KCNA2 Autoimmunity in Progressive Cognitive Impairment: Case Series and Literature Review
Abstract
1. Introduction
2. Case Reports
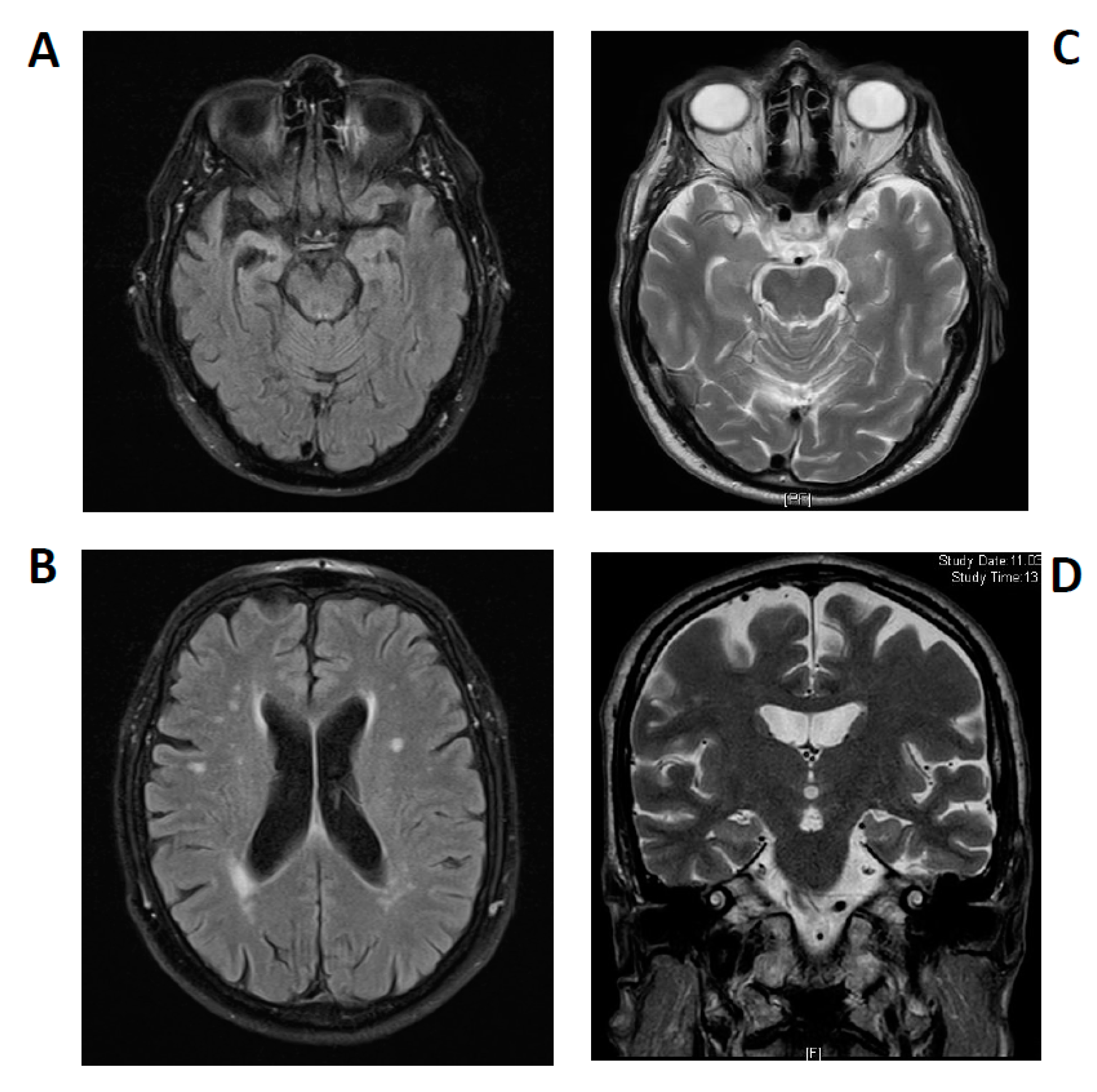
2.1. Case 1
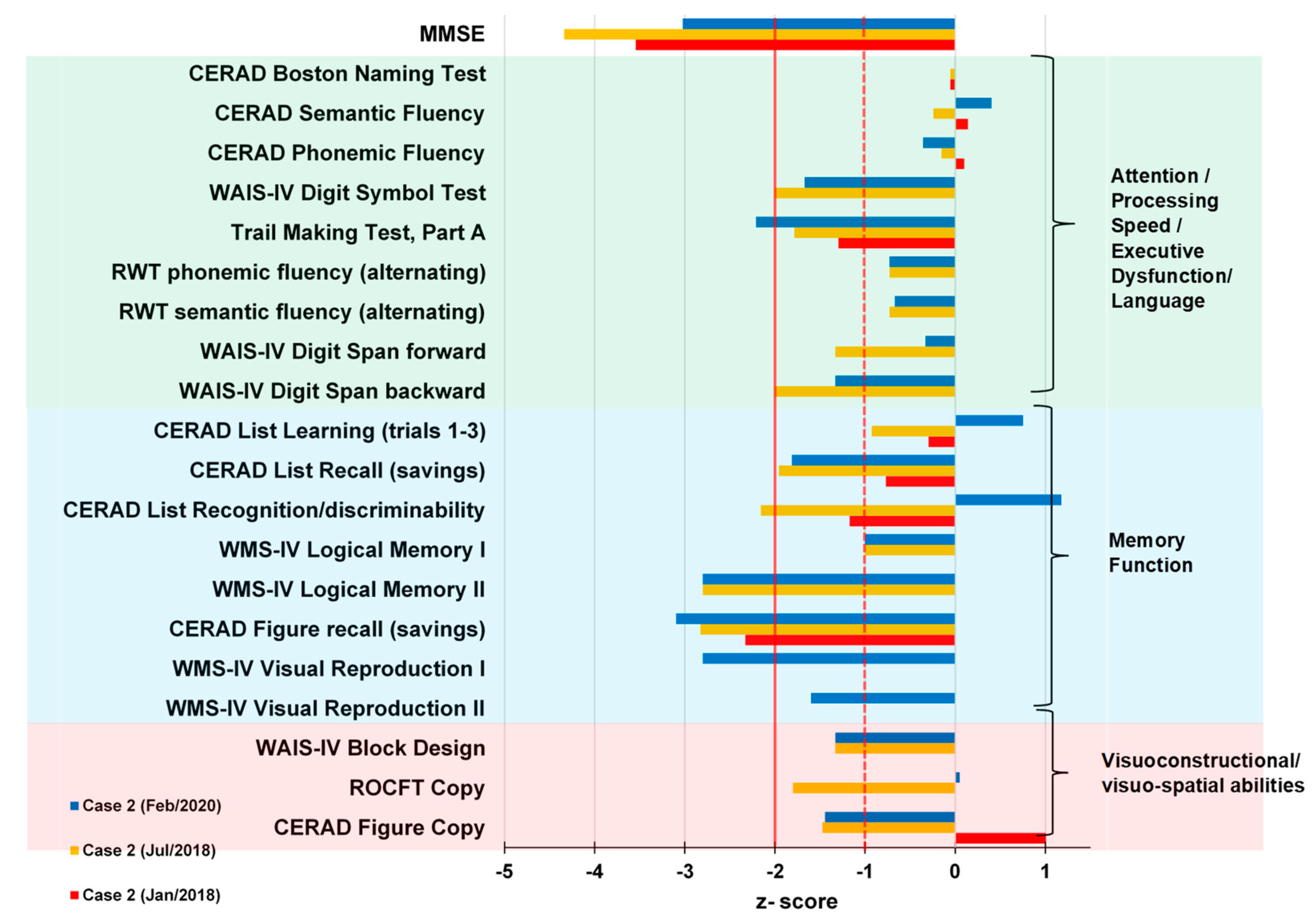
2.2. Case 2
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yellen, G. The voltage-gated potassium channels and their relatives. Nature 2002, 419, 35–42. [Google Scholar] [CrossRef]
- Jan, L.Y.; Jan, Y.N. Voltage-gated potassium channels and the diversity of electrical signalling. J. Physiol. 2012, 590, 2591–2599. [Google Scholar] [CrossRef]
- Melé, M.; Ferreira, P.G.; Reverter, F.; DeLuca, D.S.; Monlong, J.; Sammeth, M.; Young, T.R.; Goldmann, J.M.; Pervouchine, D.D.; Sullivan, T.J.; et al. Human genomics. The human transcriptome across tissues and individuals. Science 2015, 348, 660–665. [Google Scholar] [CrossRef] [PubMed]
- Sheng, M.; Tsaur, M.L.; Jan, Y.N.; Jan, L.Y. Contrasting subcellular localization of the Kv1.2 K+ channel subunit in different neurons of rat brain. J. Neurosci. 1994, 14, 2408–2417. [Google Scholar] [CrossRef] [PubMed]
- Masnada, S.; Hedrich, U.B.S.; Gardella, E.; Schubert, J.; Kaiwar, C.; Klee, E.W.; Lanpher, B.C.; Gavrilova, R.H.; Synofzik, M.; Bast, T.; et al. Clinical spectrum and genotype-phenotype associations of KCNA2-related encephalopathies. Brain 2017, 140, 2337–2354. [Google Scholar] [CrossRef] [PubMed]
- Manole, A.; Männikkö, R.; Hanna, M.G.; Kullmann, D.M.; Houlden, H. De novo KCNA2 mutations cause hereditary spastic paraplegia. Ann. Neurol. 2017, 81, 326–328. [Google Scholar] [CrossRef]
- Corbett, M.A.; Bellows, S.T.; Li, M.; Carroll, R.; Micallef, S.; Carvill, G.L.; Myers, C.T.; Howell, K.B.; Maljevic, S.; Lerche, H.; et al. Dominant KCNA2 mutation causes episodic ataxia and pharmacoresponsive epilepsy. Neurology 2016, 87, 1975–1984. [Google Scholar] [CrossRef]
- Shillito, P.; Molenaar, P.C.; Vincent, A.; Leys, K.; Zheng, W.; van den Berg, R.J.; Plomp, J.J.; van Kempen, G.T.; Chauplannaz, G.; Wintzen, A.R. Acquired neuromyotonia: Evidence for autoantibodies directed against K+ channels of peripheral nerves. Ann. Neurol. 1995, 38, 714–722. [Google Scholar] [CrossRef]
- Barber, P.A.; Anderson, N.E.; Vincent, A. Morvan’s syndrome associated with voltage-gated K+ channel antibodies. Neurology 2000, 54, 771–772. [Google Scholar] [CrossRef]
- Buckley, C.; Oger, J.; Clover, L.; Tüzün, E.; Carpenter, K.; Jackson, M.; Vincent, A. Potassium channel antibodies in two patients with reversible limbic encephalitis. Ann. Neurol. 2001, 50, 73–78. [Google Scholar] [CrossRef]
- Vincent, A.; Buckley, C.; Schott, J.M.; Baker, I.; Dewar, B.-K.; Detert, N.; Clover, L.; Parkinson, A.; Bien, C.G.; Omer, S.; et al. Potassium channel antibody-associated encephalopathy: A potentially immunotherapy-responsive form of limbic encephalitis. Brain 2004, 127, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Irani, S.R.; Alexander, S.; Waters, P.; Kleopa, K.A.; Pettingill, P.; Zuliani, L.; Peles, E.; Buckley, C.; Lang, B.; Vincent, A. Antibodies to Kv1 potassium channel-complex proteins leucine-rich, glioma inactivated 1 protein and contactin-associated protein-2 in limbic encephalitis, Morvan’s syndrome and acquired neuromyotonia. Brain 2010, 133, 2734–2748. [Google Scholar] [CrossRef]
- Lai, M.; Huijbers, M.G.M.; Lancaster, E.; Graus, F.; Bataller, L.; Balice-Gordon, R.; Cowell, J.K.; Dalmau, J. Investigation of LGI1 as the antigen in limbic encephalitis previously attributed to potassium channels: A case series. Lancet Neurol. 2010, 9, 776–785. [Google Scholar] [CrossRef]
- Lang, B.; Makuch, M.; Moloney, T.; Dettmann, I.; Mindorf, S.; Probst, C.; Stoecker, W.; Buckley, C.; Newton, C.R.; Leite, M.I.; et al. Intracellular and non-neuronal targets of voltage-gated potassium channel complex antibodies. J. Neurol. Neurosurg. Psychiatry 2017, 88, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Molloy, A.; Cassidy, E.; Ryan, A.; O’Toole, O. VGKC positive autoimmune encephalopathy mimicking dementia. BMJ Case Rep. 2011, 2011. [Google Scholar] [CrossRef] [PubMed]
- Reintjes, W.; Romijn, M.D.M.; Hollander, D.; Ter Bruggen, J.P.; van Marum, R.J. Reversible dementia: Two nursing home patients with voltage-gated potassium channel antibody-associated limbic encephalitis. J. Am. Med. Dir. Assoc. 2015, 16, 790–794. [Google Scholar] [CrossRef] [PubMed]
- Gibson, L.L.; McKeever, A.; Cullen, A.E.; Nicholson, T.R.; Aarsland, D.; Zandi, M.S.; Pollak, T.A. Neuronal surface autoantibodies in dementia: A systematic review and meta-analysis. J. Neurol. 2020. [Google Scholar] [CrossRef]
- Sechi, E.; Flanagan, E.P. Diagnosis and Management of Autoimmune Dementia. Curr. Treat. Options Neurol. 2019, 21, 11. [Google Scholar] [CrossRef]
- Wagner, J.; Witt, J.-A.; Helmstaedter, C.; Malter, M.P.; Weber, B.; Elger, C.E. Automated volumetry of the mesiotemporal structures in antibody-associated limbic encephalitis. J. Neurol. Neurosurg. Psychiatry 2015, 86, 735–742. [Google Scholar] [CrossRef]
- Heine, J.; Prüss, H.; Bartsch, T.; Ploner, C.J.; Paul, F.; Finke, C. Imaging of autoimmune encephalitis--Relevance for clinical practice and hippocampal function. Neuroscience 2015, 309, 68–83. [Google Scholar] [CrossRef]
- Graus, F.; Titulaer, M.J.; Balu, R.; Benseler, S.; Bien, C.G.; Cellucci, T.; Cortese, I.; Dale, R.C.; Gelfand, J.M.; Geschwind, M.; et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016, 15, 391–404. [Google Scholar] [CrossRef]
- Hansen, N.; Lipp, M.; Vogelgsang, J.; Vukovich, R.; Zindler, T.; Luedecke, D.; Gingele, S.; Malchow, B.; Frieling, H.; Kühn, S.; et al. Autoantibody-associated psychiatric symptoms and syndromes in adults: A narrative review and proposed diagnostic approach. Brainbehaviorimmun. Health 2020, 9, 100154. [Google Scholar] [CrossRef]
- Rössling, R.; Prüss, H. SOP: Antibody-associated autoimmune encephalitis. Neurol. Res. Pract. 2020, 2, 1. [Google Scholar] [CrossRef] [PubMed]
- Endres, D.; Leypoldt, F.; Bechter, K.; Hasan, A.; Steiner, J.; Domschke, K.; Wandinger, K.-P.; Falkai, P.; Arolt, V.; Stich, O.; et al. Autoimmune encephalitis as a differential diagnosis of schizophreniform psychosis: Clinical symptomatology, pathophysiology, diagnostic approach, and therapeutic considerations. Eur. Arch. Psychiatry Clin. Neurosci. 2020, 270, 803–818. [Google Scholar] [CrossRef]
- Flanagan, E.P.; McKeon, A.; Lennon, V.A.; Boeve, B.F.; Trenerry, M.R.; Tan, K.M.; Drubach, D.A.; Josephs, K.A.; Britton, J.W.; Mandrekar, J.N.; et al. Autoimmune dementia: Clinical course and predictors of immunotherapy response. Mayo Clin. Proc. 2010, 85, 881–897. [Google Scholar] [CrossRef]
- Constantinescu, R.; Krýsl, D.; Bergquist, F.; Andrén, K.; Malmeström, C.; Asztély, F.; Axelsson, M.; Menachem, E.B.; Blennow, K.; Rosengren, L.; et al. Cerebrospinal fluid markers of neuronal and glial cell damage to monitor disease activity and predict long-term outcome in patients with autoimmune encephalitis. Eur. J. Neurol. 2016, 23, 796–806. [Google Scholar] [CrossRef]
- Körtvelyessy, P.; Prüss, H.; Thurner, L.; Maetzler, W.; Vittore-Welliong, D.; Schultze-Amberger, J.; Heinze, H.-J.; Reinhold, D.; Leypoldt, F.; Schreiber, S.; et al. Biomarkers of Neurodegeneration in Autoimmune-Mediated Encephalitis. Front. Neurol. 2018, 9, 668. [Google Scholar] [CrossRef]
- Rössling, R.; Prüss, H. Apheresis in Autoimmune Encephalitis and Autoimmune Dementia. J. Clin. Med. 2020, 9, 2683. [Google Scholar] [CrossRef]
- Stracke, S.; Lange, S.; Bornmann, S.; Kock, H.; Schulze, L.; Klinger-König, J.; Böhm, S.; Vogelgesang, A.; von Podewils, F.; Föel, A.; et al. Immunoadsorption for Treatment of Patients with Suspected Alzheimer Dementia and Agonistic Autoantibodies against Alpha1a-Adrenoceptor-Rationale and Design of the IMAD Pilot Study. J. Clin. Med. 2020, 9, 1919. [Google Scholar] [CrossRef]
- van Sonderen, A.; Schreurs, M.W.J.; de Bruijn, M.A.A.M.; Boukhrissi, S.; Nagtzaam, M.M.P.; Hulsenboom, E.S.P.; Enting, R.H.; Thijs, R.D.; Wirtz, P.W.; Sillevis Smitt, P.A.E.; et al. The relevance of VGKC positivity in the absence of LGI1 and Caspr2 antibodies. Neurology 2016, 86, 1692–1699. [Google Scholar] [CrossRef]
- Scharf, M.; Miske, R.; Kade, S.; Hahn, S.; Denno, Y.; Begemann, N.; Rochow, N.; Radzimski, C.; Brakopp, S.; Probst, C.; et al. A Spectrum of Neural Autoantigens, Newly Identified by Histo-Immunoprecipitation, Mass Spectrometry, and Recombinant Cell-Based Indirect Immunofluorescence. Front. Immunol. 2018, 9, 1447. [Google Scholar] [CrossRef] [PubMed]
- Malter, M.P.; Frisch, C.; Schoene-Bake, J.C.; Helmstaedter, C.; Wandinger, K.P.; Stoecker, W.; Urbach, H.; Surges, R.; Elger, C.E.; Vincent, A.V.; et al. Outcome of limbic encephalitis with VGKC-complex antibodies: Relation to antigenic specificity. J. Neurol. 2014, 261, 1695–1705. [Google Scholar] [CrossRef] [PubMed]
- Butler, C.R.; Miller, T.D.; Kaur, M.S.; Baker, I.W.; Boothroyd, G.D.; Illman, N.A.; Rosenthal, C.R.; Vincent, A.; Buckley, C.J. Persistent anterograde amnesia following limbic encephalitis associated with antibodies to the voltage-gated potassium channel complex. J. Neurol. Neurosurg. Psychiatry 2014, 85, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Frisch, C.; Malter, M.P.; Elger, C.E.; Helmstaedter, C. Neuropsychological course of voltage-gated potassium channel and glutamic acid decarboxylase antibody related limbic encephalitis. Eur. J. Neurol. 2013, 20, 1297–1304. [Google Scholar] [CrossRef]
- Loane, C.; Argyropoulos, G.P.D.; Roca-Fernández, A.; Lage, C.; Sheerin, F.; Ahmed, S.; Zamboni, G.; Mackay, C.; Irani, S.R.; Butler, C.R. Hippocampal network abnormalities explain amnesia after VGKCC-Ab related autoimmune limbic encephalitis. J. Neurol. Neurosurg. Psychiatry 2019, 90, 965–974. [Google Scholar] [CrossRef]
- Kirschstein, T.; Sadkiewicz, E.; Hund-Göschel, G.; Becker, J.; Guli, X.; Müller, S.; Rohde, M.; Hübner, D.-C.; Brehme, H.; Kolbaske, S.; et al. Stereotactically Injected Kv1.2 and CASPR2 Antisera Cause Differential Effects on CA1 Synaptic and Cellular Excitability, but Both Enhance the Vulnerability to Pro-epileptic Conditions. Front. Synaptic Neurosci. 2020, 12, 13. [Google Scholar] [CrossRef]
- Johnston, D.; Hoffman, D.A.; Magee, J.C.; Poolos, N.P.; Watanabe, S.; Colbert, C.M.; Migliore, M. Dendritic potassium channels in hippocampal pyramidal neurons. J. Physiol. 2000, 525, 75–81. [Google Scholar] [CrossRef]
- Shah, N.H.; Aizenman, E. Voltage-gated potassium channels at the crossroads of neuronal function, ischemic tolerance, and neurodegeneration. Transl. Stroke Res. 2014, 5, 38–58. [Google Scholar] [CrossRef]
- Hansen, N.; Widman, G.; Hattingen, E.; Elger, C.E.; Kunz, W.S. Mesial temporal lobe epilepsy associated with KCNT1 mutation. Seizure 2017, 45, 181–183. [Google Scholar] [CrossRef]
- Rolls, E.T. Limbic systems for emotion and for memory, but no single limbic system. Cortex A J. Devoted Study Nerv. Syst. Behav. 2015, 62, 119–157. [Google Scholar] [CrossRef]
- Titulaer, M.J.; McCracken, L.; Gabilondo, I.; Armangué, T.; Glaser, C.; Iizuka, T.; Honig, L.S.; Benseler, S.M.; Kawachi, I.; Martinez-Hernandez, E.; et al. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: An observational cohort study. Lancet Neurol. 2013, 12, 157–165. [Google Scholar] [CrossRef]



| Patient Number | Patient 1 | Patient 2 |
|---|---|---|
| CSF Analysis | 1/2020 | 7/2020 |
| Cell count /µL (pathological: >5 µL) | 2 | 0 |
| Lymphocytes in % | 61 | 70 |
| Monocytes in % | 13 | 29 |
| Plasma cells in % | - | - |
| Albumin mg/L | 269 | 358 |
| IgG mg/L | 41.6 | 25.1 |
| IgA mg/L | 6.8 | 9.8 |
| IgM mg/L | 0.92 | 0.34 |
| QAlb % | 7.1 | 8.3 |
| QIgG % | 3.5 | 3.7 |
| QIgA % | 1.8 | 1.9 |
| QIgM % | 0.92 | 0.62 |
| Lactat mmol/L | 1.8 | 1.6 |
| Oligoclonal IgG (CSF/Serum) | -/- | -/- |
| NSE ng/mL (pathological: >30 ng/mL) | n.a. | 35 |
| S100 µg/L (pathological: >2.7 µg/L) | n.a. | 4.1 |
| T-tau pg/mL (pathological: >450 pg/mL) | 370 | 505 |
| P-tau181 pg/mL (pathological: >61 pg/mL) | 45 | 118 |
| Aß1-42 pg/mL (pathological: ˂450 pg/mL) | 1139 | 716 |
| Aß1-40 pg/ml | 9013 | 18,662 |
| Aß ratio (pathological: ˂0.5) | 1.3 | 0.38 |
| Specific antibody-indices (CSF) | ||
| Borreliosis-antibody-index-IgM (pathological: >1.5) | - | n.a. |
| Borreliosis-antibody-index-IgG (pathological: >1.5) | - | n.a. |
| Measles-rubella-varicella-herpes simplex-antibody-index-IgG (pathological: >1.5) | 0.9 | n.a. |
| Measles-rubella-varicella-herpes simplex-antibody-index-IgM (pathological: >1.5) | 0.9 | n.a. |
| Anitbodies against neuronal cell-surface and paraneoplastic antigens (serum/CSF) | 1/2020 | 2/2020 + 7/2020 |
| Anti-KCNA2-antibodies (pathological: >1:10) | 1:100/- | 1:32/- |
| Serum | ||
| Homocysteine (pathological: >16.2 µmol/L) | 10.9 | 16.5 |
| Cerulopasmine (pathological: >60 mg/dL) | 29.8 | n.a. |
| Cupper (pathological: <11 µmol/L) | 18.7 | n.a. |
| Vitamin B12 (pathological: <187 ng/L) | 547 | 365 |
| Holotranscobalamine (pathological: <50) | 194.5 | n.a. |
| Vitamin B1 (pathological: <28 µg/L) | 72.8 | n.a. |
| Vitamin B6 (pathological: <4 µg/L) | 7.4 | n.a. |
| Folic acid (pathological: <3.1 µg/L) | 12.9 | 10.5 |
| CRP (pathological: >5 mg/L) | 2.5 | 5.2 |
| Endocrinology Laboratory | ||
| Parathyroid hormone (reference range: 18.1–88.5 ng/L) | 54.9 | n.a. |
| Cortisol (reference range: 37–194) | 99.4 | n.a. |
| TSH (reference range: 0.35–4.94 mlU/L) | 2.72 | 0.95 |
| T3 (reference range: 1.71–3.71 ng/L) | 3.34 | 3.27 |
| T4 (reference range: 7.0–14.8 ng/L) | 10.7 | 10.0 |
| Thyroid peroxidase antibodies (pathological: >6 IU/mL) | <3 | n.a. |
| Thyroglobulin antibodies (pathological: >14 IU/mL) | <4 | n.a. |
| Thyroid-stimulating hormone receptor (pathological: >1.75 IU/L) | <0.80 | n.a. |
| Serological Diagnostics | ||
| Borreliosis-IgM-EIA (pathological: >9) | 4.8 | 1.5 |
| Borreliosis-IgG-EIA (pathological: >9) | 37 | 15.10 |
| Borreliosis-IgM-Immunoblot | - | - |
| Borreliosis-IgG-Immunoblot | + | - |
| Lues TPPA (pathological: >1:80) | - | - |
| Lues TPPA (pathological: >1:1) | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Timäus, C.; von Gottberg, P.; Hirschel, S.; Lange, C.; Wiltfang, J.; Hansen, N. KCNA2 Autoimmunity in Progressive Cognitive Impairment: Case Series and Literature Review. Brain Sci. 2021, 11, 89. https://doi.org/10.3390/brainsci11010089
Timäus C, von Gottberg P, Hirschel S, Lange C, Wiltfang J, Hansen N. KCNA2 Autoimmunity in Progressive Cognitive Impairment: Case Series and Literature Review. Brain Sciences. 2021; 11(1):89. https://doi.org/10.3390/brainsci11010089
Chicago/Turabian StyleTimäus, Charles, Philipp von Gottberg, Sina Hirschel, Claudia Lange, Jens Wiltfang, and Niels Hansen. 2021. "KCNA2 Autoimmunity in Progressive Cognitive Impairment: Case Series and Literature Review" Brain Sciences 11, no. 1: 89. https://doi.org/10.3390/brainsci11010089
APA StyleTimäus, C., von Gottberg, P., Hirschel, S., Lange, C., Wiltfang, J., & Hansen, N. (2021). KCNA2 Autoimmunity in Progressive Cognitive Impairment: Case Series and Literature Review. Brain Sciences, 11(1), 89. https://doi.org/10.3390/brainsci11010089






