Non-Immersive Virtual Reality for Post-Stroke Upper Extremity Rehabilitation: A Small Cohort Randomized Trial
Abstract
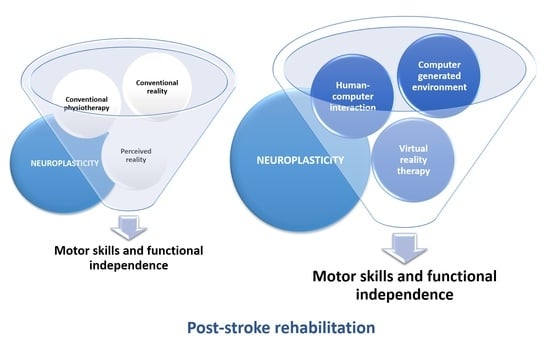
:1. Introduction
2. Materials and Methods
2.1. Participants
- (1)
- Stroke survivors after the acute phase, at least six weeks post-stroke; mild impairment (FIM ≥ 73, FMUE ≥ 13), minor cognitive impairment (Cognitive FIM ≥ 25);
- (2)
- Stroke survivors within no more than four years after a stroke, at least 30-degree flexion and scapulohumeral abduction against gravity, and at least 30-degree elbow flexion against gravity.
2.2. Outcome Measures
2.3. Procedures
2.4. Virtual Reality Software, Devices, and Exergames
2.5. Statistical Analysis
3. Results
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stroke Alliance for Europe. The burden of Stroke in Europe. 2015. Available online: https://www.stroke.org.uk/sites/default/files/the_burden_of_stroke_in_europe_-_challenges_for_policy_makers.pdf (accessed on 12 December 2019).
- Benjamin, E.J.; Muntner, P.; Alonso, A.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Das, S.R.; et al. Heart disease and stroke statistics—2017 update: A report from the American Heart Association. Circulation 2017, 135, e229–e445. [Google Scholar] [CrossRef]
- European Commission. State of health in EU. Romania, Country Health Profile. 2019. Available online: https://ec.europa.eu/health/sites/health/files/state/docs/2019_chp_romania_english.pdf (accessed on 3 January 2020).
- Stroke Alliance for Europe. The Burden of Stroke in Romania. 2017. Available online: https://www.safestroke.eu/wp-content/uploads/2017/12/SAFE_STROKE_ROMANIA.pdf (accessed on 3 January 2020).
- Crichton, S.L.; Bray, B.D.; McKevitt, C.; Rudd, A.G.; Wolfe, C.D.A. Patient outcomes up to 15 years after stroke: Survival, disability, quality of life, cognition and mental health. J. Neurol. Neurosurg. Psychiatry 2016, 87, 1091–1098. [Google Scholar] [CrossRef] [Green Version]
- Matchar, D.B.; Bilger, M.; Do, Y.K.; Eom, K. International Comparison of Poststroke Resource Use: A Longitudinal Analysis in Europe. J. Stroke Cereb. Dis. 2015, 24, 2256–2262. [Google Scholar] [CrossRef]
- Winstein, C.J.; Stein, J.; Arena, R.; Bates, B.; Cherney, L.R.; Cramer, S.C.; Deruyter, F.; Eng, J.J.; Fisher, B.; Harvey, R.L.; et al. Guidelines for Adult Stroke Rehabilitation and Recovery: A Guideline for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke 2016, 47, e98–e169. [Google Scholar] [CrossRef]
- Teasel, R.; Hussein, N. Stroke Rehabilitation Clinician Handbook. Motor Rehabilitation. 4.5 Recovery for Upper Extremity. 2016. Available online: http://www.ebrsr.com/sites/default/files/Chapter%204B_Upper%20Extremity%20Post%20Stroke_0.pdf (accessed on 12 January 2020).
- Quinn, T.J.; Paolucci, S.; Sunnerhagen, K.S.; Sivenius, J.; Walker, M.F.; Toni, D.; Lees, K.R.; European Stroke Organisation (ESO) Executive Committee; ESO Writing Committee. Evidence-based stroke r-ehabilitation: An expanded guidance document from the european stroke organization (ESO) guidelines for management of ischaemic stroke and transient ischaemic attack. J. Rehabil. Med. 2008, 41, 99–111. [Google Scholar] [CrossRef] [Green Version]
- Norouzi-Gheidari, N.; Hernandez, A.; Archambault, P.S.; Higgins, J.; Poissant, L.; Kairy, D. Feasibility, Safety and Efficacy of a Virtual Reality Exergame System to Supplement Upper Extremity Rehabilitation Post-Stroke: A Pilot Randomized Clinical Trial and Proof of Principle. Int. J. Environ. Res. Public Health 2019, 17, 113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad, M.A.; Singh, D.K.A.; Mohd Nordin, N.A.; Hooi Nee, K.; Ibrahim, N. Virtual Reality Games as an Adjunct in Improving Upper Limb Function and General Health among Stroke Survivors. Int. J. Environ. Res. Public Health 2019, 16, 5144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laver, K.E.; Lange, B.; George, S.; Deutsch, J.E.; Saposnik, G.; Crotty, M. Virtual reality for stroke rehabilitation. Cochrane Database Syst. Rev. 2017, 11, CD008349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maier, M.; Rubio Ballester, B.; Duff, A.; Duarte Oller, E.; Verschure, P. Effect of Specific Over Nonspecific VR-Based Rehabilitation on Poststroke Motor Recovery: A Systematic Meta-analysis. Neurorehabilit. Neural Repair. 2019, 33, 112–129. [Google Scholar] [CrossRef]
- Mekbib, D.B.; Han, J.; Zhang, L.; Fang, S.; Jiang, H.; Zhu, J.; Roe, A.W.; Xu, D. Virtual reality therapy for upper limb rehabilitation in patients with stroke: A meta-analysis of randomized clinical trials. Brain Inj. 2020, 34, 456–465. [Google Scholar] [CrossRef]
- Maier, M.; Ballester, B.R.; Verschure, P.F.M.J. Principles of Neurorehabilitation After Stroke Based on Motor Learning and Brain Plasticity Mechanisms. Front. Syst. Neurosci. 2019, 13, 74. [Google Scholar] [CrossRef] [PubMed]
- Livingston-Thomas, J.; Nelson, P.; Karthikeyan, S.; Antonescu, S.; Jeffers, M.S.; Marzolini, S.; Corbett, D. Exercise and Environmental Enrichment as Enablers of Task-Specific Neuroplasticity and Stroke Recovery. Neurotherapeutics 2016, 13, 395–402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kilbride, C.; Scott, D.J.M.; Butcher, T.; Norris, M.; Ryan, J.M.; Anokye, N.; Warland, A.; Baker, K.; Athanasiou, D.A.; Singla-Buxarrais, G.; et al. Rehabilitation via HOMe Based gaming exercise for the Upper-limb post Stroke (RHOMBUS): Protocol of an intervention feasibility trial. BMJ Open 2018, 8, e026620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saposnik, G.; Cohen, L.G.; Mamdani, M.; Pooyania, S.; Ploughman, M.; Cheung, D.; Shaw, J.; Hall, J.; Nord, P.; Dukelow, S.; et al. Efficacy and safety of non-immersive virtual reality exercising in stroke rehabilitation (EVREST): A randomised, multicentre, single-blind, controlled trial. Lancet Neurol. 2016, 15, 1019–1027. [Google Scholar] [CrossRef] [Green Version]
- Turolla, A.; Dam, M.; Ventura, L.; Tonin, P.; Agostini, M.; Zucconi, C.; Kiper, P.; Cagnin, A.; Piron, L. Virtual reality for the rehabilitation of the upper limb motor function after stroke: A prospective controlled trial. J. Neuroeng. Rehabil. 2013, 10, 85. [Google Scholar] [CrossRef]
- Aramaki, A.L.; Sampaio, R.F.; Cavalcanti, A.; Dutra, F. Use of client-centered virtual reality in rehabilitation after stroke: A feasibility study. Arquivos. Neuro-Psiquiatr. 2019, 77, 622–631. [Google Scholar] [CrossRef]
- Wolfe, C.D.; Taub, N.A.; Woodrow, E.J.; Burney, P.G. Assessment of scales of disability and handicap for stroke patients. Stroke 1991, 22, 1242–1244. [Google Scholar] [CrossRef] [Green Version]
- Shinohara, Y.; Minematsu, K.; Amano, T.; Ohashi, Y. Modified Rankin scale with expanded guidance scheme and interview questionnaire: Interrater agreement and reproducibility of assessment. Cerebrovasc. Dis. 2006, 21, 271–278. [Google Scholar] [CrossRef]
- Kwon, S.; Hartzema, A.G.; Duncan, P.W.; Min-Lai, S. Disability measures in stroke: Relationship among the Barthel Index, the Functional Independence Measure, and the Modified Rankin Scale. Stroke 2004, 35, 918–923. [Google Scholar] [CrossRef]
- Pollak, N.; Rheault, W.; Stoecker, J.L. Reliability and validity of the FIM for persons aged 80 years and above from a multilevel continuing care retirement community. Arch. Phy. Med. Rehabil. 1996, 77, 1056–1061. [Google Scholar] [CrossRef]
- Sharrack, B.; Hughes, R.A.; Soudain, S.; Dunn, G. The psychometric properties of clinical rating scales used in multiple sclerosis. Brain 1999, 122, 141–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehrholz, J.; Wagner, K.; Meissner, D.; Grundmann, K.; Zange, C.; Koch, R.; Pohl, M. Reliability of the Modified Tardieu Scale and the Modified Ashworth Scale in adult patients with severe brain injury: A comparison study. Clin. Rehabil. 2005, 19, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Katz, R.T.; Rovai, G.P.; Brait, C.; Rymer, W.Z. Objective quantification of spastic hypertonia: Correlation with clinical findings. Arch. Phys. Med. Rehabil. 1992, 73, 339–347. [Google Scholar] [CrossRef]
- Roman, N.; Miclaus, R.; Repanovici, A.; Nicolau, C. Equal Opportunities for Stroke Survivors’ Rehabilitation: A Study on the Validity of the Upper Extremity Fugl-Meyer Assessment Scale Translated and Adapted into Romanian. Medicina 2020, 56, 409. [Google Scholar] [CrossRef]
- Woodbury, M.L.; Velozo, C.A.; Richards, L.G.; Duncan, P.W.; Studenski, S.; Lai, S.M. Longitudinal stability of the Fugl-Meyer Assessment of the upper extremity. Arch. Phys. Med. Rehabil. 2008, 89, 1563–1569. [Google Scholar] [CrossRef]
- Cuthbert, S.C.; Goodheart, G.J. On the reliability and validity of manual muscle testing: A literature review. Chiropr. Man Therap. 2007, 15, 4. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.G.; Kim, E.K. Test-retest reliability of an active range of motion test for the shoulder and hip joints by unskilled examiners using a manual goniometer. J. Phys. Ther. Sci. 2016, 28, 722–724. [Google Scholar] [CrossRef] [Green Version]
- Duncan, P.W.; Weiner, D.K.; Chandler, J.; Studenski, S. Functional reach: A new clinical measure of balance. J. Gerontol. 1990, 45, M192–N197. [Google Scholar] [CrossRef]
- Suresh, K.P. An overview of randomization techniques: An unbiased assessment of outcome in clinical research. J. Hum. Reprod. Sci. 2011, 4, 8–11. [Google Scholar] [CrossRef]
- Cantea, A.; Mihaiu, C.; Dascalu, A.; Calin, A. Mira. In Proceedings of the 31st International BCS Human Computer Interaction Conference, British, UK, 3–6 July 2017; pp. 1–3. [Google Scholar]
- Kim, J.H. Effects of a virtual reality video game exercise program on upper extremity function and daily living activities in stroke patients. J. Phys. Ther. Sci. 2018, 30, 1408–1411. [Google Scholar] [CrossRef] [Green Version]
- Dromerick, A.W.; Edwards, D.F.; Diringer, M.N. Sensitivity to changes in disability after stroke: A comparison of four scales useful in clinical trials. J. Rehabil. Res. Dev. 2003, 40, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schuster-Amft, C.; Eng, K.; Suica, Z.; Thaler, I.; Signer, S.; Lehmann, I.; Kiper, D. Effect of a four-week virtual reality-based training versus conventional therapy on upper limb motor function after stroke: A multicenter parallel group randomized trial. PLoS ONE 2018, 13, e0204455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brunner, I.; Skouen, J.S.; Hofstad, H.; Assmuss, J.; Becker, F.; Pallesen, H.; Thijs, L.; Verheyden, G. Is upper limb virtual reality training more intensive than conventional training for patients in the subacute phase after stroke? An analysis of treatment intensity and content. BMC Neurol. 2016, 16, 219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dębska, M.; Polechoński, J.; Mynarski, A.; Polechoński, P. Enjoyment and Intensity of Physical Activity in Immersive Virtual Reality Performed on Innovative Training Devices in Compliance with Recommendations for Health. Int. J. Environ. Res. Public Health 2019, 16, 3673. [Google Scholar] [CrossRef] [Green Version]
- Bevilacqua, R.; Maranesi, E.; Riccardi, G.R.; Donna, V.D.; Pelliccioni, P.; Luzi, R.; Pelliccioni, G. Non-Immersive Virtual Reality for Rehabilitation of the Older People: A Systematic Review into Efficacy and Effectiveness. J. Clin. Med. 2019, 8, 1882. [Google Scholar] [CrossRef] [Green Version]
- Young, D.E.; Schmidt, R.A. Augmented Kinematic Feedback for Motor Learning. J. Mot. Behav. 1992, 24, 261–273. [Google Scholar] [CrossRef]
- Ronsse, R.; Puttemans, V.; Coxon, J.P.; Goble, D.J.; Wagemans, J.; Wenderoth, N.; Swinnen, S.P. Motor learning with augmented feedback: Modality-dependent behavioral and neural consequences. Cereb. Cortex. 2011, 21, 1283–1294. [Google Scholar] [CrossRef] [Green Version]
- Adomaviciene, A.; Daunoraviciene, K.; Kubilius, R.; Varzaityte, L.; Raistenskis, J. Influence of New Technologies on Post-Stroke Rehabilitation: A Comparison of Armeo Spring to the Kinect System. Medicina 2019, 55, 98. [Google Scholar] [CrossRef] [Green Version]
- Jang, S.H.; You, S.H.; Hallett, M.; Cho, Y.W.; Park, C.M.; Cho, S.H.; Lee, H.Y.; Kim, T.H. Cortical reorganization and associated functional motor recovery after virtual reality in patients with chronic stroke: An experimenter-blind preliminary study. Arch. Phys. Med. Rehabil. 2005, 86, 2218–2223. [Google Scholar] [CrossRef]
- Indreica, E.S.; Henter, R. Occupational therapy in fostering kinetic therapist’s empathy. J. Plus. Educ. 2015, 12, 158–166. [Google Scholar]
- Igna, R.; Stefan, S.; Onac, I.; Onac, I.; Ungur, R.A.; Tatar, A.S. Mindfulness-based cognitive-behavior therapy (mcbt) versus virtual reality (vr) enhanced cbt, versus treatment as usual for chronic back pain. a clinical trial. J. Evidence-Based Psychother. 2014, 14, 229–247. [Google Scholar]
- Palaus, M.; Marron, E.M.; Viejo-Sobera, R.; Redolar-Ripoll, D. Neural Basis of Video Gaming: A Systematic Review. Front. Hum. Neurosci. 2017, 11, 248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brilliant, T.D.; Nouchi, R.; Kawashima, R. Does Video Gaming Have Impacts on the Brain: Evidence from a Systematic Review. Brain Sci. 2019, 9, 251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cortés-Pérez, I.; Nieto-Escamez, F.A.; Obrero-Gaitán, E. Immersive Virtual Reality in Stroke Patients as a New Approach for Reducing Postural Disabilities and Falls Risk: A Case Series. Brain Sci. 2020, 10, 296. [Google Scholar] [CrossRef]
- Lee, K.S. peed-Interactive Pedaling Training Using Smartphone Virtual Reality Application for Stroke Patients: Single-Blinded, Randomized Clinical Trial. Brain Sci. 2019, 9, 295. [Google Scholar] [CrossRef] [Green Version]
- Ballester, B.R.; Maier, M.; Duff, A.; Cameirão, M.; Bermúdez, S.; Duarte, E.; Cuxart, A.; Rodríguez, S.; San Segundo Mozo, R.M.; Verschure, P.F.M.J. A critical time window for recovery extends beyond one-year post-stroke. J. Neurophysiol. 2019, 122, 350–357. [Google Scholar] [CrossRef] [Green Version]
- Dušica, S.P.; Devečerski, G.V.; Jovićević, M.N.; Platiša, N.M. Stroke rehabilitation: Which factors influence the outcome? Ann. Indian Acad Neurol. 2015, 18, 484–487. [Google Scholar] [CrossRef]
- Jin, H.; Hong, C.; Chen, S. Consensus for prevention and management of coronavirus disease 2019 (COVID-19) for neurologists. Stroke Vasc. Neurol. 2020, 5, 146–151. [Google Scholar] [CrossRef] [Green Version]
- Ostwald, S.K.; Davis, S.; Hersch, G.; Kelley, C.; Godwin, K.M. Evidence-based educational guidelines for stroke survivors after discharge home. J. Neurosci. Nurs. 2008, 40, 173–191. [Google Scholar] [CrossRef] [Green Version]

| Characteristic | Subacute Experimental (n = 6) | Chronic Experimental (n = 20) | Subacute Control (n = 5) | Chronic Control (n = 21) |
|---|---|---|---|---|
| Affected side (left/right) | 3/3 | 10/10 | 2/3 | 5/16 |
| Hemorrhagic/Ischemic stroke | 2/4 | 5/15 | 1/4 | 7/14 |
| Gender (male/female) | 2/4 | 10/10 | 2/3 | 12/9 |
| High blood pressure (yes/no) | 6/0 | 16/4 | 4/1 | 16/5 |
| Dyslipidemia (yes/no) | 2/4 | 11/9 | 3/2 | 7/14 |
| Ischemic coronary disease (yes/no) | 2/4 | 12/8 | 0/5 | 9/12 |
| Diabetes (yes/no) | 1/5 | 5/15 | 0/5 | 1/20 |
| Age Groups | n/(%) | n/(%) | n/(%) | n/(%) |
| 41–50 years | 2 (3.85%) | 1 (1.92%) | 3 (5.77%) | 0 (0%) |
| 51–60 years | 1 (1.92%) | 8 (15.38%) | 0 (0%) | 4 (7.69%) |
| 61–70 years | 3 (5.77%) | 10 (19.23%) | 0 (0%) | 12 (23.08%) |
| 71–80 years | 0 (0%) | 1 (1.92%) | 2 (3.85%) | 5 (9.62%) |
| Post-stroke Duration | ||||
| 0–6 months | 6 (11.54%) | 0(0%) | 5 (9.62%) | 0 (0%) |
| 7 months–1 year | (0%) | 5 (9.62%) | (0%) | 6 (11.54%) |
| 1.1–2 years | (0%) | 9(17.31%) | (0%) | 7(13.46%) |
| 2.1–4 years | (0%) | 6 (11.54%) | (0%) | 8 (15.38%) |
| Mean (minutes)/SD VR Duration | 28.46/4.01 | 25.42/3.19 | 0/0 | 0/0 |
| Total Physiotherapy Duration (minutes) | 60 | 60 | 60 | 60 |
| SE Group (n = 6) | CE Group (n = 20) | SC Group (n = 6) | CC Group (n = 20) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean/SD | Mean Rank | p | Mean/SD | Mean Rank | p | Mean/SD | Mean Rank | p | Mean/SD | Mean Rank | p | |
| AROM | 9.41/2.86 | 3.50 | 0.028 | 9.00/4.48 | 10.50 | <0.001 | 4.98/3.99 | 3.50 | 0.077 | 1.91/4.96 | 11.64 | 0.099 |
| MMT | 0.82/0.28 | 3.50 | 0.028 | 0.58/0.22 | 10.50 | <0.001 | 0.36/0.29 | 3.00 | 0.041 | 0.44/0.14 | 11.00 | <0.001 |
| FMUE | 12.66/2.42 | 3.50 | 0.027 | 8.45/3.61 | 10.50 | <0.001 | 6.00/2.35 | 3.00 | 0.042 | 7.43/3.52 | 11.00 | <0.001 |
| FIM | 10.00/2.44 | 3.50 | 0.026 | 3.65/4.33 | 9.50 | <0.001 | 4.40/3.29 | 3.00 | 0.041 | 2.52/2.93 | 9 | <0.001 |
| FRT | 5.16/2.78 | 3.50 | 0.027 | 4.88/4.54 | 9.00 | <0.001 | 3.20/2.68 | 2.50 | 0.063 | 4.62/2.39 | 11.00 | <0.001 |
| MAS | 0/0 | 0 | 1 | 0/0 | 0 | 1 | 0.40/0.55 | 0 | 1 | 0.05/0.22 | 1.50 | 0.157 |
| MRS | −0.83/0.75 | 2 | 0.102 | −0.10/0.31 | 2.5 | 0.056 | −0.20/0.45 | 1 | 0.317 | 0/0 | 0 | 1 |
| Paired Differences | |||||||
|---|---|---|---|---|---|---|---|
| Mean | Std. Deviation | Minimum | Maximum | X2 | df | p | |
| AROM | 5.79 | 5.57 | −5.58 | 15.39 | 21.21 | 3 | <0.001 |
| MMT | 0.52 | 0.24 | 0.18 | 1.13 | 13.19 | 3 | <0.001 |
| FMUE | 8.28 | 3.71 | 2.00 | 17.00 | 11.49 | 3 | 0.009 |
| FIM | 4.00 | 4.12 | 0 | 15.00 | 13.14 | 3 | 0.004 |
| FRT | 4.64 | 3.39 | 0 | 19.00 | 1.40 | 3 | 0.704 |
| MAS | 0.05 | 0.23 | 0 | 1.00 | 12.16 | 3 | 0.007 |
| MRS | −0.15 | 0.41 | −2.00 | 0 | 18.36 | 3 | <0.001 |
| Outcome Measure | Pairwise Comparison | Mean Ranks | X2 | Std. Error | p |
|---|---|---|---|---|---|
| AROM | SE > SC | 36.50/22.00 | 20.08 | 4.73 | <0.001 |
| SE > CC | 36.50/15.79 | 20.71 | 7.01 | 0.019 | |
| MMT | SE > SC | 41.50/12.40 | 29.10 | 9.17 | 0.009 |
| SE > CC | 41.50/22.12 | 19.38 | 7.01 | 0.034 | |
| FMUE | SE > SC | 44.58/17.00 | 27.58 | 9.13 | 0.015 |
| SE > CC | 44.58/23.40 | 21.17 | 6.98 | 0.015 | |
| FIM | SE > SC | 45.33/32.60 | 21.30 | 6.86 | 0.011 |
| SE > CC | 45.33/22.02 | 23.31 | 6.82 | 0.004 | |
| MAS | SE < SC | 25.00/35.40 | −10.40 | 3.70 | 0.030 |
| CE < SC | 25.00/35.40 | −10.40 | 3.06 | 0.004 | |
| CC < SC | 26.24/35.40 | 9.16 | 3.04 | 0.016 | |
| MRS | SE < CE | 12.42/27.45 | −15.03 | 4.17 | 0.002 |
| SE < CC | 12.42/30.00 | −17.58 | 4.15 | <0.001 |
| Dependent Variable | Model | R2 | p Change | Unstandardized Coefficients | Standard Coeff. | p | 95.0% Confidence Interval for B | ||
|---|---|---|---|---|---|---|---|---|---|
| B | Std. Error | Beta | Lower Bound | Upper Bound | |||||
| AROM | (Constant) | 0.47 | <0.001 | 4.08 | 0.90 | <0.001 | 2.25 | 5.91 | |
| VR Time | 0.38 | 0.06 | 0.64 | <0.001 | 0.25 | 0.51 | |||
| Dyslipidemia | −3.72 | 1.15 | −0.33 | 0.002 | −6.05 | −1.39 | |||
| MMT | (Constant) | 0.16 | <0.001 | 0.40 | 0.03 | <0.001 | 0.32 | 0.48 | |
| VR Time | 0.01 | 0.00 | 0.55 | <0.001 | 0.01 | 0.02 | |||
| FMUE | (Constant) | 0.31 | 0.001 | 8,17 | 0.68 | <0.001 | 6.79 | 9.55 | |
| VR Time | 0.17 | 0.04 | 0.42 | 0.001 | 0.07 | 0.26 | |||
| ICD | −3.13 | 0.88 | −0.42 | 0.001 | −4.90 | −1.35 | |||
| FIM | (Constant) | 0.29 | 0.011 | 4.51 | 1.07 | <0.001 | 2.36 | 6.67 | |
| VR Time | 0.16 | 0.05 | 0.37 | 0.004 | 0.05 | 0.27 | |||
| P-S.D | −1.23 | 0.46 | −0.32 | 0.011 | −2.17 | −0.30 | |||
| MRS | (Constant) | 0.23 | 0.029 | −0.19 | 0.10 | 0.069 | −0.41 | 0.01 | |
| VR Time | −0.02 | 0.01 | −0.44 | 0.001 | −0.03 | −0.01 | |||
| P-S.D | 0.10 | 0.04 | 0.28 | 0.023 | 0.01 | 0.20 | |||
| Diabetes | 0.33 | 0.14 | 0.27 | 0.029 | 0.03 | 0.62 | |||
| Descriptive Statistics | |||||
|---|---|---|---|---|---|
| Linear Regression Models | n | Minimum | Maximum | Mean | Std. Deviation |
| Model 1 AROM | 52 | 0.35 | 13.71 | 5.79 | 3.83 |
| AROM-VR | 52 | 4.08 | 13.70 | 7.44 | 3.56 |
| AROM-Dyslipidemia | 52 | 0.35 | 4.08 | 2.43 | 1.86 |
| Model 1 MMT | 52 | 0.40 | 0.76 | 0.52 | 0.13 |
| Model 1 FMUE | 52 | 5.03 | 12.47 | 8.28 | 2.07 |
| FMUE-VR | 52 | 8.17 | 12.47 | 9.67 | 1.59 |
| FMUE-ICD | 52 | 5.04 | 8.17 | 6.78 | 1.57 |
| MODEL 1 FIM | 52 | −0.43 | 8.43 | 4.00 | 2.22 |
| FIM-VR | 52 | 4.52 | 8.69 | 5.97 | 1.54 |
| FIM-P-S.D | 52 | −0.44 | 4.39 | 2.54 | 1.34 |
| MODEL 1 MRS | 52 | −0.66 | 0.32 | −0.15 | 0.23 |
| MRS-VR | 52 | −5.20 | −0.20 | −1.94 | 1.85 |
| MRS-P-S.D | 52 | −0.19 | 0.23 | −0.02 | 0.11 |
| MRS-Diabetes | 52 | −0.20 | 0.13 | −0.15 | 0.11 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miclaus, R.; Roman, N.; Caloian, S.; Mitoiu, B.; Suciu, O.; Onofrei, R.R.; Pavel, E.; Neculau, A. Non-Immersive Virtual Reality for Post-Stroke Upper Extremity Rehabilitation: A Small Cohort Randomized Trial. Brain Sci. 2020, 10, 655. https://doi.org/10.3390/brainsci10090655
Miclaus R, Roman N, Caloian S, Mitoiu B, Suciu O, Onofrei RR, Pavel E, Neculau A. Non-Immersive Virtual Reality for Post-Stroke Upper Extremity Rehabilitation: A Small Cohort Randomized Trial. Brain Sciences. 2020; 10(9):655. https://doi.org/10.3390/brainsci10090655
Chicago/Turabian StyleMiclaus, Roxana, Nadinne Roman, Silviu Caloian, Brindusa Mitoiu, Oana Suciu, Roxana Ramona Onofrei, Ecaterina Pavel, and Andrea Neculau. 2020. "Non-Immersive Virtual Reality for Post-Stroke Upper Extremity Rehabilitation: A Small Cohort Randomized Trial" Brain Sciences 10, no. 9: 655. https://doi.org/10.3390/brainsci10090655
APA StyleMiclaus, R., Roman, N., Caloian, S., Mitoiu, B., Suciu, O., Onofrei, R. R., Pavel, E., & Neculau, A. (2020). Non-Immersive Virtual Reality for Post-Stroke Upper Extremity Rehabilitation: A Small Cohort Randomized Trial. Brain Sciences, 10(9), 655. https://doi.org/10.3390/brainsci10090655