Hypertensive Crisis in Acute Cerebrovascular Diseases Presenting at the Emergency Department: A Narrative Review
Abstract
:1. Introduction
1.1. Arterial Hypertension
1.2. Hypertensive Emergency and Hypertensive Urgency
1.3. Acute Cerebrovascular Diseases and Arterial Hypertension
1.4. Aim
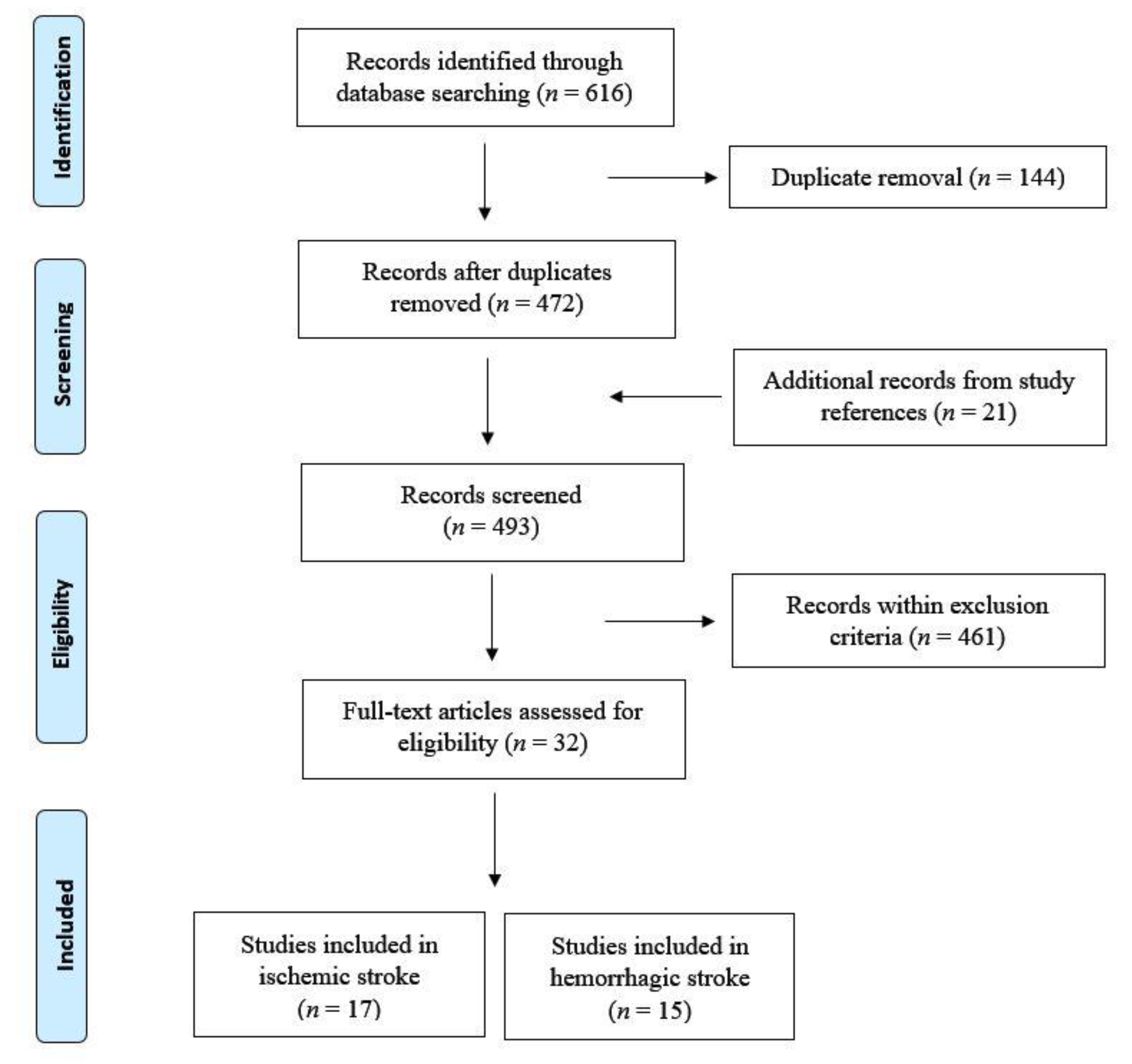
2. Data Source and Selection
3. Results
3.1. Ischemic Stroke
- (i)
- An automatic sphygmomanometer should be used instead of a manual device;
- (ii)
- If DBP is >140 mmHg in two measurements within 5 min, a continuous IV infusion of antihypertensive agents such as nitroglycerin or sodium nitroprusside (0.5–1.0 mg/kg/min) should be started. Patients at risk for cerebral edema should be constantly monitored given the possibility of increase of the intracranial pressure. Such patients are not candidates for thrombolytic treatment;
- (iii)
- If SBP is >220 mmHg, DBP is 121–140 mm Hg, or the mean BP is >130 mmHg in two measurements within 20 min, an antihypertensive drug that is easily titratable such as labetalol (10 mg IV in 1–2 min) should be administered. This dose can be repeated or doubled every 10–20 min up to a cumulative dosage of 300 mg. After this initial approach, labetalol can be administrated every 6–8 h if necessary. Labetalol is not recommended in patients with asthma, heart failure, or severe cardiac arrythmia. In these cases, urapidil (10–50 mg IV or infusion of 0.15–0.5 mg/min) should be considered. Patients requiring more than two doses of labetalol or any other antihypertensive drug to reduce SBP < 185 mmHg or DBP < 110 mmHg are generally not candidates for thrombolytic therapy;
- (iv)
- When SBP is 185–220 mmHg or DBP is 105–120 mmHg, emergency therapy should be postponed if left ventricular failure, aortic dissection, or acute myocardial infarction coexist. Patients who are candidates for thrombolytic therapy who have persistently elevated SBP (>185 mmHg) or DBP (>110 mmHg) can be treated with small doses of IV antihypertensive drugs to maintain BP values below these limits. However, the administration of more than two doses of antihypertensive drugs to keep BP under control is currently considered as a relative contraindication to thrombolytic therapy [102];
- (v)
- The use of calcium channel blockers by sublingual administration is not indicated due to the rapid and often unpredictable drop of BP that can be caused by this class of drugs;
- (vi)
- Pharmacological BP correction in the acute stroke phase should be associated with careful monitoring of the neurological status in order to promptly detect any clinical deterioration;
- (vii)
- In patients with acute ischemic stroke and SBP < 185 mmHg or DBP < 105 mmHg, antihypertensive therapy is not usually indicated [102];
- (viii)
- Although there are no data to define a stable threshold, arterial hypotension in patients with acute stroke should be treated in cases of dehydration or significantly lower BP values for that patient. Treatment options include IV administration of fluids, treatment of an underlying congestive heart failure and bradycardia, and use of vasopressor agents such as dopamine [96,103].
3.2. Hemorrhagic Stroke
4. Discussion
4.1. General Considerations
4.2. Debated Issues
- Class I recommendation and level B evidence for patients who have elevated BP and are otherwise eligible for treatment with IV rtPA should have their SBP < 185 mmHg and DBP < 110 mmHg before thrombolytic therapy is initiated. If medications are given to lower BP, clinicians should be sure that the BP is stabilized at the lower level before beginning rtPA and maintained <180/105 mmHg for at least the first 24 h after IV rtPA;
- Class I recommendation and level C evidence are given for patients who have elevated BP but are not eligible for thrombolysis to lower BP by 15% during the first 24 h after onset of stroke. The exact level of BP is not known, but consensus exists that medications should be withheld unless SBP > 220 mmHg or DBP > 120 mmHg;
- Class IIa recommendation and level B evidence for restarting antihypertensive medications after the first 24 h in patients who have pre-existing hypertension and are neurologically stable, unless a specific contraindication to restarting treatment is known.
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Banegas, J.R.; López-García, E.; Dallongeville, J.; Guallar, E.; Halcox, J.P.; Borghi, C.; Massó-González, E.L.; Jiménez, F.J.; Perk, J.; Steg, P.G.; et al. Achievement of Treatment Goals for Primary Prevention of Cardiovascular Disease in Clinical Practice across Europe: The EURIKA Study. Eur. Heart J. 2011, 32, 2143–2152. [Google Scholar] [CrossRef] [PubMed]
- Chow, C.K.; Teo, K.K.; Rangarajan, S.; Islam, S.; Gupta, R.; Avezum, A. PURE Study Investigators. Prevalence, Awareness, Treatment, and Control of Hypertension in Rural and Urban Communities in High-, Middle-, and Low-Income Countries. JAMA 2013, 310, 959–968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falaschetti, E.; Mindell, J.; Knott, C.; Poulter, N. Hypertension Management in England: A Serial Cross-Sectional Study from 1994 to 2011. Lancet 2014, 383, 1912–1919. [Google Scholar] [CrossRef]
- Tocci, G.; Agabiti Rosei, E.; Ambrosioni, E.; Borghi, C.; Ferri, C.; Ferrucci, A. Blood Pressure Control in Italy: Analysis of Clinical Data from 2005–2011 Surveys on Hypertension. J. Hypertens. 2012, 30, 1065–1074. [Google Scholar] [CrossRef] [PubMed]
- Khaw, K.T. Epidemiology of Stroke. J. Neurol. Neurosurg. Psychiatry 1996, 61, 333–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacMahon, S.; Peto, R.; Cutler, J.; Collins, R.; Sorlie, P.; Neaton, J.; Abbott, R.; Godwin, J.; Dyer, A.; Stamler, J. Blood Pressure, Stroke, and Coronary Heart Disease. Part 1, Prolonged Differences in Blood Pressure: Prospective Observational Studies Corrected for the Regression Dilution Bias. Lancet Lond. Engl. 1990, 335, 765–774. [Google Scholar] [CrossRef]
- Chobanian, A.V.; Bakris, G.L.; Black, H.R.; Cushman, W.C.; Green, L.A.; Izzo, J.L., Jr.; Jones, D.W.; Materson, B.J.; Oparil, S.; Wright, J.T., Jr.; et al. Seventh Report on the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003, 42, 1206–1252. [Google Scholar] [CrossRef] [Green Version]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M. Practice Guidelines for the Management of Arterial Hypertension of the European Society of Hypertension and the European Society of Cardiology: ESH/ESC Task Force for the Management of Arterial Hypertension. J. Hypertens. 2018, 36, 2284–2309. [Google Scholar] [CrossRef] [Green Version]
- Volpe, M.; Tocci, G.; Trimarco, B. Blood pressure control in Italy: Results of recent surveys on hypertension. J. Hypertens. 2007, 25, 1491–1498. [Google Scholar] [CrossRef]
- Ettehad, D.; Emdin, C.A.; Kiran, A.; Anderson, S.G.; Callender, T.; Emberson, J. Blood Pressure Lowering for Prevention of Cardiovascular Disease and Death: A Systematic Review and Meta-Analysis. Lancet 2016, 387, 957–967. [Google Scholar] [CrossRef] [Green Version]
- Forouzanfar, M.H.; Liu, P.; Roth, G.A.; Ng, M.; Biryukov, S.; Marczak, L. Global burden of hypertension and systolic blood pressure of at least 110 to 115mm Hg, 1990–2015. JAMA 2017, 317, 165–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- NCD Risk Factor Collaboration (NCD-RisC) Worldwide Trends in Blood Pressure from 1975 to 2015: A Pooled Analysis of 1479 Population-Based Measurement Studies with 19·1 Million Participants. Lancet Lond. Engl. 2017, 389, 37–55. [CrossRef] [Green Version]
- O’Donnell, M.J.; Chin, S.L.; Rangarajan, S.; Xavier, D.; Liu, L.; Zhang, H. INTERSTROKE Investigators. Global and Regional Effects of Potentially Variable Risk Factors Associated with Acute Stroke in 32 Countries (INTERSTROKE): A Case-Control Study. Lancet 2016, 388, 761–775. [Google Scholar] [CrossRef]
- Leonardi-Bee, J.; Bath, P.M.W.; Phillips, S.J.; Sandercock, P.A.G.; IST Collaborative Group. Blood Pressure and Clinical Outcomes in the International Stroke Trial. Stroke 2002, 33, 1315–1320. [Google Scholar] [CrossRef] [Green Version]
- Böhm, M.; Schumacher, H.; Teo, K.K.; Lonn, E.M.; Mahfoud, F.; Mann, J.F.E.; Mancia, G.; Redon, J.; Schmieder, R.E.; Sliwa, K.; et al. Achieved Blood Pressure and Cardiovascular Outcomes in High-Risk Patients: Results from ONTARGET and TRANSCEND Trials. Lancet Lond. Engl. 2017, 389, 2226–2237. [Google Scholar] [CrossRef]
- Kjeldsen, S.E.; Berge, E.; Bangalore, S.; Messerli, F.H.; Mancia, G.; Holzhauer, B.; Hua, T.A.; Zappe, D.; Zanchetti, A.; Weber, M.A.; et al. No Evidence for a J-Shaped Curve in Treated Hypertensive Patients with Increased Cardiovascular Risk: The VALUE Trial. Blood Press. 2016, 25, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Mancia, G.; Kjeldsen, S.E.; Zappe, D.H.; Holzhauer, B.; Hua, T.A.; Zanchetti, A.; Julius, S.; Weber, M.A. Cardiovascular Outcomes at Different On-Treatment Blood Pressures in the Hypertensive Patients of the VALUE Trial. Eur. Heart J. 2016, 37, 955–964. [Google Scholar] [CrossRef] [Green Version]
- Reboussin, D.M.; Allen, N.B.; Griswold, M.E.; Guallar, E.; Hong, Y.; Lackland, D.T.; Miller, E.P.R.; Polonsky, T.; Thompson-Paul, A.M.; Vupputuri, S. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018, 71, 1269–1324. [Google Scholar] [CrossRef]
- Mancia, G.; De Backer, G.; Dominiczak, A.; Cifkova, R.; Fagard, R.; Germano, G. ESH-ESC Practice Guidelines for the Management of Arterial Hypertension: ESH-ESC Task Force on the Management of Arterial Hypertension. J. Hypertens. 2007, 25, 1751–1762. [Google Scholar] [CrossRef] [Green Version]
- Martin, J.F.; Higashiama, E.; Garcia, E.; Luizon, M.R.; Cipullo, J.P. Hypertensive Crisis Profile. Prevalence and Clinical Presentation. Arq. Bras. Cardiol. 2004, 83, 125–136. [Google Scholar] [CrossRef] [Green Version]
- Adams, H.P.; del Zoppo, G.; Alberts, M.J.; Bhatt, D.L.; Brass, L.; Furlan, A.; Grubb, R.L.; Higashida, R.T.; Jauch, E.C.; Kidwell, C.; et al. Guidelines for the Early Management of Adults with Ischemic Stroke: A Guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: The American Academy of Neurology Affirms the Value of This Guideline as an Educational Tool for Neurologists. Circulation 2007, 115, e478–e534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaughan, C.J.; Delanty, N. Hypertensive emergencies. Lancet 2000, 356, 411–417. [Google Scholar] [CrossRef]
- Zampaglione, B.; Pascale, C.; Marchisio, M.; Cavallo-Perin, P. Hypertensive Urgencies and Emergencies. Prevalence and Clinical Presentation. Hypertension 1996, 27, 144–147. [Google Scholar] [CrossRef]
- Katz, J.N.; Gore, J.M.; Amin, A.; Anderson, F.A.; Dasta, J.F.; Ferguson, J.J.; Kleinschmidt, K.; Mayer, S.A.; Multz, A.S.; Peacock, W.F.; et al. Practice Patterns, Outcomes, and End-Organ Dysfunction for Patients with Acute Severe Hypertension: The Studying the Treatment of Acute HyperTension (STAT) Registry. Am. Heart J. 2009, 158, 599–606.e1. [Google Scholar] [CrossRef] [PubMed]
- Muiesan, M.L.; Salvetti, M.; Amadoro, V.; di Somma, S.; Perlini, S.; Semplicini, A.; Borghi, C.; Volpe, M.; Saba, P.S.; Cameli, M.; et al. An Update on Hypertensive Emergencies and Urgencies. J. Cardiovasc. Med. Hagerstown Md. 2015, 16, 372–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- James, P.A.; Oparil, S.; Carter, B.L.; Cushman, W.C.; Dennison-Himmelfarb, C.; Handler, J.; Lackland, D.T.; LeFevre, M.L.; MacKenzie, T.D.; Ogedegbe, O.; et al. 2014 Evidence-Based Guideline for the Management of High Blood Pressure in Adults: Report From the Panel Members Appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014, 311, 507–520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolf, S.J.; Lo, B.; Shih, R.D.; Smith, M.D.; Fesmire, F.M.; American College of Emergency Physicians Clinical Policies Committee. Clinical Policy: Critical Issues in the Evaluation and Management of Adult Patients in the Emergency Department with Asymptomatic Elevated Blood Pressure. Ann. Emerg. Med. 2013, 62, 59–68. [Google Scholar] [CrossRef]
- Members, A.F.; Mancia, G.; Fagard, R.; Narkiewicz, K.; Redon, J.; Zanchetti, A.; Böhm, M.; Christiaens, T.; Cifkova, R.; De Backer, G.; et al. 2013 ESH/ESC Guidelines for the Management of Arterial HypertensionThe Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur. Heart J. 2013, 34, 2159–2219. [Google Scholar] [CrossRef] [Green Version]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the Management of Arterial HypertensionThe Task Force for the Management of Arterial Hypertension of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH). Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef]
- Barbalić, M.; Skarić-Jurić, T.; Cambien, F.; Barbaux, S.; Poirier, O.; Turek, S.; Vrhovski-Hebrang, D.; Čubrilo-Turek, M.; Rudan, I.; Rudan, P.; et al. Gene Polymorphisms of the Renin-Angiotensin System and Early Development of Hypertension. Am. J. Hypertens. 2006, 19, 837–842. [Google Scholar] [CrossRef] [Green Version]
- Lalouel, J.M.; Rohrwasser, A. Genetic Susceptibility to Essential Hypertension: Insight from Angiotensinogen. Hypertension 2007, 49, 597–603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palatini, P.; Julius, S. The Role of Cardiac Autonomic Function in Hypertension and Cardiovascular Disease. Curr. Hypertens Rep. 2009, 11, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Gradman, A.H.; Vivas, Y. New Therapeutic Perspectives with Clevidipine: An Ultra-Short-Acting Intravenous Ca ++ Channel Blocker. Expert. Opin. Investig. Drugs 2007, 16, 1449–1457. [Google Scholar] [CrossRef] [PubMed]
- Ghali, M.G.Z. The Brainstem Network Controlling Blood Pressure: An Important Role for Pressor Sites in the Caudal Medulla and Cervical Spinal Cord. J. Hypertens. 2017, 35, 1938–1947. [Google Scholar] [CrossRef] [PubMed]
- van den Born, B.-J.H.; Löwenberg, E.C.; van der Hoeven, N.V.; de Laat, B.; Meijers, J.C.M.; Levi, M.; van Montfrans, G.A. Endothelial Dysfunction, Platelet Activation, Thrombogenesis and Fibrinolysis in Patients with Hypertensive Crisis. J. Hypertens. 2011, 29, 922–927. [Google Scholar] [CrossRef] [PubMed]
- Perez, M.I.; Musini, V.M. Pharmacological Interventions for Hypertensive Emergencies: A Cochrane Systematic Review. J. Hum. Hypertens. 2008, 22, 596–607. [Google Scholar] [CrossRef] [PubMed]
- Fisicaro, F.; Lanza, G.; Grasso, A.A.; Pennisi, G.; Bella, R.; Paulus, W.; Pennisi, M. Repetitive Transcranial Magnetic Stimulation in Stroke Rehabilitation: Review of the Current Evidence and Pitfalls. Ther. Adv. Neurol. Disord. 2019, 12, 175628641987831. [Google Scholar] [CrossRef] [Green Version]
- Hatano, S. Experience from a multicentre stroke register: A preliminary report. Bull WHO 1976, 54, 541–553. [Google Scholar]
- Albers, G.W.; Caplan, L.R.; Easton, J.D.; Fayad, P.B.; Mohr, J.P.; Saver, J.L.; Sherman, D.G.; TIA Working Group. Transient Ischemic Attack-Proposal for a New Definition. N. Engl. J. Med. 2002, 347, 1713–1716. [Google Scholar] [CrossRef]
- Cipolla, M.J.; Liebeskind, D.S.; Chan, S.-L. The Importance of Comorbidities in Ischemic Stroke: Impact of Hypertension on the Cerebral Circulation. J. Cereb. Blood Flow Metab. 2018, 38, 2129–2149. [Google Scholar] [CrossRef]
- Feigin, V.L.; Lawes, C.M.M.; Bennett, D.A.; Barker-Collo, S.L.; Parag, V. Worldwide Stroke Incidence and Early Case Fatality Reported in 56 Population-Based Studies: A Systematic Review. Lancet Neurol. 2009, 8, 355–369. [Google Scholar] [CrossRef]
- Broderick, J.P.; Brott, T.; Tomsick, T.; Miller, R.; Huster, G. Intracerebral Hemorrhage More than Twice as Common as Subarachnoid Hemorrhage. J. Neurosurg. 1993, 78, 188–191. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, A.I.; Mendelow, A.D.; Hanley, D.F. Intracerebral Haemorrhage. Lancet Lond. Engl. 2009, 373, 1632–1644. [Google Scholar] [CrossRef] [Green Version]
- Qureshi, A.I.; Tuhrim, S.; Broderick, J.P.; Batjer, H.H.; Hondo, H.; Hanley, D.F. Spontaneous Intracerebral Hemorrhage. N. Engl. J. Med. 2001, 344, 1450–1460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fisher, C.M. Lacunar Strokes and Infarcts: A Review. Neurology 1982, 32, 871–876. [Google Scholar] [CrossRef]
- Martini, S.R.; Flaherty, M.L.; Brown, W.M.; Haverbusch, M.; Comeau, M.E.; Sauerbeck, L.R.; Kissela, B.M.; Deka, R.; Kleindorfer, D.O.; Moomaw, C.J.; et al. Risk Factors for Intracerebral Hemorrhage Differ According to Hemorrhage Location. Neurology 2012, 79, 2275–2282. [Google Scholar] [CrossRef] [Green Version]
- Lamy, C.; Oppenheim, C.; Méder, J.F.; Mas, J.L. Neuroimaging in Posterior Reversible Encephalopathy Syndrome. J. Neuroimaging 2004, 14, 89–96. [Google Scholar] [CrossRef]
- Gardner, C.J.; Lee, K. Hyperperfusion Syndromes: Insight into the Pathophysiology and Treatment of Hypertensive Encephalopathy. CNS Spectr. 2007, 12, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, R.B.; Mulkern, R.V.; Gudbjartsson, H.; Jolesz, F. Diffusion weighted MR imaging in hypertensive encephalopathy: Clues to pathogenesis. AJNR Am. J. Neuroradiol. 1998, 19, 859–862. [Google Scholar]
- Trommer, B.L.; Homer, D.; Mikhael, M.A. Cerebral vasospasm and eclampsia. Stroke 1988, 19, 326–329. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, R.B.; Jones, K.M.; Kalina, P. Hypertensive encephalopathy: Findings on CT, MR imaging, and SPECT imaging in 14 cases. AJR Am. J. Roentgenol. 1992, 159, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Tsou, T.P.; Yen, Z.S.; Fang, C.C.; Chen, S.C.; Chen, W.J. Hypertensive Encephalopathy. J. Emerg. Med. 2004, 27, 85–86. [Google Scholar] [CrossRef] [PubMed]
- Servillo, G.; Bifulco, F.; De Robertis, E. Posterior Reversible Encephalopathy Syndrome in Intensive Care Medicine. Intensive Care Med. 2007, 33, 230–236. [Google Scholar] [CrossRef]
- Hinchey, J.; Chaves, C.; Appignani, B. A Reversible Posterior Leukoencephalopathy Syndrome. N. Engl. J. Med. 1996, 334, 494–500. [Google Scholar] [CrossRef]
- Mirza, A. Posterior Reversible Encephalopathy Syndrome: A Variant of Hypertensive Encephalopathy. J. Clin. Neurosci. 2006, 13, 590–595. [Google Scholar] [CrossRef] [PubMed]
- Pilato, F.; Calandrelli, R.; Distefano, M.; Panfili, M.; Della Marca, G.; Colosimo, C. Acute Radiological Pattern and Outcome in Posterior Reversible Encephalopathy Syndrome Patients. Clin. Neurol. Neurosurg. 2019, 185, 105459. [Google Scholar] [CrossRef]
- Pilato, F.; Distefano, M.; Calandrelli, R. Posterior Reversible Encephalopathy Syndrome and Reversible Cerebral Vasoconstriction Syndrome: Clinical and Radiological Considerations. Front. Neurol. 2020, 11, 34. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [Green Version]
- Barer, D.H.; Cruickshank, J.M.; Ebrahim, S.B.; Mitchell, J. Low Dose Beta Blockade in Acute Stroke (“BEST” Trial): An Evaluation. BMJ 1988, 296, 737–741. [Google Scholar] [CrossRef]
- Brott, T.; Lu, M.; Kothari, R.; Fagan, S.C.; Frankel, M.; Grotta, J.C.; Broderick, J.; Kwiatkowski, T.; Lewandowski, C.; Haley, E.C.; et al. Hypertension and Its Treatment in the NINDS Rt-PA Stroke Trial. Stroke 1998, 29, 1504–1509. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, N.; Nasman, P.; Wahlgren, N.G. Effect of Intravenous Nimodipine on Blood Pressure and Outcome after Acute Stroke. Stroke 2000, 31, 1250–1255. [Google Scholar] [CrossRef]
- PROGRESS Collaborative Group. Randomised Trial of a Perindopril-Based Blood-Pressure-Lowering Regimen among 6,105 Individuals with Previous Stroke or Transient Ischaemic Attack. Lancet Lond. Engl. 2001, 358, 1033–1041. [Google Scholar] [CrossRef]
- Rordorf, G.; Koroshetz, W.J.; Ezzeddine, M.A.; Segal, A.Z.; Buonanno, F.S. A Pilot Study of Drug-Induced Hypertension for Treatment of Acute Stroke. Neurology 2001, 56, 1213. [Google Scholar] [CrossRef] [PubMed]
- Hillis, A.E.; Ulatowski, J.A.; Barker, P.B.; Torbey, M.; Ziai, W.; Beauchamp, N.J.; Oh, S.; Wityk, R.J. A Pilot Randomized Trial of Induced Blood Pressure Elevation: Effects on Function and Focal Perfusion in Acute and Subacute Stroke. Cerebrovasc. Dis. 2003, 16, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Castillo, J.; Leira, R.; García, M.M.; Serena, J.; Blanco, M.; Dávalos, A. Blood Pressure Decrease during the Acute Phase of Ischemic Stroke Is Associated with Brain Injury and Poor Stroke Outcome. Stroke 2004, 35, 520–526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, N.; Wahlgren, N.; Brainin, M.; Castillo, J.; Ford, G.A.; Kaste, M. Relationship of Blood Pressure, Antihypertensive Therapy and Outcome in Ischemic Stroke Treated with Intravenous Thrombolysis: Retrospective Analysis from Safe Implementation of Thrombolysis in the Stroke-International Stroke Thrombolysis Register (SITS-ISTR. Stroke 2009, 40, 2442–2449. [Google Scholar]
- Potter, J.F.; Robinson, T.G.; Ford, G.A. Controlling hypertension and hypotension immediately post-stroke (CHHIPS): A randomized, placebocontrolled, double-blind pilot trial. Lancet Neurol 2009, 8, 48–56. [Google Scholar] [CrossRef]
- Robinson, T.G.; Potter, J.F.; Ford, G.A.; Bulpitt, C.J.; Chernova, J.; Jagger, C. Effects of Antihypertensive Treatment after Acute Strokes in the Continue or Stop Post-Stroke Antihypertensives Collaborative Study (COSSACS): A Prospective, Randomized, Open, Blinded-Endpoint Trial. Lancet Neurol. 2010, 9, 767–775. [Google Scholar] [CrossRef]
- Sandset, E.C.; Bath, P.M.; Boysen, G. The Angiotensin- Receptor Blocker Candesartan for Treatment of Acute Stroke (SCAST): A Randomized, Placebo-Controlled, Double-Blind Trial. Lancet 2011, 377, 741–750. [Google Scholar] [CrossRef]
- He, J.; Zhang, Y.; Xu, T. Effects of Immediate Blood Pressure Reduction on Death and Disability in Patients with Acute Ischemic Stroke: The CATIS Randomized Clinical Trial. JAMA 2014, 311, 479–489. [Google Scholar] [CrossRef] [Green Version]
- The ENOS Trial Investigators. Efficacy of Nitric Oxide, with or without Continuing Antihypertensive Treatment, for Management of High Blood Pressure in Acute Stroke (ENOS): A Partial-Factorial Randomised Controlled Trial. Lancet 2015, 385, 617–628. [Google Scholar] [CrossRef] [Green Version]
- Jusufovic, M.; Sandset, E.C.; Bath, P.M.; Karlson, B.W.; Berge, E. Effects of blood pressure lowering in patients with acute ischemic stroke and carotid artery stenosis. Int. J. Stroke 2015, 10, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Huo, X.; Zhao, X.; Liao, X.; Wang, C.; Pan, Y. Relationship between Blood Pressure and Outcomes in Acute Ischemic Stroke Patients Administered Lytic Medication in the TIMS-China Study. PLoS ONE 2016, 11, e0144260. [Google Scholar] [CrossRef] [PubMed]
- Allison, T.A.; Bowman, S.; Gulbis, B.; Hartman, H.; Schepcoff, S.; Lee, K. Comparison of Clevidipine and Nicardipine for Acute Blood Pressure Reduction in Patients With Stroke. J. Intensive Care Med. 2019, 34, 990–995. [Google Scholar] [CrossRef]
- Nasi, L.A.; Martins, S.C.O.; Gus, M.; Weiss, G.; Almeida, A.G.; Brondani, R.; Rebello, L.C.; DalPizzol, A.; Fuchs, F.D.; Valença, M.J.M.; et al. Early Manipulation of Arterial Blood Pressure in Acute Ischemic Stroke (MAPAS): Results of a Randomized Controlled Trial. Neurocrit. Care 2019, 30, 372–379. [Google Scholar] [CrossRef]
- Anderson, C.S.; Huang, Y.; Wang, J.G.; Arima, H.; Neal, B.; Peng, B.; Heeley, E.; Skulina, C.; Parsons, M.W.; Kim, J.S.; et al. Intensive Blood Pressure Reduction in Acute Cerebral Haemorrhage Trial (INTERACT): A Randomised Pilot Trial. Lancet Neurol. 2008, 7, 391–399. [Google Scholar] [CrossRef]
- Koch, S.; Roman, J.G.; Forteza, A.M.; Otero, C.M.; Rabinstein, A.A. Rapid Blood Pressure Reduction in Acute Intracerebral Hemorrhage: Feasibility and Safety. Neurocrit. Care 2008, 8, 316–321. [Google Scholar] [CrossRef]
- Suri, M.F.K.; Suarez, J.I.; Rodrigue, T.C.; Zaidat, O.O.; Vazquez, G.; Wensel, A.; Selman, W.R. Effect of Treatment of Elevated Blood Pressure on Neurological Deterioration in Patients with Acute Intracerebral Hemorrhage. Neurocrit. Care 2008, 9, 177–182. [Google Scholar] [CrossRef]
- Anderson, C.S.; Huang, Y.; Arima, H.; Heeley, E.; Skulina, C.; Parsons, M.W.; Peng, B.; Li, Q.; Su, S.; Tao, Q.L.; et al. Effects of Early Intensive Blood Pressure-Lowering Treatment on the Growth of Hematoma and Perihematomal Edema in Acute Intracerebral Hemorrhage: The Intensive Blood Pressure Reduction in Acute Cerebral Haemorrhage Trial (INTERACT). Stroke 2010, 41, 307–312. [Google Scholar] [CrossRef] [Green Version]
- Antihypertensive Treatment of Acute Cerebral Hemorrhage (ATACH) investigators Antihypertensive Treatment of Acute Cerebral Hemorrhage. Crit. Care Med. 2010, 38, 637–648. [CrossRef]
- Anderson, C.S.; Heeley, E.; Huang, Y.; Wang, J.; Stapf, C.; Delcourt, C.; Lindley, R.; Robinson, T.; Lavados, P.; Neal, B.; et al. Rapid Blood-Pressure Lowering in Patients with Acute Intracerebral Hemorrhage. N. Engl. J. Med. 2013, 368, 2355–2365. [Google Scholar] [CrossRef] [PubMed]
- Butcher, K.S.; Jeerakathil, T.; Hill, M.; Demchuk, A.M.; Dowlatshahi, D.; Coutts, S.B.; ICH ADAPT Investigators. The Intracerebral Hemorrhage Acutely Decreasing Arterial Pressure Trial. Stroke 2013, 44, 620–626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakamoto, Y.; Koga, M.; Yamagami, H.; Okuda, S.; Okada, Y.; Kimura, K. Systolic Blood Pressure after Intravenous Antihypertensive Treatment and Clinical Outcomes in Hyperacute Intracerebral Hemorrhage: Stroke Acute Management with Urgent Risk-Factor Assessment and Improvement- Intracerebral Hemorrhage Study. Stroke 2013, 44, 1846–1851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez-Luna, D.; Pineiro, S.; Rubiera, M.; Ribo, M.; Coscojuela, P.; Pagola, J. Impact of Blood Pressure Changes and Course on Hematoma Growth in Acute Intracerebral Hemorrhage. Eur. J. Neurol. 2013, 20, 1277–1283. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, K.; Scutt, P.; Woodhouse, L.; Adami, A.; Becker, J.L.; Cala, L.A.; Casado, A.M.; Chen, C.; Dineen, R.A.; Gommans, J.; et al. Continuing versus Stopping Prestroke Antihypertensive Therapy in Acute Intracerebral Hemorrhage: A Subgroup Analysis of the Efficacy of Nitric Oxide in Stroke Trial. J. Stroke Cerebrovasc. Dis. 2016, 25, 1017–1026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qureshi, A.I.; Palesch, Y.Y.; Barsan, W.G.; Hanley, D.F.; Hsu, C.Y.; Martin, R.L. Intensive Blood-Pressure Lowering in Patients with Acute Cerebral Hemorrhage. N. Engl. J. Med. 2016, 375, 1033–1043. [Google Scholar] [CrossRef] [PubMed]
- Bozzano, V.; Carandini, T.; Gruppo di Autoformazione Metodologica (GrAM). Intensive Lowering of Blood Pressure in the Acute Phase of Intracranial Haemorrhage. Intern. Emerg. Med. 2017, 12, 379–380. [Google Scholar] [CrossRef]
- Hatcher, S.; Chen, C.; Govindarajan, P. Prehospital Systolic Hypertension and Outcomes in Patients with Spontaneous Intracerebral Hemorrhage. Cureus 2017, 9. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Luna, D.; Rodriguez-Villatoro, N.; Juega, J.M.; Boned, S.; Muchada, M.; Sanjuan, E.; Pagola, J.; Rubiera, M.; Ribo, M.; Coscojuela, P.; et al. Prehospital Systolic Blood Pressure Is Related to Intracerebral Hemorrhage Volume on Admission. Stroke 2018, 49, 204–206. [Google Scholar] [CrossRef]
- Zhu, Z.; Bower, M.; Stern-Nezer, S.; Atallah, S.; Stradling, D.; Groysman, L.; Dastur, C.K.; Akbari, Y.; Yu, W. Early Initiation of Oral Antihypertensives Reduces Intensive Care Unit Stay and Hospital Cost for Patients with Hypertensive Intracerebral Hemorrhage. Neurocrit. Care 2020, 32, 707–714. [Google Scholar] [CrossRef]
- Bravata, D.M.; Ho, S.Y.; Brass, L.M.; Concato, J.; Scinto, J.; Meehan, T.P. Long-Term Mortality in Cerebrovascular Disease. Stroke 2003, 34, 699–704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayer, S.A.; Kurtz, P.; Wyman, A.; Sung, G.Y.; Multz, A.S.; Varon, J.; Granger, C.B.; Kleinschmidt, K.; Lapointe, M.; Peacock, W.F.; et al. Clinical Practices, Complications, and Mortality in Neurological Patients with Acute Severe Hypertension: The Studying the Treatment of Acute HyperTension Registry. Crit. Care Med. 2011, 39, 2330–2336. [Google Scholar] [CrossRef] [PubMed]
- Bath, P.M.; Krishnan, K. Interventions for deliberately altering blood pressure in acute stroke. Cochrane Database Syst. Rev. 2014, 10, 1883–1889. [Google Scholar] [CrossRef]
- Lee, M.; Ovbiagele, B.; Hong, K.S.; Wu, Y.L.; Lee, J.E.; Rao, N.M. Effect of blood pressure lowering in early ischemic stroke: Meta-analysis. Stroke 2015, 46, 1883–1889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, R.; Liu, F.D.; Wang, S.; Peng, J.L.; Tao, X.X.; Zhang, B. Blood pressure reduction in acute phase of ischemic stroke does not improve short- or long-term dependency or mortality: A meta-analysis of current literature. Med. Baltim. 2015, 94, 896. [Google Scholar] [CrossRef] [PubMed]
- Jauch, E.C.; Saver, J.L.; Adams, H.P., Jr. Guidelines for the Management of Patients with Acute Ischemic Strokes: A Guideline for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke 2013, 44, 870–947. [Google Scholar] [CrossRef] [PubMed]
- Sandset, E.C.; Murray, G.D.; Bath, P.M.; Kjeldsen, S.E.; Berge, K.S.E. Relationship between Change in Blood Pressure and the Risk of Early Adverse Events and Poor Outcome. Stroke 2012, 43, 2108–2114. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.S.; Shimazu, K.; Fukuuchi, Y.; Ouchi, T.; Okamoto, S.; Koto, A. Impaired Neurogenic Cerebrovascular Control and Dysautoregulation after Stroke. Stroke 1973, 4, 169–186. [Google Scholar] [CrossRef] [Green Version]
- Powers, W.J.; Videen, T.O.; Diringer, M.N.; Aiyagari, V.; Zazulia, A.R. Autoregulation after ischaemic stroke. J. Hypertens. 2009, 27, 2218–2222. [Google Scholar] [CrossRef] [Green Version]
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B.; et al. 2018 Guidelines for the Early Management of Patients With Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2018, 49, e46–e99. [Google Scholar] [CrossRef]
- SPREAD-Stroke Prevention and Educational Awareness Diffusion Ictus Cerebrale: Linee Guida Italiane di Prevenzione e Trattamento, VIII ed.; 2016; Available online: http://www.iso-spread.it/index.php?azione=capitoli#end (accessed on 1 January 2021).
- Toni, D.; Mangiafico, S.; Agostoni, E.; Bergui, M.; Cerrato, P.; Ciccone, A.; Vallone, S.; Zini, A.; Inzitari, D. Intravenous Thrombolysis and Intra-Arterial Interventions in Acute Ischemic Stroke: Italian Stroke Organisation (ISO)-SPREAD Guidelines. Int. J. Stroke Off. J. Int. Stroke Soc. 2015, 10, 1119–1129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira-Filho, J.; Martins, S.C.O.; Pontes-Neto, O.M.; Longo, A.; Evaristo, E.F.; de Carvalho, J.J.F.; Fernandes, J.G.; Zétola, V.F.; Gagliardi, R.J.; Vedolin, L.; et al. Guidelines for Acute Ischemic Stroke Treatment: Part I. Arq. Neuropsiquiatr. 2012, 70, 621–629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morgenstern, L.B.; Hemphill, J.C., III; Anderson, C.; Becker, K.; Broderick, J.P.; Connolly, E.S., Jr.; Greenberg, S.M.; Huang, J.N.; Macdonald, R.L.; Messe, S.R.; et al. Guidelines for the Management of Spontaneous Intracerebral Hemorrhage: A Guideline for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke 2010, 41, 2108–2129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hemphill, J.C., III; Greenberg, S.M.; Anderson, C.S.; Becker, K.; Bendok, B.R.; Cushman, M.; Fung, G.L.; Goldstein, J.N.; Macdonald, R.L.; Mitchell, P.H.; et al. Guidelines for the Management of Spontaneous Intracerebral Hemorrhage. Stroke 2015, 46, 2032–2060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsivgoulis, G.; Katsanos, A.H.; Butcher, K.S.; Boviatsis, E.; Triantafyllou, N.; Rizos, I.; Alexandrov, A.V. Intensive Blood Pressure Reduction in Acute Intracerebral Hemorrhage: A Meta-Analysis. Neurology 2014, 83, 1523–1529. [Google Scholar] [CrossRef]
- Gazzeri, R.; Galarza, M.; Callovini, G.; Alfieri, A. Biosurgical Hemostatic Agents in Neurosurgical Intracranial Procedures. Surg. Technol. Int. 2017, 30, 468–476. [Google Scholar]
- Zahra, K.; Turnbull, M.T.; Zubair, A.C.; Siegel, J.L.; Venegas-Borsellino, C.P.; Tawk, R.G.; Freeman, W.D. A Combined Approach to Intracerebral Hemorrhage: Intravenous Mesenchymal Stem Cell Therapy with Minimally Invasive Hematoma Evacuation. J. Stroke Cerebrovasc. Dis. Off. J. Natl. Stroke Assoc. 2020, 29, 104931. [Google Scholar] [CrossRef]
- Kuramatsu, J.B.; Gerner, S.T.; Schellinger, P.D.; Glahn, J.; Endres, M.; Sobesky, J.; Flechsenhar, J.; Neugebauer, H.; Jüttler, E.; Grau, A.; et al. Anticoagulant Reversal, Blood Pressure Levels, and Anticoagulant Resumption in Patients with Anticoagulation-Related Intracerebral Hemorrhage. JAMA 2015, 313, 824–836. [Google Scholar] [CrossRef]
- Qureshi, A.I.; Palesch, Y.Y.; Foster, L.D.; Barsan, W.G.; Goldstein, J.N.; Hanley, D.F.; Hsu, C.Y.; Moy, C.S.; Qureshi, M.H.; Silbergleit, R.; et al. Blood Pressure-Attained Analysis of ATACH 2 Trial. Stroke 2018, 49, 1412–1418. [Google Scholar] [CrossRef]
- Gong, S.; Lin, C.; Zhang, D.; Kong, X.; Chen, J.; Wang, C.; Li, Z.; Chen, R.; Sheng, P.; Dong, Y.; et al. Effects of Intensive Blood Pressure Reduction on Acute Intracerebral Hemorrhage: A Systematic Review and Meta-Analysis. Sci. Rep. 2017, 7, 10694. [Google Scholar] [CrossRef] [Green Version]
- Schreuder, F.H.; Sato, S.; Klijn, C.J.; Anderson, C.S. Medical Management of Intracerebral Haemorrhage. J. Neurol. Neurosurg. Psychiatry 2016, 88, 76–84. [Google Scholar] [CrossRef]
- Hill, M.D.; Muir, K.W. INTERACT-2: Should Blood Pressure Be Aggressively Lowered Acutely after Intracerebral Hemorrhage? Stroke 2013, 44, 2951–2952. [Google Scholar] [CrossRef] [Green Version]
- Carandini, T.; Bozzano, V.; Scarpini, E.; Montano, N.; Solbiati, M. Intensive versus Standard Lowering of Blood Pressure in the Acute Phase of Intracranial Haemorrhage: A Systematic Review and Meta-Analysis. Intern. Emerg. Med. 2018, 13, 95–105. [Google Scholar] [CrossRef]
- Smith, E.E.; Saver, J.L.; Alexander, D.N.; Furie, K.L.; Hopkins, L.N.; Katzan, I.L.; Mackey, J.S.; Miller, E.L.; Schwamm, L.H.; Williams, L.S.; et al. Clinical Performance Measures for Adults Hospitalized with Acute Ischemic Stroke: Performance Measures for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke 2014, 45, 3472–3498. [Google Scholar] [CrossRef]
- Qureshi, A.I. Acute Hypertensive Response in Patients with Stroke: Pathophysiology and Management. Circulation 2008, 118, 176–187. [Google Scholar] [CrossRef] [Green Version]
- Veglio, F.; Paglieri, C.; Rabbia, F.; Bisbocci, D.; Bergui, M.; Cerrato, P. Hypertension and Cerebrovascular Damage. Atherosclerosis 2009, 205, 331–341. [Google Scholar] [CrossRef]
- Phillips, S.J. Pathophysiology and management of hypertension in acute ischemic stroke. Hypertension 1994, 23, 131–136. [Google Scholar] [CrossRef] [Green Version]
- Johnston, K.C.; Mayer, S.A. Blood Pressure Reduction in Ischemic Stroke: A Two-Edged Sword? Neurology 2003, 61, 1047–1051. [Google Scholar] [CrossRef] [PubMed]
- Shah, Q.A.; Ezzeddine, M.A.; Qureshi, A.I. Acute Hypertension in Intracerebral Hemorrhage: Pathophysiology and Treatment. J. Neurol. Sci. 2007, 261, 74–79. [Google Scholar] [CrossRef]
- Saver, J.L. Blood Pressure Management in Early Ischemic Stroke. JAMA 2014, 311, 469–470. [Google Scholar] [CrossRef]
- Aslanyan, S.; Fazekas, F.; Weir, C.J.; Horner, S.; Lees, K.R.; GAIN International Steering Committee and Investigators. Effect of Blood Pressure during the Acute Period of Ischemic Stroke on Stroke Outcome: A Tertiary Analysis of the GAIN International Trial. Stroke 2003, 34, 2420–2425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olsen, T.S.; Larsen, B.; Herning, M.; Skriver, E.B.; Lassen, N.A. Blood Flow and Vascular Reactivity in Collaterally Perfused Brain Tissue. Evidence of an Ischemic Penumbra in Patients with Acute Stroke. Stroke 1983, 14, 332–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mattle, H.P.; Kappeler, L.; Arnold, M.; Fischer, U.; Nedeltchev, K.; Remonda, L.; Jakob, S.M.; Schroth, G. Schroth Gerhard Blood Pressure and Vessel Recanalization in the First Hours After Ischemic Stroke. Stroke 2005, 36, 264–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caponnetto, P.; Maglia, M.; Pistritto, L.; Ferlito, S.; Cannella, M.C. Family Violence and Its Psychological Management at the Emergency Department: A Review. Health Psychol. Res. 2019, 7. [Google Scholar] [CrossRef]
- Kvistad, C.E.; Oygarden, H.; Logallo, N.; Thomassen, L.; Waje-Andreassen, U.; Moen, G.; Naess, H. A Stress-Related Explanation to the Increased Blood Pressure and Its Course Following Ischemic Stroke. Vasc. Health Risk Manag. 2016, 12, 435–442. [Google Scholar] [CrossRef] [Green Version]
- Arboix, A.; Roig, H.; Rossich, R.; Martínez, E.M.; García-Eroles, L. Differences between Hypertensive and Non-Hypertensive Ischemic Stroke. Eur. J. Neurol. 2004, 11, 687–692. [Google Scholar] [CrossRef]
- Qureshi, A.I.; Geocadin, R.G.; Suarez, J.I.; Ulatowski, J.A. Long-Term Outcome after Medical Reversal of Transtentorial Herniation in Patients with Supratentorial Mass Lesions. Crit. Care Med. 2000, 28, 1556–1564. [Google Scholar] [CrossRef]
- Cheung, R.T.F.; Hachinski, V. Cardiac Effects of Stroke. Curr. Treat. Options Cardiovasc. Med. 2004, 6, 199–207. [Google Scholar] [CrossRef]
- Nakagawa, K.; Yamaguchi, T.; Seida, M.; Yamada, S.; Imae, S.; Tanaka, Y.; Yamamoto, K.; Ohno, K. Plasma Concentrations of Brain Natriuretic Peptide in Patients with Acute Ischemic Stroke. Cerebrovasc. Dis. 2005, 19, 157–164. [Google Scholar] [CrossRef]
- Stevens, S.L.; Wood, S.; Koshiaris, C.; Law, K.; Glasziou, P.; Stevens, R.J.; McManus, R.J. Blood Pressure Variability and Cardiovascular Disease: Systematic Review and Meta-Analysis. BMJ 2016, 354, i4098. [Google Scholar] [CrossRef] [Green Version]
- Rothwell, P.M.; Howard, S.C.; Dolan, E.; O’Brien, E.; Dobson, J.E.; Dahlöf, B.; Sever, P.S.; Poulter, N.R. Prognostic Significance of Visit-to-Visit Variability, Maximum Systolic Blood Pressure, and Episodic Hypertension. Lancet Lond. Engl. 2010, 375, 895–905. [Google Scholar] [CrossRef]
- Rothwell, P.M.; Howard, S.C.; Dolan, E.; O’Brien, E.; Dobson, J.E.; Dahlöf, B.; Poulter, N.R.; Sever, P.S.; ASCOT-BPLA and MRC Trial Investigators. Effects of Beta Blockers and Calcium-Channel Blockers on within-Individual Variability in Blood Pressure and Risk of Stroke. Lancet Neurol. 2010, 9, 469–480. [Google Scholar] [CrossRef]
- Zheng, J.; Xie, Y.; Wang, Y.; Guo, R.; Dai, Y.; Sun, Z.; Xing, L.; Zhang, X.; Sun, Y.; Zheng, L. Short- and Long-Term Systolic Blood Pressure Changes Have Different Impacts on Major Adverse Cardiovascular Events: Results from a 12.5 Years Follow-up Study. Int. J. Cardiol. 2020, 306, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Meeks, J.R.; Bambhroliya, A.B.; Meyer, E.G.; Slaughter, K.B.; Fraher, C.J.; Sharrief, A.Z.; Bowry, R.; Ahmed, W.O.; Tyson, J.E.; Miller, C.C.; et al. High In-Hospital Blood Pressure Variability and Severe Disability or Death in Primary Intracerebral Hemorrhage Patients. Int. J. Stroke Off. J. Int. Stroke Soc. 2019, 14, 987–995. [Google Scholar] [CrossRef]
- Penn, A.M.; Croteau, N.S.; Votova, K.; Sedgwick, C.; Balshaw, R.F.; Coutts, S.B.; Penn, M.; Blackwood, K.; Bibok, M.B.; Saly, V.; et al. Systolic Blood Pressure as a Predictor of Transient Ischemic Attack/Minor Stroke in Emergency Department Patients under Age 80: A Prospective Cohort Study. BMC Neurol. 2019, 19, 251. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.W.; Cadilhac, D.A.; Donnan, G.A.; O’Callaghan, C.; Dewey, H.M. Hypertension and TIA. Int. J. Stroke Off. J. Int. Stroke Soc. 2009, 4, 206–214. [Google Scholar] [CrossRef]
- Zonneveld, T.P.; Richard, E.; Vergouwen, M.D.; Nederkoorn, P.J.; de Haan, R.; Roos, Y.B.; Kruyt, N.D. Blood Pressure-Lowering Treatment for Preventing Recurrent Stroke, Major Vascular Events, and Dementia in Patients with a History of Stroke or Transient Ischaemic Attack. Cochrane Database Syst. Rev. 2018, 7, CD007858. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Zhong, C.; Zhang, Y.; Xie, X.; Zhu, Z.; Wang, A.; Chen, C.-S.; Peng, Y.; Peng, H.; Li, Q.; et al. Immediate Antihypertensive Treatment for Patients With Acute Ischemic Stroke With or Without History of Hypertension: A Secondary Analysis of the CATIS Randomized Clinical Trial. JAMA Netw. Open 2019, 2, e198103. [Google Scholar] [CrossRef]
- Appleton, J.P.; Sprigg, N.; Bath, P.M. Blood Pressure Management in Acute Stroke. Stroke Vasc. Neurol. 2016, 1, 72–82. [Google Scholar] [CrossRef]
- Ankolekar, S.; Fuller, M.; Cross, I.; Renton, C.; Cox, P.; Sprigg, N.; Siriwardena, A.N.; Bath, P.M. Feasibility of an Ambulance-Based Stroke Trial, and Safety of Glyceryl Trinitrate in Ultra-Acute Stroke: The Rapid Intervention with Glyceryl Trinitrate in Hypertensive Stroke Trial (RIGHT, ISRCTN66434824). Stroke 2013, 44, 3120–3128. [Google Scholar] [CrossRef] [Green Version]
- Shaw, L.; Price, C.; McLure, S.; Howel, D.; McColl, E.; Younger, P.; Ford, G.A. Paramedic Initiated Lisinopril For Acute Stroke Treatment (PIL-FAST): Results from the Pilot Randomised Controlled Trial. Emerg. Med. J. EMJ 2014, 31, 994–999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saver, J.L.; Starkman, S.; Eckstein, M.; Stratton, S.J.; Pratt, F.D.; Hamilton, S.; Conwit, R.; Liebeskind, D.S.; Sung, G.; Kramer, I.; et al. Prehospital Use of Magnesium Sulfate as Neuroprotection in Acute Stroke. N. Engl. J. Med. 2015, 372, 528–536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bath, P.M.; Scutt, P.; Anderson, C.S.; Appleton, J.P.; Berge, E.; Cala, L.; Dixon, M.; England, T.M.; Godolphin, P.J.; Havard, D.; et al. Prehospital Transdermal Glyceryl Trinitrate in Patients with Ultra-Acute Presumed Stroke (RIGHT-2): An Ambulance-Based, Randomised, Sham-Controlled, Blinded, Phase 3 Trial. Lancet 2019, 393, 1009–1020. [Google Scholar] [CrossRef] [Green Version]
- Bath, P.M.; Woodhouse, L.J.; Krishnan, K.; Appleton, J.P.; Anderson, C.S.; Berge, E.; Cala, L.; Dixon, M.; England, T.J.; Godolphin, P.J.; et al. Prehospital Transdermal Glyceryl Trinitrate for Ultra-Acute Intracerebral Hemorrhage. Stroke 2019, 50, 3064–3071. [Google Scholar] [CrossRef] [PubMed]
- Gioia, L.C.; Zewude, R.T.; Kate, M.P.; Liss, K.; Rowe, B.H.; Buck, B.; Jeerakathil, T.; Butcher, K. Prehospital Systolic Blood Pressure Is Higher in Acute Stroke Compared with Stroke Mimics. Neurology 2016, 86, 2146–2153. [Google Scholar] [CrossRef] [Green Version]
- Fassbender, K.; Grotta, J.C.; Walter, S.; Grunwald, I.Q.; Ragoschke-Schumm, A.; Saver, J.L. Mobile Stroke Units for Prehospital Thrombolysis, Triage, and beyond: Benefits and Challenges. Lancet Neurol. 2017, 16, 227–237. [Google Scholar] [CrossRef]
- Aiyagari, V.; Gorelick, P.B. Management of Blood Pressure for Acute and Recurrent Stroke. Stroke 2009, 40, 2251–2256. [Google Scholar] [CrossRef]
- Jeong, H.-G.; Kim, B.J.; Kim, H.; Jung, C.; Han, M.-K.; Liebeskind, D.S.; Bae, H.-J. Blood Pressure Drop and Penumbral Tissue Loss in Nonrecanalized Emergent Large Vessel Occlusion. Stroke 2019, 50, 2677–2684. [Google Scholar] [CrossRef]
- Law, M.R.; Morris, J.K.; Wald, N.J. Use of Blood Pressure Lowering Drugs in the Prevention of Cardiovascular Disease: Meta-Analysis of 147 Randomized Trials in the Context of Expectations from Prospective Epidemiological Studies. BMJ 2009, 338, 1665. [Google Scholar] [CrossRef] [Green Version]
- Tyrrell, P.; Swain, S.; Rudd, A.; Acute, S. NICE Guideline on Acute Stroke and TIA: Commentary. Heart 2009, 95, 843–845. [Google Scholar] [CrossRef]
- Qureshi, A.I.; Qureshi, M.H. Acute Hypertensive Response in Patients with Intracerebral Hemorrhage Pathophysiology and Treatment. J. Cereb. Blood Flow Metab. 2018, 38, 1551–1563. [Google Scholar] [CrossRef] [PubMed]
- Vitt, J.R.; Trillanes, M.; Hemphill, J.C.I. Management of Blood Pressure During and After Recanalization Therapy for Acute Ischemic Stroke. Front. Neurol. 2019, 10, 138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blech, B.; Chong, B.W.; Sands, K.A.; Wingerchuk, D.M.; Jackson, W.T.; Marks, L.A.; O’Carroll, C.B. Are Postprocedural Blood Pressure Goals Associated With Clinical Outcome After Mechanical Thrombectomy for Acute Ischemic Stroke? Neurologist 2019, 24, 44–47. [Google Scholar] [CrossRef] [PubMed]
- Talke, P.O.; Sharma, D.; Heyer, E.J.; Bergese, S.D.; Blackham, K.A.; Stevens, R.D. Republished: Society for Neuroscience in Anesthesiology and Critical Care Expert Consensus Statement: Anesthetic Management of Endovascular Treatment for Acute Ischemic Stroke*. Stroke 2014, 45, e138–e150. [Google Scholar] [CrossRef]
- Bang, O.Y.; Chung, J.-W.; Kim, S.-K.; Kim, S.J.; Lee, M.J.; Hwang, J.; Seo, W.-K.; Ha, Y.S.; Sung, S.M.; Kim, E.-G.; et al. Therapeutic-Induced Hypertension in Patients with Noncardioembolic Acute Stroke. Neurology 2019, 93, e1955–e1963. [Google Scholar] [CrossRef]
- Lanza, G.; Bella, R.; Giuffrida, S.; Cantone, M.; Pennisi, G.; Spampinato, C.; Giordano, D.; Malaguarnera, G.; Raggi, A.; Pennisi, M. Preserved Transcallosal Inhibition to Transcranial Magnetic Stimulation in Nondemented Elderly Patients with Leukoaraiosis. BioMed. Res. Int. 2013, 2013, 351680. [Google Scholar] [CrossRef]
- Vinciguerra, L.; Lanza, G.; Puglisi, V.; Pennisi, M.; Cantone, M.; Bramanti, A.; Pennisi, G.; Bella, R. Transcranial Doppler Ultrasound in Vascular Cognitive Impairment-No Dementia. PLoS ONE 2019, 14, e0216162. [Google Scholar] [CrossRef] [Green Version]
- Puglisi, V.; Bramanti, A.; Lanza, G.; Cantone, M.; Vinciguerra, L.; Pennisi, M.; Bonanno, L.; Pennisi, G.; Bella, R. Impaired Cerebral Haemodynamics in Vascular Depression: Insights From Transcranial Doppler Ultrasonography. Front. Psychiatry 2018, 9, 316. [Google Scholar] [CrossRef] [Green Version]
- Astarita, A.; Covella, M.; Vallelonga, F.; Cesareo, M.; Totaro, S.; Ventre, L.; Aprà, F.; Veglio, F.; Milan, A. Hypertensive Emergencies and Urgencies in Emergency Departments: A Systematic Review and Meta-Analysis. J. Hypertens. 2020, 38, 1203–1210. [Google Scholar] [CrossRef]
- Guiga, H.; Decroux, C.; Michelet, P.; Loundou, A.; Cornand, D.; Silhol, F.; Vaisse, B.; Sarlon-Bartoli, G. Hospital and Out-of-Hospital Mortality in 670 Hypertensive Emergencies and Urgencies. J. Clin. Hypertens. Greenwich Conn. 2017, 19, 1137–1142. [Google Scholar] [CrossRef]
- Lobanova, I.; Qureshi, A.I. Blood Pressure Goals in Acute Stroke-How Low Do You Go? Curr. Hypertens. Rep. 2018, 20, 28. [Google Scholar] [CrossRef] [PubMed]

| Study | Design | Patients n | Aim/Outcomes | Methods | Main Findings |
|---|---|---|---|---|---|
| Barer et al., 1988 [59] | Prospective randomized trial (BEST) | 302 | To test the protective effect of propranolol on cerebral function in patients with acute stroke. | Patients with clinically diagnosed hemispheric stroke within the previous 48 h were randomly assigned to atenolol, propranolol, or placebo for 3 weeks. | More mortality among patients allocated to β-blockers. The outcome in those taking beta-blockers at the time of stroke was considerably better, suggesting that prior treatment might be protective. |
| Brott et al., 1998 [60] | Prospective Randomized trial | 624 | To examine the frequency, course, and treatment of hypertension in the NINDS recombinant tissue plasminogen activator stroke trial. | BP was measured at the time of admission, at randomization, and then 36 times during the first 24 h after randomization to correlate antihypertensive therapy (not randomized) with clinical outcomes. | Treated patients who were hypertensive after randomization and received antihypertensive therapy were less likely to have a favorable outcome at three months than those who were hypertensive and did not receive antihypertensive therapy. |
| Ahmed et al., 2000 [61] | Prospective INWEST study analysis | 250 | To correlate nimodipine-induced reduction in BP and an unfavorable outcome in acute stroke with and without adjustment for prognostic variables; to investigate outcome in subgroups with increasing levels of BP reduction. | Patients with ischemic stroke (within 24 h) received placebo, 1 mg/h (low-dose), or 2 mg/h (high-dose) of nimodipine. | BP, but not SBP, reduction was associated with neurological worsening after the intravenous administration of high-dose nimodipine after acute stroke. The results related to low-dose nimodipine were not conclusive. |
| Arima et al., 2001 [62] | Prospective randomized trial (PROGRESS) | 6105 | To determine the effects of a flexible BP-lowering regimen with an angiotensin-converting enzyme inhibitor (perindopril) and a diuretic (indapamide) on the risk of stroke and other major vascular events among individuals with a history of stroke or transient ischemic attack. | Patients were randomly assigned to active treatment (perindopril, with the addition of indapamide) or placebo. | The BP-lowering regimen reduced the risk of stroke in both hypertensive and non-hypertensive individuals with a history of stroke or transient ischemic attack. Combination therapy produced larger BP reductions and larger risk reductions than perindopril alone. |
| Rordorf et al., 2001 [63] | Prospective | 13 | To assess whether induced hypertension with acute stroke would safely identify a subgroup of patients with a BP-dependent neurologic deficit. | Patients underwent induced hypertension for 30 min using accelerating doses of intravenous phenylephrine within 12 h of stroke onset. | The use of induced hypertension in the context of careful clinical setting was likely to be safe and associated with low morbidity and mortality. |
| Hillis et al., 2003 [64] | Prospective | 15 | To evaluate the effects of pharmacologically induced BP elevation on function and perfusion in acute ischemic stroke. | Patients with large diffusion–perfusion mismatch were randomly assigned to BP elevation or conventional management. | Positive effect of induced BP elevation on neurological function, associated with a reduction in hypoperfused tissue on perfusion weighted imaging scans. |
| Castillo et al., 2004 [65] | Observational study | 304 | To explore the association of SBP and DBP during acute stroke with early neurological deterioration, infarct volume, neurological outcome, and mortality at three months. | SBP and DBP values on admission and on the first day were the average values of all readings obtained in the emergency department and during a 24 h period after patient allocation in the stroke unit. | High and low SBP and DBP, as well as a relevant drop in BP, were associated with poor prognosis in patients with ischemic stroke. The effects disappeared after adjustment for the use of antihypertensive drugs and BP drop >20 mm Hg within the first day. |
| Ahmed et al., 2009 [66] | Retrospective analysis of the SITS-ISTR | 11,080 | To examine the relationship between BP and antihypertensive therapy with outcomes in patients with and without a history of hypertension treated with intravenous thrombolysis using the “Safe Implementation of Thrombolysis in the Stroke—International Stroke Thrombolysis Register” (SITS-ISTR). | Patients were categorized in 4 groups according to a history of hypertension and antihypertensive therapy within 7 days after thrombolysis. BP values were recorded at baseline, 2 h, and 24 h after thrombolysis. | There was a strong association of high SBP after thrombolysis with poor outcome. Antihypertensive therapy up to 7 days in patients with a history of hypertension was associated with worse outcome, whereas initiation of antihypertensive therapy in newly recognized moderate hypertension was associated with a favorable outcome. |
| Potter et al., 2009 [67] | Prospective randomized trial (CHHIPS) | 179 | To assess the feasibility, safety, and effects of two regimens for lowering BP in patients who had a ischemic or hemorrhagic stroke. | Patients with cerebral infarction or hemorrhage and hypertension (SBP > 160 mmHg) were randomly assigned to oral labetalol, lisinopril, or placebo if they were non-dysphagic; or to intravenous labetalol, sublingual lisinopril, or placebo if they had dysphagia within 36 h of symptom onset. | Labetalol and lisinopril were effective antihypertensive drugs in acute stroke and did not increase serious adverse events. Early lowering of BP with lisinopril and labetalol after an acute stroke was a promising approach to reduce mortality and future disability. |
| Robinson et al., 2010 [68] | Prospective randomized trial (COSSACS) | 763 | To assess the efficacy and safety of continuing or stopping pre-existing antihypertensive drugs in patients who recently had a stroke. | Patients taking antihypertensive drugs enrolled within 48 h of stroke and the last dose of antihypertensive drug and randomly assigned to either continuing or stopping pre-existing antihypertensive drugs for 2 weeks. | Continuation of antihypertensive drugs did not reduce 2-week death or dependency, cardiovascular event rate, or mortality at 6 months. Lower BP levels in those who continued treatment after an acute mild stroke were not associated with more adverse events. |
| Sandset et al., 2011 [69] | Prospective randomized trial (SCAST) | 2029 | To examine whether a careful BP-lowering treatment with the angiotensin-receptor blocker candesartan was beneficial in patients with acute stroke and increased BP. | Patients with acute stroke (ischemic or hemorrhagic) and SBP ≥140 mmHg included within 30 h from symptom onset and randomly allocated to candesartan or placebo for 7 days, with increasing dosages. | There was no indication that careful BP-lowering treatment with the angiotensin-receptor blocker candesartan was beneficial in patients with acute stroke and raised BP. |
| He et al., 2014 [70] | Prospective randomized trial (CATIS) | 4071 | To evaluate whether an immediate BP reduction in patients with acute ischemic stroke would reduce death or major disability at 14 days or at hospital discharge. | Patients with non-thrombolyzed ischemic stroke within 48 h of onset and elevated SBP were randomly assigned to receive antihypertensive treatment or to discontinue all antihypertensive medications (control) during hospitalization. | Among patients with acute ischemic stroke, BP reduction with antihypertensive medications did not reduce the likelihood of death or major disability at 14 days, or hospital discharge compared with the absence of antihypertensive medication. |
| Bath et al., 2015 [71] | Prospective randomized trial (ENOS) | 4011 | To assess the safety and efficacy of glyceryl trinitrate within 48 h in patients with acute ischemic or hemorrhagic stroke and high BP. To assess outcomes for a subset of patients who continued or stopped taking antihypertensive drugs for 1 week after their stroke. | Patients hospitalized with an acute ischemic or hemorrhagic stroke and increased SBP (140–220 mm Hg) were randomly assigned to 7 days of transdermal glyceryl trinitrate (5 mg per day) within 48 h of stroke onset or to no treatment. | In patients with acute stroke and high BP, transdermal glyceryl trinitrate lowered B and had acceptable safety, although it did not improve functional outcome. There was no evidence to support continuing pre-stroke antihypertensive drugs in patients in the first few days after the stroke. |
| Jusufovic et al., 2015 [72] | Prospective subgroup analysis of SCAST | 993 | To assess whether the effects of BP lowering with the angiotensin receptor blocker candesartan in the acute phase of stroke were harmful in the subgroup of patients with carotid artery stenosis. | Patients with carotid artery stenosis presenting within 30 h from acute ischemic or hemorrhagic stroke and with SBP ≥140 mmHg were treated with candesartan or placebo for 7 days. | No clear evidence that candesartan was qualitatively different in patients with carotid stenosis, but patients with severe stenosis were at particularly high risk of stroke progression and poor functional outcome. |
| Wu et al., 2016 [73] | Prospective observational (TIMS-China Registry) | 1128 | To identify the association between BP and clinical outcomes in acute ischemic stroke patients treated with a thrombolytic medication (recombinant tissue plasminogen activator). | SBP and DBP at baseline, 2 h, and 24 h after treatment and changes from baseline were analyzed in patients hospitalized within 4.5 h from acute ischemic stroke for intravenous thrombolysis. | Lower BP within the first 24 h was associated with a more favorable outcome and less frequent spontaneous ICH in patients with acute ischemic stroke undergoing thrombolytic medication. |
| Allison et al., 2019 [74] | Retrospective | 210 | To determine whether clevidipine achieved faster BP control compared to nicardipine in patients with acute ischemic stroke or ICH. | Patients receiving clevidipine or continuous infusion of nicardipine for acute BP management. | No difference in the mean time from infusion initiation to SBP goal between the agents or secondary outcomes (door-to-needle time, length of stay, mortality) |
| Nasi et al., 2019 [75] | Prospective (MAPAS) | 218 | To determine the efficacy of the early manipulation of SBP in non-thrombolyzed patients. | Patients randomized within 12 h from acute ischemic stroke to maintain SBP during 24 h within 3 ranges (group 1: 140–160 mmHg; group 2: 161–180 mmHg; group 3: 181–200 mmHg) using vasoactive drugs and fluids. | The modified Rankin Scale at 90 days did not differ among the groups. None had neurological deterioration due to BP reduction in 24 h. ICH occurred more frequently in higher SBP (181–200 mmHg). More chance of good outcome in Group 2. |
| Study | Design | Patients n | Aim/Location | Main Findings |
|---|---|---|---|---|
| Anderson et al., 2008 [76] | Prospective randomized trial (INTERACT) | 404 | Lobar (9.0%), basal ganglia (82.5), brainstem (4.5%), cerebellum (4%), intraventricular extension (23.5%). | The relative risk of hematoma growth was lower with an intensive BP-lowering treatment. Clinical outcomes were not different with intensive blood-pressure-lowering treatment. |
| Koch et al., 2008 [77] | Prospective | 42 | Feasibility and safety of reducing BP to lower than presently recommended levels in acute ICH. | Aggressive lowering of BP did not affect hematoma, edema expansion, neurological deterioration, or outcome. |
| Suri et al., 2008 [78] | Retrospective | 122 | Drop in systolic BP and mean arterial pressures over 24 h were divided into quartiles to determine the risk of neurological deterioration among quartiles. | The reduction of BP in patients with acute ICH is safe. An aggressive decrease in BP might reduce the risk of neurological deterioration in the first 24 h of admission. |
| Anderson et al., 2010 [79] | Prospective randomized trial (INTERACT) | 404 | To determine the effects of intensive BP reduction on hematoma and perihematomal edema over 72 h. | The early intensive BP-lowering treatment attenuated hematoma growth over 72 h. There was no appreciable effect on perihematomal edema. |
| Qureshi et al., 2010 [80] | Prospective (ATACH) | 774 | Lobar hemorrhages. | Reduced mortality rate with systolic BP-lowering treatment. |
| Anderson et al., 2013 [81] | Prospective randomized trial (INTERACT-2) | 2839 | Deep location of hematoma (83.5%), intraventricular extension of hemorrhage (28.3%). | Death or severe disability was not reduced with intensive BP-lowering treatment. Lower modified Rankin scale with intensive BP-lowering treatment. |
| Butcher et al., 2013 [82] | Prospective randomized trial (ICH ADAPT) | 75 | Basal ganglia (74.5%), lobar (22.5%), brainstem (3.0%), intraventricular extension (38.5%). | Acute BP lowering did not worsen cerebral ischemia. |
| Sakamoto et al., 2013 [83] | Prospective, observational (SAMURAI) | 211 | Putamen (57%), thalamus (36%), lobar (7%). | Aggressive BP lowering may ameliorate clinical outcomes. |
| Rodriguez-Luna et al., 2013 [84] | Prospective | 117 | Supratentorial, intraventricular extension of hemorrhage (47%). | Systolic BP and variability predict hematoma growth and early neurological deterioration. |
| Krishnan et al., 2016 [85] | Prospective randomized trial (ENOS subanalysis) | 246 | To continue or stop antihypertensive treatment during the acute phase of ICH. | Among patients with acute ICH, immediate continuation of antihypertensive drugs during the first week did not reduce death or major disability in comparison to stopping treatment temporarily. |
| Qureshi et al., 2016 [86] | Prospective randomized trial (ATACH-2) | 1000 | Thalamus (37.8%), basal ganglia (51.2%), cerebral lobe (11.0%), cerebellum (0.1%) | A target BP values from 110 to 139 mmHg did not result in a lower rate of death or disability than a standard reduction. |
| Bozzano et al., 2017 [87] | Randomized, controlled, multicenter trial | 1000 | To determine the efficacy and safety of early and rapid BP lowering in patients with spontaneous ICH. | No clinical benefits from intensive and rapid lowering of BP. |
| Hatcher et al., 2017 [88] | Retrospective observational | 243 | Intraventricular extension of hemorrhage (64%), infratentorial bleeding (20%). | Elevated pre-hospital systolic BP (≥140 mmHg) was associated with larger hematoma volumes. |
| Rodriguez-Luna et al., 2018 [89] | Retrospective | 219 | Lobar hemorrhages (40.6%). Intraventricular extension (48.9%), subarachnoid extension (35.2%). | Pre-hospital BP was correlated with hematoma volume at admission. |
| Zhu et al., 2020 [90] | Single-center retrospective study | 166 | To compare early versus late initiation of oral antihypertensives on intensive care unit length of stay and cost of hospitalization in patients with ICH. | Early initiation of oral antihypertensives is safe and may have significant clinical and socio-economic impacts on patients with hypertensive ICH. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cantone, M.; Lanza, G.; Puglisi, V.; Vinciguerra, L.; Mandelli, J.; Fisicaro, F.; Pennisi, M.; Bella, R.; Ciurleo, R.; Bramanti, A. Hypertensive Crisis in Acute Cerebrovascular Diseases Presenting at the Emergency Department: A Narrative Review. Brain Sci. 2021, 11, 70. https://doi.org/10.3390/brainsci11010070
Cantone M, Lanza G, Puglisi V, Vinciguerra L, Mandelli J, Fisicaro F, Pennisi M, Bella R, Ciurleo R, Bramanti A. Hypertensive Crisis in Acute Cerebrovascular Diseases Presenting at the Emergency Department: A Narrative Review. Brain Sciences. 2021; 11(1):70. https://doi.org/10.3390/brainsci11010070
Chicago/Turabian StyleCantone, Mariagiovanna, Giuseppe Lanza, Valentina Puglisi, Luisa Vinciguerra, Jaime Mandelli, Francesco Fisicaro, Manuela Pennisi, Rita Bella, Rosella Ciurleo, and Alessia Bramanti. 2021. "Hypertensive Crisis in Acute Cerebrovascular Diseases Presenting at the Emergency Department: A Narrative Review" Brain Sciences 11, no. 1: 70. https://doi.org/10.3390/brainsci11010070
APA StyleCantone, M., Lanza, G., Puglisi, V., Vinciguerra, L., Mandelli, J., Fisicaro, F., Pennisi, M., Bella, R., Ciurleo, R., & Bramanti, A. (2021). Hypertensive Crisis in Acute Cerebrovascular Diseases Presenting at the Emergency Department: A Narrative Review. Brain Sciences, 11(1), 70. https://doi.org/10.3390/brainsci11010070







