Executive Functions and Attention Processes in Adolescents and Young Adults with Intellectual Disability
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample
2.2. Instruments
2.2.1. Behavioral Assessment of the Dysexecutive Syndrome (BADS)
2.2.2. Attention and Concentration Battery
2.2.3. Tests Used
- (1)
- Simple Reaction Times Test
- (2)
- Choice Reaction Times Test
- (3)
- Continuous Performance Test
- 3A)
- Auditory Continuous Performance subtest
- 3B)
- Visual Continuous Performance subtest
- 3C)
- Spatial Continuous Performance subtest
- (4)
- Divided Attention (Dual Task) Test
- (5)
- Color–Word Interference Test (Stroop Test)
- 5A)
- Naming colors
- 5B)
- Naming word colors
- (6)
- Shifting of Attention Test
- 6A)
- Shifting of Attention with verbal targets
- 6B)
- Shifting of Attention with visual targets
2.3. Procedure
2.4. Data Analysis
3. Results
3.1. Intelligence Tests
3.2. Executive Tests
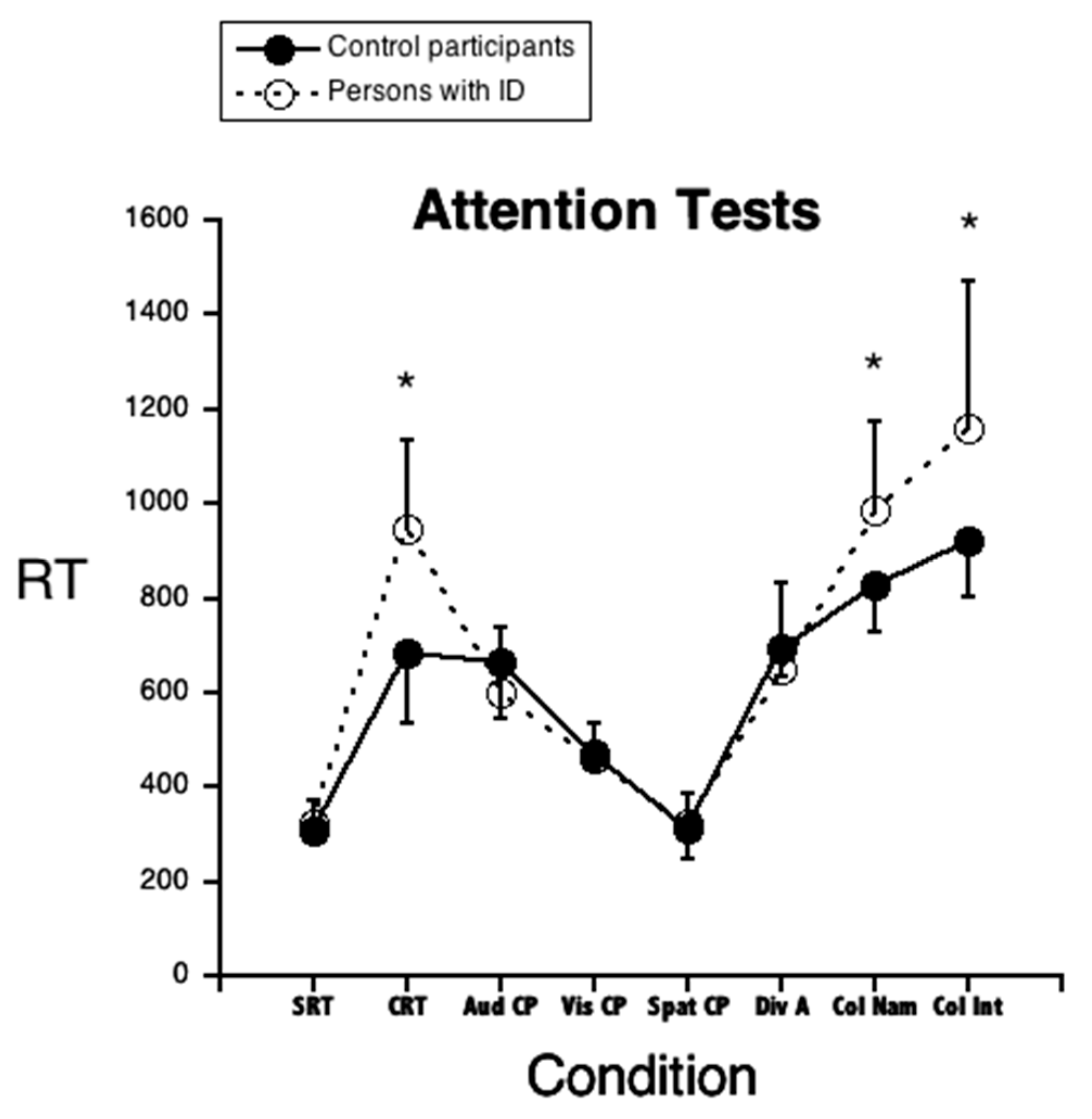
3.3. Attention Tests
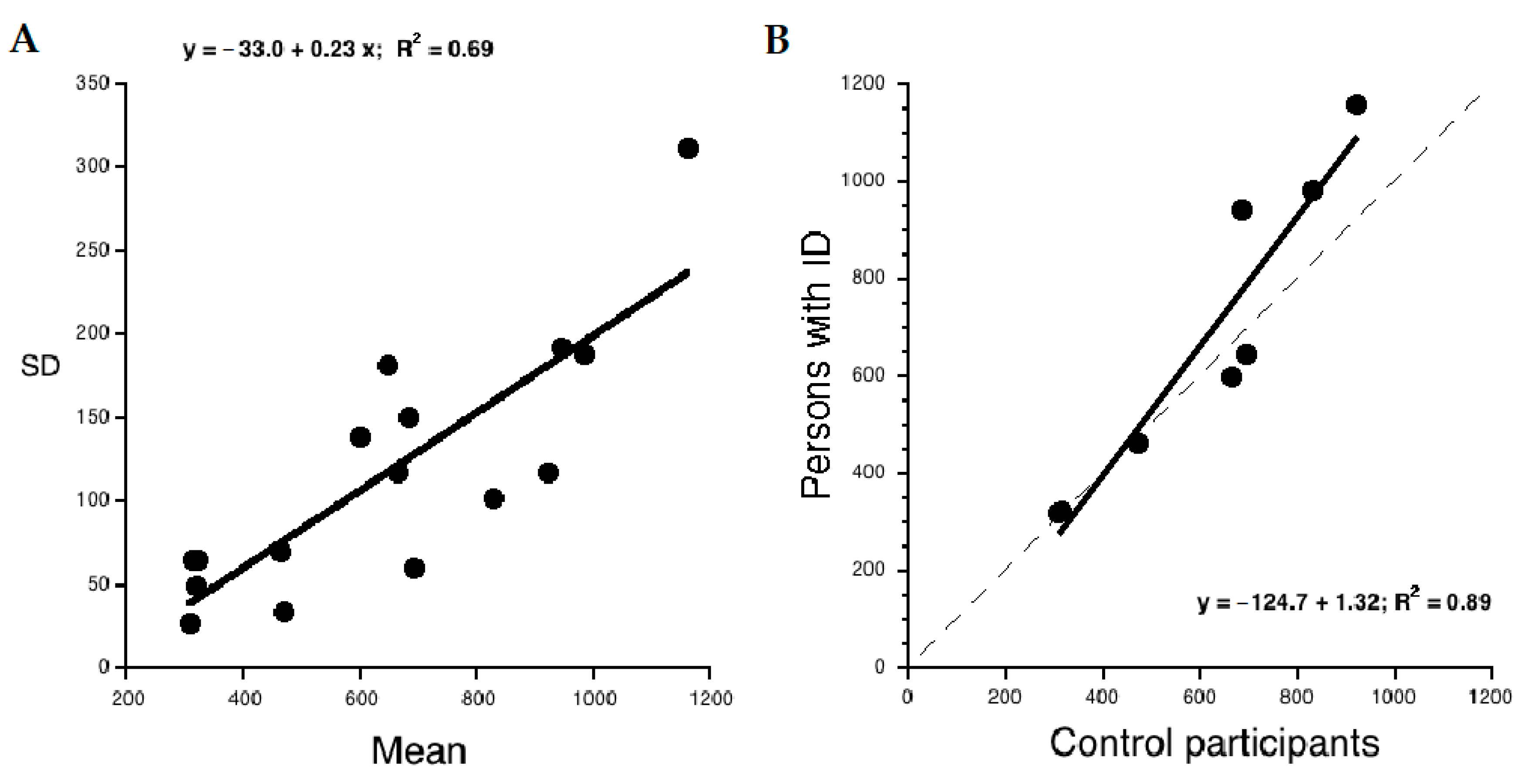
3.4. Analysis of Global Components in RT Tasks
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gilbert, S.J.; Burgess, P.W. Executive function. Curr. Biol. 2008, 18, R110–R114. [Google Scholar] [CrossRef] [PubMed]
- Lezak, M.D. The problem of assessing executive functions. Int. J. Psychol. 1982, 17, 281–297. [Google Scholar] [CrossRef]
- Pennington, B.F.; Ozonoff, S. Executive functions and developmental psychopathology. J. Child Psychol. Psychiatry 1996, 37, 51–87. [Google Scholar] [CrossRef] [PubMed]
- Miyake, A.; Friedman, N.P.; Emerson, M.J.; Witzki, A.H.; Howerter, A.; Wager, T.D. The unity and diversity of executive functions and their contributions to complex ‘‘frontal lobe” tasks: A latent variable analysis. Cogn. Psychol. 2000, 41, 49–100. [Google Scholar] [CrossRef]
- Spikman, J.; Van Zomeren, E. Assessment of attention. In The Handbook of Clinical Neuropsychology; Gurd, J., Kischka, U., Marshall, J., Eds.; Oxford University Press: Oxford, UK, 2010; pp. 81–96. [Google Scholar]
- Blair, C. Developmental science and executive function. Curr. Dir. Psychol. Sci. 2016, 25, 3–7. [Google Scholar] [CrossRef]
- Blankenship, T.L.; Slough, M.A.; Calkins, S.D.; Deater-Deckard, K.; Kim-Spoon, J.; Bell, M.A. Attention and executive functioning in infancy: Links to childhood executive function and reading achievement. Dev. Sci. 2019, 22, e12824. [Google Scholar] [CrossRef]
- Brandes-Aitken, A.; Braren, S.; Swingler, M.; Voegtline, K.; Blair, C. Sustained attention in infancy: A foundation for the development of multiple aspects of self-regulation for children in poverty. J. Exp. Child Psychol. 2019, 184, 192–209. [Google Scholar] [CrossRef]
- Peltopuro, M.; Ahonen, T.; Kaartinen, J.; Seppälä, H.; Närhi, V. Borderline intellectual functioning: a systematic literature review. Intell. Dev. Dis. 2014, 52, 419–443. [Google Scholar] [CrossRef]
- Ettenhofer, M.L.; Stavro, G.M.; Melrose, R.J. Return to work in mild cognitive disorders. In Handbook of Return to Work: From Research to Practice; Schultz, I.Z., Gatchel, R.J., Eds.; Springer: New York, NY, USA, 2016. [Google Scholar]
- Duncan, J.; Burgess, P.; Emslie, H. Fluid intelligence after frontal lobe lesions. Neuropsychologia 1995, 33, 261–268. [Google Scholar] [CrossRef]
- Friedman, N.P.; Miyake, A.; Corley, R.P.; Young, S.E.; De Fries, J.C.; Hewitt, J.K. Not all executive functions are related to intelligence. Psychol. Sci. 2006, 17, 172–179. [Google Scholar] [CrossRef]
- Chevalère, J.; Postal, V.; Jauregui, J.; Copet, P.; Laurier, V.; Thuilleaux, D. Assessment of executive functions in Prader-Willi syndrome and relationship with intellectual level. J. Appl. Res. Intellect. Disabil. 2013, 26, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Rowe, J.; Lavender, A.; Turk, V. Cognitive executive function in Down’s syndrome. Br. J. Clin. Psychol. 2006, 45, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Maehler, C.; Schuchardt, K. Working memory functioning in children with learning disabilities: Does intelligence make a difference? J. Intellect. Disabil. Res. 2009, 53, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Happé, F.; Booth, R.; Charlton, R.; Hughes, C. Executive function deficits in autism spectrum disorders and attention-deficit/hyperactivity disorder: examining profiles across domains and ages. Brain Cogn. 2006, 61, 25–39. [Google Scholar] [CrossRef]
- Wilding, J.; Cornish, K.; Munir, F. Further delineation of the executive deficit in males with fragile-X syndrome. Neuropsychologia 2002, 40, 1343–1349. [Google Scholar] [CrossRef]
- Hooper, S.R.; Hatton, D.; Sideris, J.; Sullivan, K.; Hammer, J.; Schaaf, J.; Mirrett, P.; Ornstein, P.A.; Bailey, D.P., Jr. Executive functions in young males with fragile X syndrome in comparison to mental age-matched controls: baseline findings from a longitudinal study. Neuropsychology 2008, 22, 36–47. [Google Scholar] [CrossRef]
- Cornish, K.M.; Kogan, C.S.; Li, L.; Turk, J.; Jacquemont, S.; Hagerman, R.J. Lifespan changes in working memory in fragile X premutation males. Brain Cogn. 2009, 69, 551–558. [Google Scholar] [CrossRef]
- Woodcock, K.A.; Oliver, C.; Humphreys, G.W. Task switching deficits and repetitive behaviour in genetic neurodevelopmental disorders: data from children with Prader-Willi syndrome chromosome 15 q11–q13 deletion and boys with Fragile-X syndrome. Cogn. Neuropsychol. 2009, 26, 172–194. [Google Scholar] [CrossRef]
- Das, J.P.; Divis, B.; Alexander, J.; Parrila, R.K.; Naglieri, J.A. Cognitive decline due to aging among persons with down syndrome. Res. Dev. Disabil. 1995, 16, 461–478. [Google Scholar] [CrossRef]
- Pennington, B.F.; Moon, J.; Edgin, J.; Stedron, J.; Nadel, L. The neuropsychology of down syndrome: evidence for hippocampal dysfunction. Child Dev. 2003, 74, 75–93. [Google Scholar] [CrossRef]
- Ball, S.L.; Holland, A.J.; Treppner, P.; Watson, P.C.; Huppert, F.A. Executive dysfunction and its association with personality and behaviour changes in the development of Alzheimer’s disease in adults with Down syndrome and mild to moderate learning disabilities. Br. J. Clin. Psychol. 2008, 47, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Lanfranchi, S.; Jerman, O.; Dal Pont, E.; Alberti, A.; Vianello, R. Executive function in adolescents with Down Syndrome. J. Ntellect. Disabil. Res. 2010, 54, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, F.; Varuzza, C.; Menghini, D.; Addona, F.; Gianesini, T.; Vicari, S. Executive functions in intellectual disabilities: A comparison between Williams syndrome and Down syndrome. Res. Dev. Disabil. 2013, 34, 1770–1780. [Google Scholar] [CrossRef] [PubMed]
- Gross-Tsur, V.; Landau, Y.E.; Benarroch, F.; Wertman-Elad, R.; Shalev, R.S. Cognition, attention, and behavior in Prader–Willi syndrome. J. Child Neurol. 2001, 16, 288–290. [Google Scholar] [CrossRef] [PubMed]
- Walley, R.M.; Donaldson, M.D. An investigation of executive function abilities in adults with Prader–Willi syndrome. J. Intellect. Disabil. Res. 2005, 49, 613–625. [Google Scholar] [CrossRef] [PubMed]
- Jauregi, J.; Arias, C.; Vegas, O.; Alén, F.; Martinez, S.; Copet, P.; Thuilleaux, D. A neuropsychological assessment of frontal cognitive functions in Prader-Willi syndrome. J. Intellect. Disabil. Res. 2007, 51, 350–365. [Google Scholar] [CrossRef] [PubMed]
- Menghini, D.; Addona, F.; Costanzo, F.; Vicari, S. Executive functions in individuals with Williams syndrome. J. Intellect. Disabil. Res. 2010, 54, 418–432. [Google Scholar] [CrossRef] [PubMed]
- Van der Molen, M.J. Working Memory in Children with Mild Intellectual Disabilities: Abilities and Training Potential (Unpublished Doctoral Dissertation); Institute for the Study of Education and Human Development: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Jensen, A.R. Process differences and individual differences in some cognitive tasks. Intelligence 1987, 11, 107–136. [Google Scholar] [CrossRef]
- Nettelbeck, T. Inspection time and intelligence. In Speed of Information Processing and Intelligence; Vernon, P.A., Ed.; Ablex: New York, NY, USA, 1987. [Google Scholar]
- Vernon, P.A. Speed of information processing and intelligence. Intelligence 1983, 7, 53–70. [Google Scholar] [CrossRef]
- Anderson, M. Intelligence and Development: A Cognitive Theory; Blackwell: Oxford, UK, 1992. [Google Scholar]
- Kranzler, J.H.; Jensen, A.R. Inspection time and intelligence: A meta-analysis. Intelligence 1989, 13, 329–348. [Google Scholar] [CrossRef]
- Hronis, A.; Roberts, L.; Kneebone, I.I. A review of cognitive impairments in children with intellectual disabilities: Implications for cognitive behaviour therapy. Br. J. Clin. Psychol. 2017, 56, 189–207. [Google Scholar] [CrossRef] [PubMed]
- Baumeister, A.A.; Kellas, G. Reaction time and mental retardation. In International Review of Research in Mental Retardation; Norman, R.E., Ed.; Academic Press: Cambridge, MA, USA, 1968; Volume 3, pp. 163–193. [Google Scholar]
- Burack, J.A.; Evans, D.W.; Klaiman, C.; Larocci, G. The mysterious myth of attention deficits and other defect stories: Contemporary issues in the developmental approach to mental retardation. Int. Rev. Res. Ment. Retard. 2001, 24, 299–320. [Google Scholar]
- Bonifacci, P.; Snowling, M.J. Speed of processing and reading disability: A cross- linguistic investigation of dyslexia and border-line intellectual functioning. Cognition 2008, 107, 999–1017. [Google Scholar] [CrossRef] [PubMed]
- Van der Meer, D.; Van der Meere, J. Response inhibition in children with conduct disorder and borderline intellectual functioning. Child Neuropsychol. 2004, 10, 189–194. [Google Scholar] [CrossRef]
- Pearson, D.A.; Yaffee, L.S.; Loveland, K.A.; Lewis, K.R. Comparison of sustained and selective attention in children who have mental retardation with and without attention deficit hyperactivity disorder. Am. J. Ment. Retard. 1996, 100, 592–607. [Google Scholar]
- Celeste, P.M.; Esteban, V.P.; Mariana, L.; José, G.B.M.; Florencia, B.; Christy, E.; Isabel, L.M.; Guillermo, A. Continuous performance test in children with intellectual disability and attention deficit hyperactivity disorder. Appl. Neuropsychol. Child 2019, 8, 246–252. [Google Scholar] [CrossRef]
- Van Zomeren, A.H.; Brouwer, W.H. Clinical Neuropsychology of Attention; Oxford University Press: Oxford, UK, 1994. [Google Scholar]
- Zoccolotti, P.; Matano, A.; Deloche, G.; Cantagallo, A.; Passadori, A.; Leclercq, M.; Braga, L.; Cremel, N.; Pittau, P.; Renom, M.; et al. Patterns of attentional impairments following a closed head injury: A collaborative European study. Cortex 2000, 36, 93–107. [Google Scholar] [CrossRef]
- Kaufmann, L.; Zieren, N.; Zotter, S.; Karall, D.; Scholl-Burgi, S.; Haberlandt, E.; Fimm, B. Predictive validity of attentional functions in differentiating children with and without ADHD: A componential analysis. Dev. Med. Child Neurol. 2010, 52, 371–378. [Google Scholar] [CrossRef]
- Faust, M.E.; Balota, D.A.; Spieler, D.H.; Ferraro, F.R. Individual differences in information processing rate and amount: implications for group differences in response latency. Psychol. Bull. 1999, 125, 777–799. [Google Scholar] [CrossRef]
- Myerson, J.; Hale, S.; Zheng, Y.; Jenkins, L.; Widaman, K.F. The difference engine: a model of diversity in speeded cognition. Psychon. Bull. Rev. 2003, 10, 262–288. [Google Scholar] [CrossRef]
- Verhaeghen, P.; Cerella, J. Aging, executive control, and attention: A review of meta-analyses. Neurosci. Biobehav. Rev. 2002, 26, 849–857. [Google Scholar] [CrossRef]
- Kail, R. General slowing of information-processing by persons with mental retardation. Am. J. Ment. Retard. 1992, 97, 333–341. [Google Scholar] [PubMed]
- Burgess, P.W. Theory and methodology in executive function and research. In Methodology of Frontal and Executive Function; Rabbitt, P., Ed.; Psychology Press: Hove, UK, 1997; pp. 81–116. [Google Scholar]
- Burgess, P.W.; Alderman, N.; Evans, J.; Emslie, H.; Wilson, B.A. The ecological validity of tests of executive function. J. Int. Neuropsychol. Soc. 1998, 4, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Wilson, B.A.; Alderman, N.; Burgess, P.; Emslie, H.; Evans, J.J. Behavioural Assessment of the Dysexecutive Syndrome (BADS); Thames Valley Test Company: Bury St Edmunds, UK, 1996. [Google Scholar]
- Orsini, A.; Pezzuti, L.; WAIS-IV. Contributo alla Taratura Italiana; Contribution to the Italian Standardization; WAIS-IV, Translator; Giunti OS: Firenze, Italy, 2013. [Google Scholar]
- Orsini, A.; Pezzuti, L.; Picone, L.; WISC-4. Contributo alla Taratura Italiana; Contribution to the Italian Standardization; WISC-4, Translator; Giunti OS: Firenze, Italy, 2012. [Google Scholar]
- Di Nuovo, S. Attenzione e Concentrazione; Attention and Concentration, Translator; Erickson: Trento, Italy, 2000. [Google Scholar]
- Antonucci, G.; Spitoni, G.; Orsini, A.; D’Olimpio, F.; Cantagallo, A. Taratura Italiana della Batteria per la valutazione della Sindrome Disesecutiva: BADS; ItalianNormalization of the Battery for the BehaviouralAssessment of the DysexecutiveSyndrome (BADS), Translator; Giunti, O.S. Organizzazioni Speciali: Firenze, Italy, 2014. [Google Scholar]
- Willner, P.; Bailey, R.; Parry, R.; Dymond, S. Evaluation of executive functioning in people with intellectual disabilities. J. Intellect. Disabil. Res. 2010, 54, 366–379. [Google Scholar] [CrossRef]
- Danielsson, H.; Henry, L.; Rönnberg, J.; Nilsson, L.G. Executive functions in individuals with intellectual disability. Res. Dev. Disabil. 2010, 31, 1299–1304. [Google Scholar] [CrossRef]
| Individuals with ID | Controls | |||||
|---|---|---|---|---|---|---|
| Condition | Mean | SD | Mean | SD | p | d |
| Rule Shift Card | 2.04 | 1.16 | 3.44 | 0.70 | 0.00000 | 1.52 |
| Action Programme | 3.00 | 0.96 | 3.89 | 0.32 | 0.00007 | 1.39 |
| Key Search | 0.96 | 0.94 | 2.00 | 1.00 | 0.00026 | 1.07 |
| Temporal Judgement | 2.11 | 1.12 | 3.00 | 0.92 | 0.00249 | 0.87 |
| Zoo Map Test | 1.89 | 0.97 | 3.11 | 0.89 | 0.00001 | 1.31 |
| Modified Six Elements | 2.30 | 1.46 | 3.44 | 0.75 | 0.00082 | 1.04 |
| Total | 12.30 | 3.68 | 18.85 | 1.92 | 0.00000 | 2.34 |
| IQ score | 76.48 | 18.39 | 109.26 | 9.58 | 0.00000 | 2.34 |
| Individuals with ID | Controls | |||||
|---|---|---|---|---|---|---|
| Test/Measure | ||||||
| RTs (ms) | Mean | SD | Mean | SD | p | d |
| Simple Reaction Times | 320 | 50 | 308 | 27 | 0.28 | 0.31 |
| Choice Reaction Times | 944 | 193 | 684 | 151 | 0.000001 * | 1.51 |
| Auditory Continuous Performance | 601 | 139 | 664 | 118 | 0.08 | −0.49 |
| Visual Continuous Performance | 463 | 70 | 469 | 34 | 0.69 | −0.11 |
| Spatial Continuous Performance | 323 | 65 | 313 | 65 | 0.57 | 0.15 |
| Divided Attention | 648 | 182 | 693 | 60 | 0.23 | −0.37 |
| Color Naming | 984 | 189 | 829 | 102 | 0.0006 * | 1.06 |
| Color–Word Interference | 1161 | 312 | 921 | 118 | 0.0007 * | 1.12 |
| Time measures (s) | ||||||
| Shifting of Attention (verbal targets) | 61.7 | 12.5 | 46.4 | 13.7 | 0.00008 * | 1.17 |
| Shifting of Attention (visual targets) | 88.3 | 21.4 | 66.4 | 11.4 | 0.00003 * | 1.33 |
| Errors | ||||||
| Choice Reaction Times | 3.19 | 3.48 | 2.30 | 1.90 | 0.25 | 0.33 |
| Auditory Continuous Performance | 0.70 | 0.99 | 0.15 | 0.36 | 0.01 | 0.82 |
| Visual Continuous Performance | 0.78 | 1.37 | 0.26 | 0.53 | 0.07 | 0.55 |
| Spatial Continuous Performance | 0.26 | 0.59 | 0.15 | 0.60 | 0.50 | 0.19 |
| Divided Attention | 1.15 | 1.41 | 0.56 | 0.85 | 0.07 | 0.53 |
| Color Naming | 2.19 | 2.13 | 1.00 | 0.88 | 0.01 | 0.79 |
| Color–Word Interference | 3.30 | 3.22 | 1.96 | 3.47 | 0.15 | 0.40 |
| Omissions | ||||||
| Choice Reaction Times | 1.93 | 2.79 | 0.41 | 0.80 | 0.01 | 0.85 |
| Auditory Continuous Performance | 0.11 | 0.32 | 0.00 | 0.00 | 0.08 | 0.69 |
| Visual Continuous Performance | 0.26 | 0.53 | 0.11 | 0.32 | 0.22 | 0.35 |
| Spatial Continuous Performance | 0.07 | 0.27 | 0.11 | 0.42 | 0.70 | −0.11 |
| Divided Attention | 1.33 | 1.33 | 0.48 | 0.85 | 0.007 | 0.78 |
| Shifting of Attention (verbal targets) | 3.19 | 2.43 | 1.70 | 2.03 | 0.02 | 0.66 |
| Shifting of Attention (visual targets) | 3.11 | 2.74 | 1.63 | 1.55 | 0.02 | 0.69 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zagaria, T.; Antonucci, G.; Buono, S.; Recupero, M.; Zoccolotti, P. Executive Functions and Attention Processes in Adolescents and Young Adults with Intellectual Disability. Brain Sci. 2021, 11, 42. https://doi.org/10.3390/brainsci11010042
Zagaria T, Antonucci G, Buono S, Recupero M, Zoccolotti P. Executive Functions and Attention Processes in Adolescents and Young Adults with Intellectual Disability. Brain Sciences. 2021; 11(1):42. https://doi.org/10.3390/brainsci11010042
Chicago/Turabian StyleZagaria, Tommasa, Gabriella Antonucci, Serafino Buono, Marilena Recupero, and Pierluigi Zoccolotti. 2021. "Executive Functions and Attention Processes in Adolescents and Young Adults with Intellectual Disability" Brain Sciences 11, no. 1: 42. https://doi.org/10.3390/brainsci11010042
APA StyleZagaria, T., Antonucci, G., Buono, S., Recupero, M., & Zoccolotti, P. (2021). Executive Functions and Attention Processes in Adolescents and Young Adults with Intellectual Disability. Brain Sciences, 11(1), 42. https://doi.org/10.3390/brainsci11010042






