (27; 33) | N/A | 29 (27; 33) | <0.001 * | N/A | N/A |
| FMA-Balance | 11 (5; 13) | 12 (12; 14) | N/A | 12 (12; 14) | 0.010 * | N/A | N/A |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koroleva, E.S.; Tolmachev, I.V.; Alifirova, V.M.; Boiko, A.S.; Levchuk, L.A.; Loonen, A.J.M.; Ivanova, S.A. Serum BDNF’s Role as a Biomarker for Motor Training in the Context of AR-Based Rehabilitation after Ischemic Stroke. Brain Sci. 2020, 10, 623. https://doi.org/10.3390/brainsci10090623
Koroleva ES, Tolmachev IV, Alifirova VM, Boiko AS, Levchuk LA, Loonen AJM, Ivanova SA. Serum BDNF’s Role as a Biomarker for Motor Training in the Context of AR-Based Rehabilitation after Ischemic Stroke. Brain Sciences. 2020; 10(9):623. https://doi.org/10.3390/brainsci10090623
Chicago/Turabian StyleKoroleva, Ekaterina S., Ivan V. Tolmachev, Valentina M. Alifirova, Anastasiia S. Boiko, Lyudmila A. Levchuk, Anton J. M. Loonen, and Svetlana A. Ivanova. 2020. "Serum BDNF’s Role as a Biomarker for Motor Training in the Context of AR-Based Rehabilitation after Ischemic Stroke" Brain Sciences 10, no. 9: 623. https://doi.org/10.3390/brainsci10090623
APA StyleKoroleva, E. S., Tolmachev, I. V., Alifirova, V. M., Boiko, A. S., Levchuk, L. A., Loonen, A. J. M., & Ivanova, S. A. (2020). Serum BDNF’s Role as a Biomarker for Motor Training in the Context of AR-Based Rehabilitation after Ischemic Stroke. Brain Sciences, 10(9), 623. https://doi.org/10.3390/brainsci10090623






