Bypass Procedure Performed in the Field of a Decompressive Craniectomy in the Case of an MCA Dissecting Aneurysm: Case Report and Review of the Literature
Abstract
:1. Introduction
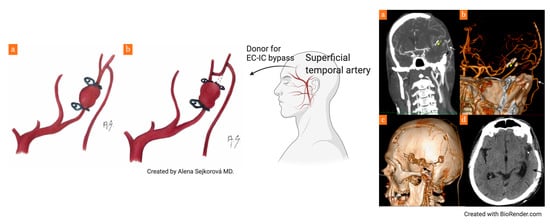
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lawton, M.T.; Hamilton, M.G.; Morcos, J.J.; Spetzler, R.F. Revascularization and Aneurysm Surgery: Current techniques, Indications and Outcome. Neurosurgery 1996, 38, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Kalani, M.Y.S.; Zabramski, J.M.; Hu, Y.C.; Spetzler, R.F. Extracranial-Intracranial Bypass and Vessel Occlusion for the Treatment of Unclippable Giant Middle Cerebral Artery Aneurysms. Neurosurgery 2012, 72, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Lawton, M.T. Seven Aneurysms: Tenets and Techniques for Clipping, 1st ed.; Thieme: New York, NY, USA, 2010. [Google Scholar] [CrossRef]
- Lawton, M.T. Seven Bypasses: Tenets and Techniques for Revascularization, 1st ed.; Thieme: New York, NY, USA, 2018. [Google Scholar] [CrossRef]
- Hernesniemi, J.; Dashti, R.; Niemelä, M.; Romani, R.; Rinne, J.; Jääskeläinen, J.E. Microsurgical and Angiographic Anatomy of Middle Cerebral Artery Aneurysm. Neurosurgery 2010, 66, E1030. [Google Scholar] [CrossRef] [PubMed]
- Krisht, A.F.; Krayenbühl, N.; Sercl, D.; Bikmaz, K.; Kadri, P.A. Results of Microsurgical Clipping of 50 High Complexity Basilarapex Aneurysms. Neurosurgery 2007, 60, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Ota, N.; Matsukawa, H.; Noda, K.; Sato, H.; Hatano, Y.; Hashimoto, A.; Miyazaki, T.; Kondo, T.; Kinoshita, Y.; Saito, N.; et al. Evaluation of Microsurgery for Managing Giant or Complex Cerebral Aneurysms: A Retrospective Study. World Neurosurg. 2018, 115, e190–e199. [Google Scholar] [CrossRef] [PubMed]
- Solomon, R.A.; Fukushima, T. New aneurysm clip appliers for “key-hole” neurosurgery. Neurosurgery 1991, 28, 474–476. [Google Scholar] [CrossRef]
- Meybodi, A.T.; Huang, W.; Benet, A.; Kola, O.; Lawton, M. Bypass surgery for complex middle cerebral artery aneurysms: An algorithmic approach to revascularization. J. Neurosurg. 2017, 127, 463–479. [Google Scholar] [CrossRef]
- Kivipelto, L.; Niemelä, M.; Meling, T.; Lehecka, M.; Lehto, H.; Hernesniemi, J. Bypass surgery for complex middle cerebral artery aneurysms: Impact of the exact location in the MCA tree. J. Neurosurg. 2014, 120, 398–408. [Google Scholar] [CrossRef] [PubMed]
- Burkhardt, J.-K.; Yousef, S.; Tabani, H.; Benet, A.; Rubio, R.R.; Lawton, M.T. Combination Superficial Temporal Artery-Middle Cerebral Artery Bypass and M2–M2 Reanastomosis With Trapping of a Stented Distal Middle Cerebral Artery Aneurysm: 3-Dimensional Operative Video. Oper. Neurosurg. 2018, 15, E67–E68. [Google Scholar] [CrossRef] [PubMed]
- Stambolija, V.; Mrak, G.; Lozic, M.; Ljevak, J.; Bublic, M.M.; Scap, M. Intraoperative Eptifibatide Administration During Urgent Arterial Bypass in Neurosurgery. World Neurosurg. 2017, 103, 952.e5–952.e9. [Google Scholar] [CrossRef] [PubMed]
- Saito, H.; Ogasawara, K.; Kubo, Y.; Saso, M.; Otawara, Y.; Ogawa, A. Treatement of Ruptured Spontaneous Saccular Aneurysm in the Central Artery of the Middle Cerebral Artery Using Bypass Surgery with Trapping. Neurol. Med. Chir. (Tokyo) 2007, 47, 471–474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakamoto, S.; Ikawa, F.; Kawamoto, H.; Ohbayashi, N.; Inagawa, T. Acute surgery for ruptured dissecting aneurysm of the M3 portion of the middle cerebral artery. Neurol. Med. Chir. 2003, 43, 188–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakahara, I.; Taha, M.M.; Higashi, T.; Iwamuro, Y.; Iwaasa, M.; Watanabe, Y.; Tsunetoshi, K.; Munemitsu, T. Different modalities of treatment of intracranial mycotic aneurysms: Report of 4 cases. Surg. Neurol. 2006, 66, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Qian, H.; Shi, X.; Brooks, K.S.; Zhao, X.; Shi, X.; Lei, T. MCA-to-MCA Bypass with Interposition Graft for Ruptured Mycotic Middle Cerebral Artery Aneurysm. World Neurosurg. 2019, 122, 195. [Google Scholar] [CrossRef] [PubMed]
- Aljuboori, Z.; Meyer, K.; Ding, D.; James, R.; Meyer, K. Endovascular Treatment of a Traumatic Middle Cerebral Artery Pseudoaneurysm with the Pipeline Flex Embolization Device. World Neurosurg. 2019, 133, 201–204. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartoš, R.; Lodin, J.; Hejčl, A.; Humhej, I.; Concepción, I.; Cihlář, F.; Sameš, M. Bypass Procedure Performed in the Field of a Decompressive Craniectomy in the Case of an MCA Dissecting Aneurysm: Case Report and Review of the Literature. Brain Sci. 2021, 11, 29. https://doi.org/10.3390/brainsci11010029
Bartoš R, Lodin J, Hejčl A, Humhej I, Concepción I, Cihlář F, Sameš M. Bypass Procedure Performed in the Field of a Decompressive Craniectomy in the Case of an MCA Dissecting Aneurysm: Case Report and Review of the Literature. Brain Sciences. 2021; 11(1):29. https://doi.org/10.3390/brainsci11010029
Chicago/Turabian StyleBartoš, Robert, Jan Lodin, Aleš Hejčl, Ivan Humhej, Ingrid Concepción, Filip Cihlář, and Martin Sameš. 2021. "Bypass Procedure Performed in the Field of a Decompressive Craniectomy in the Case of an MCA Dissecting Aneurysm: Case Report and Review of the Literature" Brain Sciences 11, no. 1: 29. https://doi.org/10.3390/brainsci11010029