Oscillatory EEG Signatures of Affective Processes during Interaction with Adaptive Computer Systems
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. General Study Design and Cover Story
- Supportive adaptation: The system helps the participant to reach the target faster by rearranging the menu layout so that the number of remaining navigation steps is reduced. This kind of system adaptation is expected to induce a positive affective user reaction.
- Impeding adaptation: The system hinders the participant reaching their target by rearranging the menu layout so that the number of remaining navigation steps is increased. In this condition, we assume induced negative affective user reactions.
- No adaptation: As a baseline, the system does not perform any adaptive behavior.
2.3. Experimental Design and Trial Procedure
2.4. Measurement Set-Up and Data Recording
2.5. Data Analysis of Subjective Affective Experience
2.6. EEG Data Pre-Processing
2.7. Estimation of Event-Related Spectral Pertubation
2.8. Estimation of Functional Cortical Networks
2.9. Statistical Analysis of Event-Related Spectral Pertubations and Functional Connectivity
3. Results
3.1. Subjective Affective Reations to Adaptive System Behavior
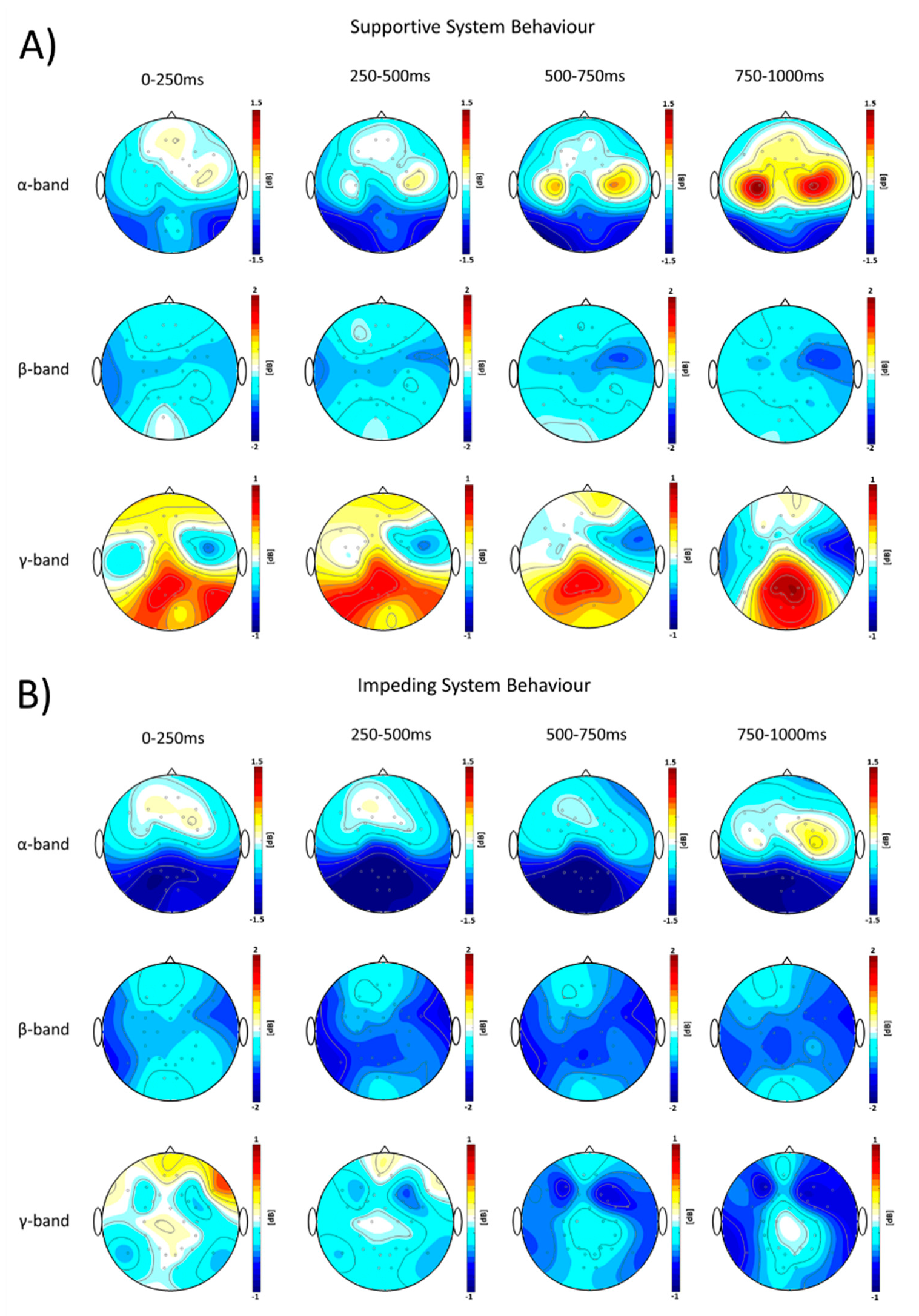
3.2. Regional Frequency Domain Specific Neuronal Signatures of Affective Processes to Adaptive System Behavior
3.3. Global Frequency Domain Specific Neuronal Signatures of Affective Processes to Adaptive System Behavior
4. Discussion
4.1. Difference in Regional and Global Oscillatory Neuronal Signatures
4.2. Processing of Computer-Generated Feedback
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Picard, R.W.; Vyzas, E.; Healey, J. Toward Machine Emotional Intelligence: Analysis of Affective Physiological State. IEEE Trans. Pattern Anal. Mach. Intell. 2001, 23, 1175–1191. [Google Scholar] [CrossRef]
- Vermeeren, A.P.O.S.; Law, E.L.-C.; Roto, V.; Obrist, M.; Hoonhout, J.; Väänänen-Vainio-Mattila, K. User experience evaluation methods: Current state and development needs. In Proceedings of the 6th Nordic Conference on Human-Computer Interaction Extending Boundaries—NordiCHI ’10, New York, NY, USA, 16–20 October 2010; Association for Computing Machinery (ACM): New York, NY, USA, 2010; pp. 521–530. [Google Scholar]
- Grissmann, S.; Zander, T.O.; Faller, J.; Brönstrup, J.; Kelava, A.; Gramann, K.; Gerjets, P. Affective Aspects of Perceived Loss of Control and Potential Implications for Brain-Computer Interfaces. Front. Hum. Neurosci. 2017, 11, 370. [Google Scholar] [CrossRef]
- Mühl, C.; Allison, B.Z.; Nijholt, A.; Chanel, G. A survey of affective brain computer interfaces: Principles, state-of-the-art, and challenges. Brain Comput. Interfaces 2014, 1, 66–84. [Google Scholar] [CrossRef]
- Partala, T.; Surakka, V. The effects of affective interventions in human–computer interaction. Interact. Comput. 2004, 16, 295–309. [Google Scholar] [CrossRef]
- Reuderink, B.; Mühl, C.; Poel, M. Valence, arousal and dominance in the EEG during game play. Int. J. Auton. Adapt. Commun. Syst. 2013, 6, 45. [Google Scholar] [CrossRef]
- Scheirer, J.; Fernandez, R.; Klein, J.; Picard, R.W. Frustrating the User on Purpose: A Step Toward Building an Affective Computer. Interact. Comput. 2002, 14, 93–118. [Google Scholar] [CrossRef]
- He, Z.; Li, Z.; Yang, F.; Wang, L.; Li, J.; Zhou, C.; Pan, J. Advances in Multimodal Emotion Recognition Based on Brain–Computer Interfaces. Brain Sci. 2020, 10, 687. [Google Scholar] [CrossRef] [PubMed]
- Al-Nafjan, A.; Alharthi, K.; Kurdi, H. Lightweight Building of an Electroencephalogram-Based Emotion Detection System. Brain Sci. 2020, 10, 781. [Google Scholar] [CrossRef] [PubMed]
- Fairclough, S.H. Fundamentals of Physiological Computing. Interact. Comput. 2009, 21, 133–145. [Google Scholar] [CrossRef]
- Hettinger, L.J.; Branco, P.; Encarnação, L.M.; Bonato, P. Neuroadaptive technologies: Applying Neuroergonomics to the Design of Advanced Interfaces. Theor. Issues Ergon. Sci. 2003, 4, 220–237. [Google Scholar] [CrossRef]
- Zander, T.O.; Krol, L.R.; Birbaumer, N.P.; Gramann, K. Neuroadaptive Technology Enables Implicit Cursor Control Based on Medial Prefrontal Cortex Activity. Proc. Natl. Acad. Sci. USA 2016, 113, 14898–14903. [Google Scholar] [CrossRef] [PubMed]
- Barrett, L.F.; Satpute, A.B. Large-scale Brain Networks in Affective and Social Neuroscience: Towards an Integrative Functional Architecture of the Brain. Curr. Opin. Neurobiol. 2013, 23, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Cromwell, H.C.; Panksepp, J. Rethinking the Cognitive Revolution from a Neural Perspective: How Overuse/Misuse of the Term ‘Cognition’ and the Neglect of Affective Controls in Behavioral Neuroscience could be Delaying Progress in Understanding the BrainMind. Neurosci. Biobehav. Rev. 2011, 35, 2026–2035. [Google Scholar] [CrossRef] [PubMed]
- Balconi, M.; Grippa, E.; Vanutelli, M.E. What Hemodynamic (fNIRS), Electrophysiological (EEG) and Autonomic Integrated Measures can tell us about Emotional Processing. Brain Cogn. 2015, 95, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Cuthbert, B.N.; Schupp, H.T.; Bradley, M.M.; Birbaumer, N.; Lang, P.J. Brain Potentials in Affective Picture Processing: Covariation with Autonomic Arousal and Affective Report. Biol. Psychol. 2000, 52, 95–111. [Google Scholar] [CrossRef]
- Koelstra, S.; Muhl, C.; Soleymani, M.; Lee, J.-S.; Yazdani, A.; Ebrahimi, T.; Pun, T.; Nijholt, A.; Patras, I. DEAP: A Database for Emotion Analysis;Using Physiological Signals. IEEE Trans. Affect. Comput. 2011, 3, 18–31. [Google Scholar] [CrossRef]
- Lang, P.J.; Bradley, M.M.; Cuthbert, B.N. International Affective Picture System (IAPS): Affective Ratings of Pictures and Instruction Manual; Technical Report-8; University of Florida: Gainesville, FL, USA, 2008. [Google Scholar]
- Lang, P.J.; Bradley, M.M. Emotion and the Motivational Brain. Biol. Psychol. 2010, 84, 437–450. [Google Scholar] [CrossRef]
- Nummenmaa, L.; Niemi, P. Inducing Affective States with Success-Failure Manipulations: A meta-analysis. Emotion 2004, 4, 207–214. [Google Scholar] [CrossRef]
- Damasio, A.; Carvalho, G.B. The Nature of Feelings: Evolutionary and Neurobiological Origins. Nat. Rev. Neurosci. 2013, 14, 143–152. [Google Scholar] [CrossRef]
- Panksepp, J.; Lane, R.D.; Solms, M.; Smith, R. Reconciling Cognitive and Affective Neuroscience Perspectives on the Brain Basis of Emotional Experience. Neurosci. Biobehav. Rev. 2017, 76, 187–215. [Google Scholar] [CrossRef]
- Mauri, M.; Magagnin, V.; Cipresso, P.; Mainardi, L.T.; Brown, E.N.; Cerutti, S.; Villamira, M.; Barbieri, R. Psychophysiological Signals Associated with Affective States. In Proceedings of the 2010 Annual International Conference of the IEEE Engineering in Medicine and Biology, Buenos Aires, Argentina, 31 August–4 September 2010; Institute of Electrical and Electronics Engineers (IEEE): Piscataway, NJ, USA, 2010; Volume 2010, pp. 3563–3566. [Google Scholar] [CrossRef]
- Prendinger, H.; Mori, J.; Ishizuka, M. Using Human Physiology to Evaluate Subtle Expressivity of a Virtual Quizmaster in a Mathematical Game. Int. J. Hum. Comput. Stud. 2005, 62, 231–245. [Google Scholar] [CrossRef]
- Rani, P.; Liu, C.; Sarkar, N.; Vanman, E.J. An Empirical Study of Machine Learning Techniques for Affect Recognition in Human–Robot Interaction. Pattern Anal. Appl. 2006, 9, 58–69. [Google Scholar] [CrossRef]
- Vasiljevic, G.A.M.; De Miranda, L.C. Brain–Computer Interface Games Based on Consumer-Grade EEG Devices: A Systematic Literature Review. Int. J. Hum. Comput. Interact. 2019, 36, 105–142. [Google Scholar] [CrossRef]
- Phan, K.L.; Wager, T.; Taylor, S.F.; Liberzon, I. Functional Neuroanatomy of Emotion: A Meta-Analysis of Emotion Activation Studies in PET and fMRI. NeuroImage 2002, 16, 331–348. [Google Scholar] [CrossRef]
- Sabatinelli, D.; Lang, P.J.; Keil, A.; Bradley, M.M. Emotional Perception: Correlation of Functional MRI and Event-Related Potentials. Cereb. Cortex 2006, 17, 1085–1091. [Google Scholar] [CrossRef]
- Sitaram, R.; Lee, S.; Ruiz, S.; Rana, M.; Veit, R.; Birbaumer, N. Real-time Support Vector Classification and Feedback of Multiple Emotional Brain States. NeuroImage 2011, 56, 753–765. [Google Scholar] [CrossRef]
- Suhaimi, N.S.; Mountstephens, J.; Teo, J. EEG-Based Emotion Recognition: A State-of-the-Art Review of Current Trends and Opportunities. Comput. Intell. Neurosci. 2020, 2020, 1–19. [Google Scholar] [CrossRef]
- Torres, P.E.P.; Torres, E.A.; Hernández-Álvarez, M.; Yoo, S.G. EEG-Based BCI Emotion Recognition: A Survey. Sensors 2020, 20, 5083. [Google Scholar] [CrossRef]
- Appriou, A.; Cichocki, A.; Lotte, F. Modern Machine Learning Algorithms to Classify Cognitive and Affective States from Electroencephalography Signals. In IEEE Systems, Man and Cybernetics Magazine; Institute of Electrical and Electronics Engineers: Piscataway, NJ, USA, 2020. [Google Scholar]
- Zheng, W.-L.; Zhu, J.-Y.; Lu, B.-L. Identifying Stable Patterns over Time for Emotion Recognition from EEG. IEEE Trans. Affect. Comput. 2019, 10, 417–429. [Google Scholar] [CrossRef]
- Kim, M.-K.; Kim, M.; Oh, E.; Kim, S.-P. A Review on the Computational Methods for Emotional State Estimation from the Human EEG. Comput. Math. Methods Med. 2013, 2013, 1–13. [Google Scholar] [CrossRef]
- Brouwer, A.-M.; Stuldreher, I.; Penen, S.H.; Lingelbach, K.; Vukelić, M. Combining eye tracking and physiology for detection of emotion and workload. In Proceedings of the 12th International Conference on Measurement and Behavioural and 6th International Seminar on Behavioral Methods, Krakow, Poland , 15–18 October 2021; Volume 1, pp. 2–11. [Google Scholar] [CrossRef]
- Dolcos, F.; Cabeza, R. Event-Related Potentials of Emotional Memory: Encoding pleasant, unpleasant, and neutral pictures. Cogn. Affect. Behav. Neurosci. 2002, 2, 252–263. [Google Scholar] [CrossRef] [PubMed]
- Olofsson, J.K.; Nordin, S.; Sequeira, H.; Polich, J. Affective Picture Processing: An Integrative Review of ERP Findings. Biol. Psychol. 2008, 77, 247–265. [Google Scholar] [CrossRef] [PubMed]
- Schupp, H.T.; Junghöfer, M.; Weike, A.I.; Hamm, A. The Selective Processing of Briefly Presented Affective Pictures: An ERP Analysis. Psychophysiology 2004, 41, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, A.; Hajcak, G. Beyond Good and Evil: The Time-Course of Neural Activity Elicited by Specific Picture Content. Emotion 2010, 10, 767–782. [Google Scholar] [CrossRef] [PubMed]
- Aftanas, L.I.; Varlamov, A.; Pavlov, S.; Makhnev, V.; Reva, N. Event-Related Synchronization and Desynchronization during Affective Processing: Emergence of Valence-Related Time-Dependent Hemispheric Asymmetries in Theta and Upper Alpha Band. Int. J. Neurosci. 2001, 110, 197–219. [Google Scholar] [CrossRef] [PubMed]
- Balconi, M.; Lucchiari, C. EEG Correlates (Event-Related Desynchronization) of Emotional Face Elaboration: A Temporal Analysis. Neurosci. Lett. 2006, 392, 118–123. [Google Scholar] [CrossRef]
- Balconi, M.; Mazza, G. Brain Oscillations and BIS/BAS (Behavioral Inhibition/Activation System) Effects on Processing Masked Emotional Cues. Int. J. Psychophysiol. 2009, 74, 158–165. [Google Scholar] [CrossRef]
- Baumgartner, T.; Esslen, M.; Jäncke, L. From Emotion Perception to Emotion Experience: Emotions Evoked by Pictures and Classical Music. Int. J. Psychophysiol. 2006, 60, 34–43. [Google Scholar] [CrossRef]
- Bekkedal, M.Y.; Rossi, J.; Panksepp, J. Human Brain EEG Indices of Emotions: Delineating Responses to Affective Vocalizations by Measuring Frontal Theta Event-Related Synchronization. Neurosci. Biobehav. Rev. 2011, 35, 1959–1970. [Google Scholar] [CrossRef]
- Garcia-Garcia, M.; Yordanova, J.; Kolev, V.; Domínguez-Borràs, J.; Escera, C. Tuning the Brain for Novelty Detection Under Emotional Threat: The Role of Increasing Gamma Phase-Synchronization. NeuroImage 2010, 49, 1038–1044. [Google Scholar] [CrossRef]
- Keil, A.; Sabatinelli, D.; Ding, M.; Lang, P.J.; Ihssen, N.; Heim, S. Re-entrant Projections Modulate Visual Cortex in Affective Perception: Evidence from Granger Causality Analysis. Hum. Brain Mapp. 2007, 30, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Keil, A.; Costa, V.D.; Smith, J.C.; Sabatinelli, D.; McGinnis, E.M.; Bradley, M.M.; Lang, P.J. Tagging Cortical Networks in Emotion: A Topographical Analysis. Hum. Brain Mapp. 2011, 33, 2920–2931. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Liu, C.; Chen, X. Emotional Modulation of Conflict Processing in the Affective Domain: Evidence from Event-related Potentials and Event-related Spectral Perturbation Analysis. Sci. Rep. 2016, 6, 31278. [Google Scholar] [CrossRef] [PubMed]
- Martini, N.; Menicucci, D.; Sebastiani, L.; Bedini, R.; Pingitore, A.; Vanello, N.; Milanesi, M.; Landini, L.; Gemignani, A. The Dynamics of EEG Gamma Responses to Unpleasant Visual Stimuli: From Local Activity to Functional Connectivity. NeuroImage 2012, 60, 922–932. [Google Scholar] [CrossRef]
- Miskovic, V.; Schmidt, L.A. Frontal Brain Electrical Asymmetry and Cardiac Vagal Tone Predict Biased Attention to Social Threat. Int. J. Psychophysiol. 2010, 75, 332–338. [Google Scholar] [CrossRef]
- Miskovic, V.; Schmidt, L.A. Cross-regional Cortical Synchronization During Affective Image Viewing. Brain Res. 2010, 1362, 102–111. [Google Scholar] [CrossRef]
- Müller, M.M.; Keil, A.; Gruber, T.; Elbert, T. Processing of Affective Pictures Modulates Right-Hemispheric Gamma Band EEG Activity. Clin. Neurophysiol. 1999, 110, 1913–1920. [Google Scholar] [CrossRef]
- Wyczesany, M.; Grzybowski, S.J.; Barry, R.J.; Kaiser, J.; Coenen, A.M.L.; Potoczek, A. Covariation of EEG Synchronization and Emotional State as Modified by Anxiolytics. J. Clin. Neurophysiol. 2011, 28, 289–296. [Google Scholar] [CrossRef]
- Wu, X.; Zheng, W.-L.; Lu, B.-L. Investigating EEG-Based Functional Connectivity Patterns for Multimodal Emotion Recognition. arXiv 2020, arXiv:2004.01973. [Google Scholar]
- Pollmann, K.; Ziegler, D.; Peissner, M.; Vukelić, M. A New Experimental Paradigm for Affective Research in Neuro-Adaptive Technologies. In Proceedings of the 2017 ACM Workshop on An Application-oriented Approach to BCI out of the Laboratory, BCIforReal ’17, Limassol, Cyprus, 13 March 2017. [Google Scholar] [CrossRef]
- Brendl, C.M.; Higgins, E.T. Principles of Judging Valence: What Makes Events Positive or Negative. In Advances in Experimental Social Psychology; Elsevier BV: Amsterdam, The Netherlands, 1996; Volume 28, pp. 95–160. [Google Scholar] [CrossRef]
- Sander, D.; Grandjean, D.; Scherer, K.R. A Systems Approach to Appraisal Mechanisms in Emotion. Neural Netw. 2005, 18, 317–352. [Google Scholar] [CrossRef]
- Scherer, K.R.; Schorr, A.; Johnstone, T. (Eds.) Appraisal Processes in Emotion: Theory, Methods, Research; Oxford University Press: Oxford, UK; New York, NY, USA, 2001. [Google Scholar]
- Krol, L.R.; Zander, T.O.; Ham, J.; Spagnolli, A.; Blankertz, B.; Gamberini, L.; Jacucci, G. Towards a Conceptual Framework for Cognitive Probing. In Symbiotic Interaction; Springer International Publishing: Cham, Switzerland, 2018; Volume 10727, pp. 74–78. [Google Scholar]
- Lee, Y.-Y.; Hsieh, S. Classifying Different Emotional States by Means of EEG-Based Functional Connectivity Patterns. PLoS ONE 2014, 9, e95415. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Liu, H.; Xu, P.; Si, Y.; Li, C.; Li, F.; Zhu, X.; Huang, X.; Zeng, Y.; Yao, D.; et al. EEG Based Emotion Recognition by Combining Functional Connectivity Network and Local Activations. IEEE Trans. Biomed. Eng. 2019, 66, 2869–2881. [Google Scholar] [CrossRef] [PubMed]
- Peissner, M.; Edlin-White, R.; Kotzé, P.; Marsden, G.; Lindgaard, G.; Wesson, J.; Winckler, M. User Control in Adaptive User Interfaces for Accessibility. In Human-Computer Interaction—INTERACT 2013; Springer: Berlin/Heidelberg, Germany, 2013; Volume 8117, pp. 623–640. [Google Scholar]
- Bradley, M.M.; Lang, P.J. Measuring Emotion: The Self-Assessment Manikin and the Semantic Differential. J. Behav. Ther. Exp. Psychiatry 1994, 25, 49–59. [Google Scholar] [CrossRef]
- Nunez, P.L.; Srinivasan, R. Electric Fields of the Brain: The Neurophysics of EEG, 2nd ed.; Oxford University Press: Oxford, UK; New York, NY, USA, 2006. [Google Scholar]
- Delorme, A.; Makeig, S. EEGLAB: An Open Source Toolbox for Analysis of Single-Trial EEG Dynamics Including Independent Component Analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Chaumon, M.; Bishop, D.V.; Busch, N.A. A Practical Guide to the Selection of Independent Components of the Electroencephalogram for Artifact Correction. J. Neurosci. Methods 2015, 250, 47–63. [Google Scholar] [CrossRef] [PubMed]
- Hipp, J.; Siegel, M. Dissociating Neuronal Gamma-Band Activity from Cranial and Ocular Muscle Activity in EEG. Front. Hum. Neurosci. 2013, 7, 338. [Google Scholar] [CrossRef] [PubMed]
- Nolte, G.; Bai, O.; Wheaton, L.; Mari, Z.; Vorbach, S.; Hallett, M. Identifying True Brain Interaction from EEG Data Using the Imaginary Part of Coherency. Clin. Neurophysiol. 2004, 115, 2292–2307. [Google Scholar] [CrossRef]
- Ewald, A.; Marzetti, L.; Zappasodi, F.; Meinecke, F.C.; Nolte, G. Estimating True Brain Connectivity from EEG/MEG Data Invariant to Linear and Static Transformations in Sensor Space. NeuroImage 2012, 60, 476–488. [Google Scholar] [CrossRef]
- Mitra, P.; Bokil, H. Observed Brain Dynamics; Oxford University Press (OUP): Oxford, UK, 2007; ISBN 9780195178081. [Google Scholar]
- Percival, D.B.; Walden, A.T. Spectral Analysis for Physical Applications: Multitaper and Conventional Univariate Techniques. J. Am. Stat. Assoc. 1997, 92, 1226. [Google Scholar] [CrossRef]
- Rosenberg, J.; Amjad, A.; Breeze, P.; Brillinger, D.; Halliday, D. The Fourier Approach to the Identification of Functional Coupling between Neuronal Spike Trains. Prog. Biophys. Mol. Biol. 1989, 53, 1–31. [Google Scholar] [CrossRef]
- Notturno, F.; Marzetti, L.; Pizzella, V.; Uncini, A.; Zappasodi, F. Local and Remote Effects of Transcranial Direct Current Stimulation on the Electrical Activity of the Motor Cortical Network. Hum. Brain Mapp. 2013, 35, 2220–2232. [Google Scholar] [CrossRef] [PubMed]
- Vukelić, M.; Gharabaghi, A. Oscillatory Entrainment of the Motor Cortical Network During Motor Imagery is Modulated by the Feedback Modality. NeuroImage 2015, 111, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Maris, E.; Schoffelen, J.-M.; Fries, P. Nonparametric Statistical Testing of Coherence Differences. J. Neurosci. Methods 2007, 163, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Maris, E.; Oostenveld, R. Nonparametric Statistical Testing of EEG- and MEG-Data. J. Neurosci. Methods 2007, 164, 177–190. [Google Scholar] [CrossRef]
- Oostenveld, R.; Fries, P.; Maris, E.; Schoffelen, J.-M. FieldTrip: Open Source Software for Advanced Analysis of MEG, EEG, and Invasive Electrophysiological Data. Comput. Intell. Neurosci. 2010, 2011, 1–9. [Google Scholar] [CrossRef]
- Engel, A.K.; Gerloff, C.; Hilgetag, C.C.; Nolte, G. Intrinsic Coupling Modes: Multiscale Interactions in Ongoing Brain Activity. Neuron 2013, 80, 867–886. [Google Scholar] [CrossRef]
- Güntekin, B.; Başar, E. A Review of Brain Oscillations in Perception of Faces and Emotional Pictures. Neuropsychologia 2014, 58, 33–51. [Google Scholar] [CrossRef]
- Siegel, M.; Donner, T.H.; Engel, A.K. Spectral Fingerprints of Large-Scale Neuronal Interactions. Nat. Rev. Neurosci. 2012, 13, 121–134. [Google Scholar] [CrossRef]
- Pfurtscheller, G.; Da Silva, F.L. Event-Related EEG/MEG Synchronization and Desynchronization: Basic Principles. Clin. Neurophysiol. 1999, 110, 1842–1857. [Google Scholar] [CrossRef]
- Davis, N.J.; Tomlinson, S.P.; Morgan, H.M. The Role of Beta-Frequency Neural Oscillations in Motor Control. J. Neurosci. 2012, 32, 403–404. [Google Scholar] [CrossRef]
- Engel, A.K.; Fries, P. Beta-band Oscillations—Signalling the Status Quo? Curr. Opin. Neurobiol. 2010, 20, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Hipp, J.; Engel, A.K.; Siegel, M. Oscillatory Synchronization in Large-Scale Cortical Networks Predicts Perception. Neuron 2011, 69, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Kilavik, B.E.; Zaepffel, M.; Brovelli, A.; Mackay, W.A.; Riehle, A. The ups and Downs of Beta Oscillations in Sensorimotor Cortex. Exp. Neurol. 2013, 245, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.J.; Schalk, G.; Fetz, E.E.; Nijs, M.D.; Ojemann, J.G.; Rao, R.P.N. Cortical Activity During Motor Execution, Motor Imagery, and Imagery-Based Online Feedback. Proc. Natl. Acad. Sci. USA 2010, 107, 4430–4435. [Google Scholar] [CrossRef]
- Jensen, O.; Kaiser, J.; Lachaux, J.-P. Human Gamma-Frequency Oscillations Associated with Attention and Memory. Trends Neurosci. 2007, 30, 317–324. [Google Scholar] [CrossRef]
- Klimesch, W.; Efellinger, R.; Efreunberger, R. Alpha Oscillations and Early Stages of Visual Encoding. Front. Psychol. 2011, 2, 118. [Google Scholar] [CrossRef]
- Palva, S.; Palva, J.M. New Vistas for α-Frequency Band Oscillations. Trends Neurosci. 2007, 30, 150–158. [Google Scholar] [CrossRef]
- Siegel, M.; Donner, T.H.; Oostenveld, R.; Fries, P.; Engel, A.K. Neuronal Synchronization along the Dorsal Visual Pathway Reflects the Focus of Spatial Attention. Neuron 2008, 60, 709–719. [Google Scholar] [CrossRef]
- Siegel, M.; Donner, T.H.; Oostenveld, R.; Fries, P.; Engel, A.K. High-Frequency Activity in Human Visual Cortex Is Modulated by Visual Motion Strength. Cereb. Cortex 2006, 17, 732–741. [Google Scholar] [CrossRef]
- Engel, A.K.; Singer, W. Temporal Binding and the Neural Correlates of Sensory Awareness. Trends Cogn. Sci. 2001, 5, 16–25. [Google Scholar] [CrossRef]
- Miller, K.J.; Hermes, D.; Honey, C.J.; Hebb, A.O.; Ramsey, N.F.; Knight, R.T.; Ojemann, J.G.; Fetz, E.E. Human Motor Cortical Activity Is Selectively Phase-Entrained on Underlying Rhythms. PLoS Comput. Biol. 2012, 8, e1002655. [Google Scholar] [CrossRef] [PubMed]
- Schulz, H.; Übelacker, T.; Keil, J.; Müller, N.; Weisz, N. Now I am Ready—Now I am not: The Influence of Pre-TMS Oscillations and Corticomuscular Coherence on Motor-Evoked Potentials. Cereb. Cortex 2014, 24, 1708–1719. [Google Scholar] [CrossRef] [PubMed]
- De Cesarei, A.; Codispoti, M. Affective Modulation of the LPP and α-ERD during Picture Viewing. Psychophysiology 2011, 48, 1397–1404. [Google Scholar] [CrossRef] [PubMed]
- Güntekin, B.; Emek-Savaş, D.D.; Kurt, P.; Yener, G.G.; Başar, E. Beta Oscillatory Responses in Healthy Subjects and Subjects with Mild Cognitive Impairment. NeuroImage Clin. 2013, 3, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Onoda, K.; Okamoto, Y.; Shishida, K.; Hashizume, A.; Ueda, K.; Yamashita, H.; Yamawaki, S. Anticipation of Affective Images and Event-Related Desynchronization (ERD) of Alpha Activity: An MEG Study. Brain Res. 2007, 1151, 134–141. [Google Scholar] [CrossRef][Green Version]
- Schutter, D.J.L.G.; Putman, P.; Hermans, E.; Van Honk, J. Parietal Electroencephalogram Beta Asymmetry and Selective Attention to Angry Facial Expressions in Healthy Human Subjects. Neurosci. Lett. 2001, 314, 13–16. [Google Scholar] [CrossRef]
- Woodruff, C.C.; Daut, R.; Brower, M.; Bragg, A. Electroencephalographic α-band and β-band Correlates of Perspective-Taking and Personal Distress. Neuro Rep. 2011, 22, 744–748. [Google Scholar] [CrossRef]
- Jung, J.; Bayle, D.; Jerbi, K.; Vidal, J.R.; Hénaff, M.-A.; Ossandon, T.; Bertrand, O.; Mauguière, F.; Lachaux, J.-P. Intracerebral Gamma Modulations Reveal Interaction between Emotional Processing and Action Outcome Evaluation in the Human Orbitofrontal Cortex. Int. J. Psychophysiol. 2011, 79, 64–72. [Google Scholar] [CrossRef]
- Luo, Q.; Mitchell, D.; Cheng, X.; Mondillo, K.; McCaffrey, D.; Holroyd, T.; Carver, F.; Coppola, R.; Blair, J. Visual Awareness, Emotion, and Gamma Band Synchronization. Cereb. Cortex 2008, 19, 1896–1904. [Google Scholar] [CrossRef]
- Sato, W.; Kochiyama, T.; Uono, S.; Matsuda, K.; Usui, K.; Inoue, Y.; Toichi, M. Rapid Amygdala Gamma Oscillations in Response to Fearful Facial Expressions. Neuropsychologia 2011, 49, 612–617. [Google Scholar] [CrossRef]
- Senkowski, D.; Kautz, J.; Hauck, M.; Zimmermann, R.; Engel, A.K. Emotional Facial Expressions Modulate Pain-Induced Beta and Gamma Oscillations in Sensorimotor Cortex. J. Neurosci. 2011, 31, 14542–14550. [Google Scholar] [CrossRef] [PubMed]
- Keil, A.; Stolarova, M.; Moratti, S.; Ray, W.J. Adaptation in Human Visual Cortex as a Mechanism for Rapid Discrimination of Aversive Stimuli. NeuroImage 2007, 36, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Michalareas, G.; Vezoli, J.; Van Pelt, S.; Schoffelen, J.-M.; Kennedy, H.; Fries, P. Alpha-Beta and Gamma Rhythms Subserve Feedback and Feedforward Influences among Human Visual Cortical Areas. Neuron 2016, 89, 384–397. [Google Scholar] [CrossRef] [PubMed]
- Dalgleish, T. The Emotional Brain. Nat. Rev. Neurosci. 2004, 5, 583–589. [Google Scholar] [CrossRef] [PubMed]
- LeDoux, J.E. Emotion Circuits in the Brain. Annu. Rev. Neurosci. 2000, 23, 155–184. [Google Scholar] [CrossRef] [PubMed]
- Kelly, A.M.C.; Garavan, H. Human Functional Neuroimaging of Brain Changes Associated with Practice. Cereb. Cortex 2004, 15, 1089–1102. [Google Scholar] [CrossRef] [PubMed]
- Müsch, K.; Hamamé, C.M.; Perrone-Bertolotti, M.; Minotti, L.; Kahane, P.; Engel, A.K.; Lachaux, J.-P.; Schneider, T.R. Selective Attention Modulates High-Frequency Activity in the Face-Processing Network. Cortex 2014, 60, 34–51. [Google Scholar] [CrossRef]
- Lisiecka, D.M.; Carballedo, A.; Fagan, A.J.; Ferguson, Y.; Ryan, D.J.; Frodl, T. Recruitment of the Left Hemispheric Emotional Attention Neural Network in Risk for and Protection from Depression. J. Psychiatry Neurosci. 2013, 38, 117–128. [Google Scholar] [CrossRef]
- Brosch, T.; Pourtois, G.; Sander, D.; Vuilleumier, P. Additive Effects of Emotional, Endogenous, and Exogenous Attention: Behavioral and Electrophysiological Evidence. Neuropsychologia 2011, 49, 1779–1787. [Google Scholar] [CrossRef]
- Wisniewski, D.; Reverberi, C.; Momennejad, I.; Kahnt, T.; Haynes, J.-D. The Role of the Parietal Cortex in the Representation of Task-Reward Associations. J. Neurosci. 2015, 35, 12355–12365. [Google Scholar] [CrossRef]
- Ernst, L.H.; Plichta, M.M.; Lutz, E.; Zesewitz, A.K.; Tupak, S.V.; Dresler, T.; Ehlis, A.-C.; Fallgatter, A.J. Prefrontal Activation Patterns of Automatic and Regulated Approach–Avoidance Reactions – A Functional Near-Infrared Spectroscopy (fNIRS) Study. Cortex 2013, 49, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Tupak, S.V.; Dresler, T.; Guhn, A.; Ehlis, A.-C.; Fallgatter, A.J.; Pauli, P.; Herrmann, M.J. Implicit Emotion Regulation in the Presence of Threat: Neural and Autonomic Correlates. NeuroImage 2014, 85, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Marzetti, L.; Basti, A.; Chella, F.; D’Andrea, A.; Syrjälä, J.; Pizzella, V. Brain Functional Connectivity Through Phase Coupling of Neuronal Oscillations: A Perspective From Magnetoencephalography. Front. Neurosci. 2019, 13, 964. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Sohrabpour, A.; Brown, E.; Liu, Z. Electrophysiological Source Imaging: A Noninvasive Window to Brain Dynamics. Annu. Rev. Biomed. Eng. 2018, 20, 171–196. [Google Scholar] [CrossRef]
- He, B.; Astolfi, L.; Valdes-Sosa, P.A.; Marinazzo, D.; Palva, S.; Bénar, C.-G.; Michel, C.M.; Koenig, T. Electrophysiological Brain Connectivity: Theory and Implementation. IEEE Trans. Biomed. Eng. 2019, 66, 2115–2137. [Google Scholar] [CrossRef]
- Vukelić, M.; Gharabaghi, A. Self-Regulation of Circumscribed Brain Activity Modulates Spatially Selective and Frequency Specific Connectivity of Distributed Resting State Networks. Front. Behav. Neurosci. 2015, 9, 181. [Google Scholar] [CrossRef]
- Sheridan, T.B.; Parasuraman, R. Human-Automation Interaction. Rev. Hum. Factors Ergon. 2005, 1, 89–129. [Google Scholar] [CrossRef]
- Schindler, S.; Wegrzyn, M.; Steppacher, I.; Kissler, J. Perceived Communicative Context and Emotional Content Amplify Visual Word Processing in the Fusiform Gyrus. J. Neurosci. 2015, 35, 6010–6019. [Google Scholar] [CrossRef]
- Schindler, S.; Kissler, J. People Matter: Perceived Sender Identity Modulates Cerebral Processing of Socio-Emotional Language Feedback. NeuroImage 2016, 134, 160–169. [Google Scholar] [CrossRef]
- Hussein, A.; ElSawah, S.; Abbass, H.A. Trust Mediating Reliability–Reliance Relationship in Supervisory Control of Human–Swarm Interactions. Hum. Factors J. Hum. Factors Ergon. Soc. 2020, 62, 1237–1248. [Google Scholar] [CrossRef]
- Lee, J.; Moray, N. Trust, Control Strategies and Allocation of Function in Human-Machine Systems. Ergonmics 1992, 35, 1243–1270. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Nikolaidis, S.; Soh, H.; Hsu, D.; Srinivasa, S. Planning with Trust for Human-Robot Collaboration. In Proceedings of the 2018 ACM/IEEE International Conference on Human-Robot Interaction, HRI ’18, Chicago, IL, USA, 5–8 March 2018; Association for Computing Machinery (ACM): New York, NY, USA, 2018; pp. 307–315. [Google Scholar] [CrossRef]
- Master, R.; Jiang, S.; Khasawneh, M.T.; Bowling, S.R.; Grimes, L.; Gramopadhye, A.K.; Melloy, B.J. Measurement of Trust Over Time in Hybrid Inspection Systems. Hum. Factors Ergon. Manuf. 2005, 15, 177–196. [Google Scholar] [CrossRef]
- Xu, A.; Dudek, G. OPTIMo: Online Probabilistic Trust Inference Model for Asymmetric Human-Robot Collaborations. In Proceedings of the Tenth Annual ACM/IEEE International Conference on Human-Robot Interaction, HRI ’15, Portland, Oregon, 2–5 March 2015; Association for Computing Machinery (ACM): New York, NY, USA, 2015; pp. 221–228. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vukelić, M.; Lingelbach, K.; Pollmann, K.; Peissner, M. Oscillatory EEG Signatures of Affective Processes during Interaction with Adaptive Computer Systems. Brain Sci. 2021, 11, 35. https://doi.org/10.3390/brainsci11010035
Vukelić M, Lingelbach K, Pollmann K, Peissner M. Oscillatory EEG Signatures of Affective Processes during Interaction with Adaptive Computer Systems. Brain Sciences. 2021; 11(1):35. https://doi.org/10.3390/brainsci11010035
Chicago/Turabian StyleVukelić, Mathias, Katharina Lingelbach, Kathrin Pollmann, and Matthias Peissner. 2021. "Oscillatory EEG Signatures of Affective Processes during Interaction with Adaptive Computer Systems" Brain Sciences 11, no. 1: 35. https://doi.org/10.3390/brainsci11010035
APA StyleVukelić, M., Lingelbach, K., Pollmann, K., & Peissner, M. (2021). Oscillatory EEG Signatures of Affective Processes during Interaction with Adaptive Computer Systems. Brain Sciences, 11(1), 35. https://doi.org/10.3390/brainsci11010035





