Biological Bases of Empathy and Social Cognition in Patients with Attention-Deficit/Hyperactivity Disorder: A Focus on Treatment with Psychostimulants
Abstract
1. Introduction
1.1. Empathy and Related Constructs
1.2. Social Cognition in ADHD
1.3. The Systematic Review
2. Materials and Methods
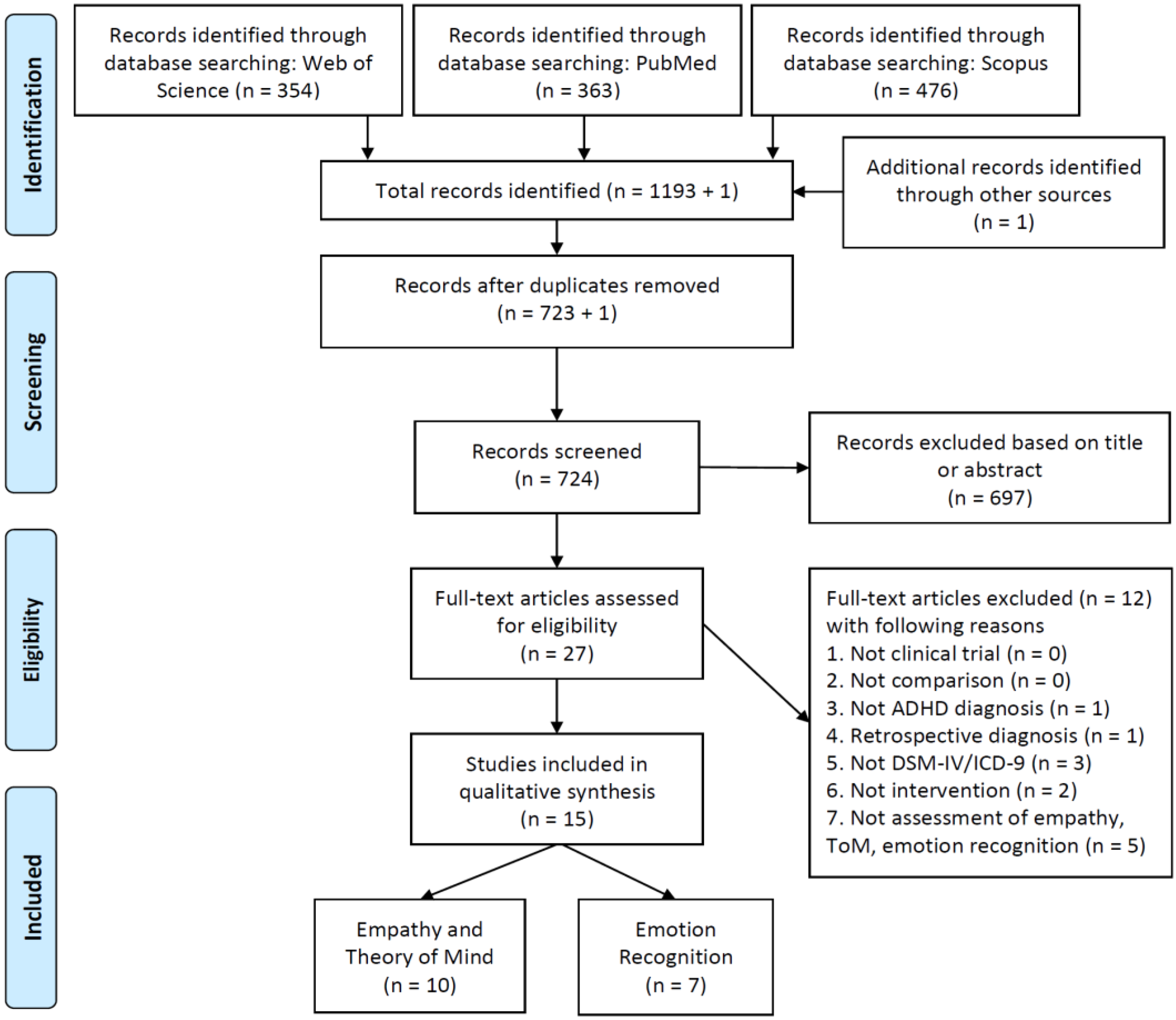
2.1. Search Strategy
2.2. Screening Procedure
- (1)
- Study design: any type of clinical trial;
- (2)
- Comparison: either case versus control, drug versus placebo or pre-to peri-/post-treatment;
- (3)
- Participants: patients non-retrospectively diagnosed with ADHD according to the international classification systems DSM-IV, ICD-9, or later versions; no restriction for participants’ age, gender, or IQ;
- (4)
- Intervention: either one-day, single-dose administration or prolonged daily administration of psychostimulants (e.g., Methylphenidate) or nonstimulant drugs (e.g., Atomoxetine);
- (5)
- Measures: any type of measurement (i.e., tasks, rating scales, and parent- or self-rated questionnaires) assessing empathy, theory of mind, and emotion recognition.
2.3. Data Collection
3. Results
3.1. Empathy and Theory of Mind
3.2. Emotion Recognition
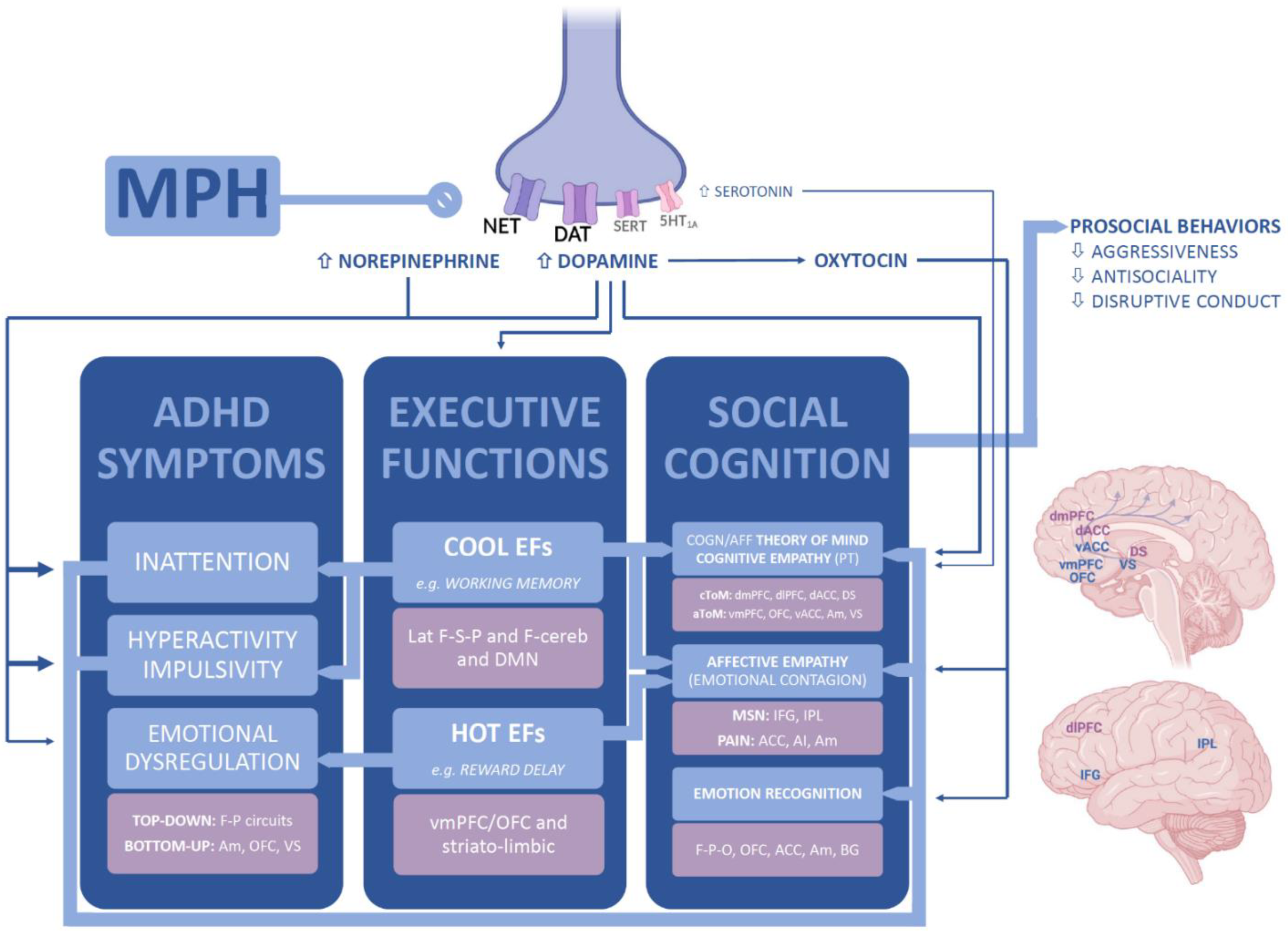
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thomas, R.; Sanders, S.; Doust, J.; Beller, E.; Glasziou, P. Prevalence of attention-deficit/hyperactivity disorder: A systematic review and meta-analysis. Pediatrics 2015, 135, e994–e1001. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, DSM-5, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013; ISBN 0-89042-555-8. [Google Scholar]
- Uekermann, J.; Kraemer, M.; Abdel-Hamid, M.; Schimmelmann, B.G.; Hebebrand, J.; Daum, I.; Wiltfang, J.; Kis, B. Social cognition in attention-deficit hyperactivity disorder (ADHD). Neurosci. Biobehav. Rev. 2010, 34, 734–743. [Google Scholar] [CrossRef] [PubMed]
- Andrade, B.F.; Waschbusch, D.A.; Doucet, A.; King, S.; MacKinnon, M.; McGrath, P.J.; Stewart, S.H.; Corkum, P. Social Information Processing of Positive and Negative Hypothetical Events in Children with ADHD and Conduct Problems and Controls. J. Atten. Disord. 2012, 16, 491–504. [Google Scholar] [CrossRef] [PubMed]
- Sinzig, J.; Morsch, D.; Lehmkuhl, G. Do hyperactivity, impulsivity and inattention have an impact on the ability of facial affect recognition in children with autism and ADHD? Eur. Child Adolesc. Psychiatry 2008, 17, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Nijmeijer, J.S.; Minderaa, R.B.; Buitelaar, J.K.; Mulligan, A.; Hartman, C.A.; Hoekstra, P.J. Attention-deficit/hyperactivity disorder and social dysfunctioning. Clin. Psychol. Rev. 2008, 28, 692–708. [Google Scholar] [CrossRef] [PubMed]
- Cristofani, C.; Sesso, G.; Cristofani, P.; Fantozzi, P.; Inguaggiato, E.; Muratori, P.; Narzisi, A.; Pfanner, C.; Pisano, S.; Polidori, L.; et al. The role of executive functions in the development of empathy and its association with externalizing behaviors in children with neurodevelopmental disorders and other psychiatric comorbidities. Brain Sci. 2020, 10, 489. [Google Scholar] [CrossRef] [PubMed]
- Crick, N.R.; Dodge, K.A. Social Information-Processing Mechanisms in Reactive and Proactive Aggression. Child Dev. 1996, 67, 993–1002. [Google Scholar] [CrossRef]
- Blair, R.J.R. Fine Cuts of Empathy and the Amygdala: Dissociable Deficits in Psychopathy and Autism. Q. J. Exp. Psychol. 2008, 61, 157–170. [Google Scholar] [CrossRef] [PubMed]
- de Waal, F.B.M. Putting the Altruism Back into Altruism: The Evolution of Empathy. Annu. Rev. Psychol. 2008, 59, 279–300. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.H. A multidimensional approach to individual difference in empathy. JSAS Cat. Sel. Doc. Psychol. 1980, 10, 85–94. [Google Scholar]
- Decety, J.; Moriguchi, Y. The empathic brain and its dysfunction in psychiatric populations: Implications for intervention across different clinical conditions. Biopsychosoc. Med. 2007, 1, 22. [Google Scholar] [CrossRef] [PubMed]
- Decety, J.; Jackson, P.L. The Functional Architecture of Human Empathy. Behav. Cogn. Neurosci. Rev. 2004, 3, 71–100. [Google Scholar] [CrossRef]
- Shamay-Tsoory The neural bases for empathy. Neuroscientist 2011, 17, 18–24. [CrossRef]
- Shamay-Tsoory, S.; Harari, H.; Szepsenwol, O.; Levkovitz, Y. Neuropsychological evidence of impaired cognitive empathy in euthymic bipolar disorder. J. Neuropsychiatry Clin. Neurosci. 2009, 21, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Singer, T.; Seymour, B.; O’Doherty, J.P.; Stephan, K.E.; Dolan, R.J.; Frith, C.D. Empathic neural responses are modulated by the perceived fairness of others. Nature 2006, 439, 466–469. [Google Scholar] [CrossRef]
- Shamay-Tsoory, S.; Tomer, R.; Goldsher, D.; Berger, B.D.; Aharon-Peretz, J. Impairment in cognitive and affective empathy in patients with brain lesions: Anatomical and cognitive correlates. J. Clin. Exp. Neuropsychol. 2004, 26, 1113–1127. [Google Scholar] [CrossRef] [PubMed]
- Hurlemann, R.; Patin, A.; Onur, O.A.; Cohen, M.X.; Baumgartner, T.; Metzler, S.; Dziobek, I.; Gallinat, J.; Wagner, M.; Maier, W.; et al. Oxytocin enhances amygdala-dependent, socially reinforced learning and emotional empathy in humans. J. Neurosci. 2010, 30, 4999–5007. [Google Scholar] [CrossRef]
- Lackner, C.L.; Bowman, L.C.; Sabbagh, M.A. Dopaminergic functioning and preschoolers’ theory of mind. Neuropsychologia 2010, 48, 1767–1774. [Google Scholar] [CrossRef] [PubMed]
- Preston, S.D.; de Waal, F.B.M. Empathy: Its ultimate and proximate bases. Behav. Brain Sci. 2002, 25, 1–20; discussion 20–71. [Google Scholar]
- Decety, J.; Skelly, L.R.; Kiehl, K.A. Brain response to empathy-eliciting scenarios involving pain in incarcerated individuals with psychopathy. JAMA Psychiatry 2013, 70, 638–645. [Google Scholar] [CrossRef]
- Frith, U. Autism and “Theory of Mind.” In Diagnosis and Treatment of Autism; Springer US: Boston, MA, USA, 1989; pp. 33–52. [Google Scholar]
- Shamay-Tsoory, S.; Aharon-Peretz, J.; Perry, D. Two systems for empathy: A double dissociation between emotional and cognitive empathy in inferior frontal gyrus versus ventromedial prefrontal lesions. Brain 2009, 132, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Abu-Akel, A.; Shamay-Tsoory, S. Neuroanatomical and neurochemical bases of theory of mind. Neuropsychologia 2011, 49, 2971–2984. [Google Scholar]
- Frith, U.; Frith, C.D. Development and neurophysiology of mentalizing. Philos. Trans. R. Soc. B Biol. Sci. 2003, 358, 459–473. [Google Scholar] [CrossRef]
- Siegal, M.; Varley, R. Neural systems involved in “theory of mind”. Nat. Rev. Neurosci. 2002, 3, 463–471. [Google Scholar] [CrossRef]
- Blair, R.J.R. Neurocognitive models of aggression, the antisocial personality disorders, and psychopathy. J. Neurol. Neurosurg. Psychiatry 2001, 71, 727–731. [Google Scholar] [CrossRef]
- Hermens, D.F.; Rowe, D.L.; Gordon, E.; Williams, L.M. Integrative neuroscience approach to predict ADHD stimulant response. Expert Rev. Neurother. 2006, 6, 753–763. [Google Scholar]
- Nelson, A.L.; Combs, D.R.; Penn, D.L.; Basso, M.R. Subtypes of social perception deficits in schizophrenia. Schizophr. Res. 2007, 94, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Pera-Guardiola, V.; Contreras-Rodriguez, O.; Batalla, I.; Kosson, D.; Menchon, J.M.; Pifarre, J.; Bosque, J.; Cardoner, N.; Soriano-Mas, C. Brain Structural Correlates of Emotion Recognition in Psychopaths. PLoS ONE 2016, 11, e0149807. [Google Scholar] [CrossRef]
- Beyer Von Morgenstern, S.; Becker, I.; Sinzig, J. Improvement of facial affect recognition in children and adolescents with attention-deficit/hyperactivity disorder under methylphenidate. Acta Neuropsychiatry 2014, 26, 202–208. [Google Scholar] [CrossRef][Green Version]
- Muratori, P.; Lochman, J.E.; Lai, E.; Milone, A.; Nocentini, A.; Pisano, S.; Righini, E.; Masi, G. Which dimension of parenting predicts the change of callous unemotional traits in children with disruptive behavior disorder? Compr. Psychiatry 2016, 69, 202–210. [Google Scholar] [CrossRef]
- Gonzalez-Liencres, C.; Shamay-Tsoory, S.G.; Brüne, M. Towards a neuroscience of empathy: Ontogeny, phylogeny, brain mechanisms, context and psychopathology. Neurosci. Biobehav. Rev. 2013, 37, 1537–1548. [Google Scholar] [CrossRef]
- Blair, R.J.R. Responding to the emotions of others: Dissociating forms of empathy through the study of typical and psychiatric populations. Conscious. Cogn. 2005, 14, 698–718. [Google Scholar] [CrossRef]
- Gillberg, C.L. The Emanuel Miller Memorial Lecture 1991: Autism and Autistic-like Conditions: Subclasses among Disorders of Empathy. J. Child Psychol. Psychiatry 1992, 33, 813–842. [Google Scholar] [CrossRef] [PubMed]
- Preti, A.; Vellante, M.; Baron-Cohen, S.; Zucca, G.; Petretto, D.R.; Masala, C. The Empathy Quotient: A cross-cultural comparison of the Italian version. Cogn. Neuropsychiatry 2011, 16, 50–70. [Google Scholar] [CrossRef] [PubMed]
- Vellante, M.; Baron-Cohen, S.; Melis, M.; Marrone, M.; Petretto, D.R.; Masala, C.; Preti, A. The “reading the Mind in the Eyes” test: Systematic review of psychometric properties and a validation study in Italy. Cogn. Neuropsychiatry 2013, 18, 326–354. [Google Scholar] [CrossRef] [PubMed]
- Jolliffe, D.; Farrington, D.P. Development and validation of the Basic Empathy Scale. J. Adolesc. 2006, 29, 589–611. [Google Scholar] [CrossRef] [PubMed]
- Milone, A.; Cerniglia, L.; Cristofani, C.; Inguaggiato, E.; Levantini, V.; Masi, G.; Paciello, M.; Simone, F.; Muratori, P. Empathy in youths with conduct disorder and callous-unemotional traits. Neural Plast. 2019, 2019, 9638973. [Google Scholar] [CrossRef] [PubMed]
- Abikoff, H.; Hechtman, L.; Klein, R.G.; Gallagher, R.; Fleiss, K.; Etcovitch, J.; Cousins, L.; Greenfield, B.; Martin, D.; Pollack, S. Social functioning in children with ADHD treated with long-term methylphenidate and multimodal psychosocial treatment. J. Am. Acad. Child Adolesc. Psychiatry 2004, 43, 820–829. [Google Scholar] [CrossRef] [PubMed]
- Braaten, E.B.; Rosén, L.A. Self-regulation of affect in attention deficit-hyperactivity disorder (ADHD) and non-ADHD boys: Differences in empathic responding. J. Consult. Clin. Psychol. 2000, 68, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Cordier, R.; Bundy, A.; Hocking, C.; Einfeld, S. Empathy in the play of children with attention deficit hyperactivity disorder. OTJR Occup. Particip. Heal. 2010, 30, 122–132. [Google Scholar] [CrossRef]
- Bora, E.; Pantelis, C. Meta-analysis of social cognition in attention-deficit/hyperactivity disorder (ADHD): Comparison with healthy controls and autistic spectrum disorder. Psychol. Med. 2016, 46, 699–716. [Google Scholar]
- Parke, E.M.; Becker, M.L.; Graves, S.J.; Baily, A.R.; Paul, M.G.; Freeman, A.J.; Allen, D.N. Social Cognition in Children with ADHD. J. Atten. Disord. 2018, 25, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Marton, I.; Wiener, J.; Rogers, M.; Moore, C.; Tannock, R. Empathy and social perspective taking in children with attention-deficit/ hyperactivity disorder. J. Abnorm. Child Psychol. 2009, 37, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Maoz, H.; Gvirts, H.Z.; Sheffer, M.; Bloch, Y. Theory of Mind and Empathy in Children with ADHD. J. Atten. Disord. 2019, 23, 1331–1338. [Google Scholar] [CrossRef]
- Maoz, H.; Tsviban, L.; Gvirts, H.Z.; Shamay-Tsoory, S.G.; Levkovitz, Y.; Watemberg, N.; Bloch, Y. Stimulants improve theory of mind in children with attention deficit/hyperactivity disorder. J. Psychopharmacol. 2014, 28, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Staff, A.I.; Luman, M.; van der Oord, S.; Bergwerff, C.E.; van den Hoofdakker, B.J.; Oosterlaan, J. Facial emotion recognition impairment predicts social and emotional problems in children with (subthreshold) ADHD. Eur. Child Adolesc. Psychiatry 2021. [Google Scholar] [CrossRef]
- Pelc, K.; Kornreich, C.; Foisy, M.L.; Dan, B. Recognition of Emotional Facial Expressions in Attention-Deficit Hyperactivity Disorder. Pediatr. Neurol. 2006, 35, 93–97. [Google Scholar] [CrossRef]
- Williams, L.M.; Hermens, D.F.; Palmer, D.; Kohn, M.; Clarke, S.; Keage, H.; Clark, C.R.; Gordon, E. Misinterpreting Emotional Expressions in Attention-Deficit/Hyperactivity Disorder: Evidence for a Neural Marker and Stimulant Effects. Biol. Psychiatry 2008, 63, 917–926. [Google Scholar] [CrossRef]
- Barkley, R.A. The relevance of the Still lectures to attention-deficit/hyperactivity disorder: A commentary. J. Atten. Disord. 2006, 10, 137–140. [Google Scholar] [CrossRef]
- Yan, Z.; Hong, S.; Liu, F.; Su, Y. A meta-analysis of the relationship between empathy and executive function. PsyCh J. 2020, 9, 34–43. [Google Scholar] [CrossRef]
- Abdel-Hamid, M.; Niklewski, F.; Heßmann, P.; Guberina, N.; Kownatka, M.; Kraemer, M.; Scherbaum, N.; Dziobek, I.; Bartels, C.; Wiltfang, J.; et al. Impaired empathy but no theory of mind deficits in adult attention deficit hyperactivity disorder. Brain Behav. 2019, 9, e01401. [Google Scholar] [CrossRef] [PubMed]
- Pineda-Alhucema, W.; Aristizabal, E.; Escudero-Cabarcas, J.; Acosta-López, J.E.; Vélez, J.I. Executive Function and Theory of Mind in Children with ADHD: A Systematic Review. Neuropsychol. Rev. 2018, 28, 341–358. [Google Scholar] [CrossRef] [PubMed]
- Cortese, S.; Adamo, N.; Del Giovane, C.; Mohr-Jensen, C.; Hayes, A.J.; Carucci, S.; Atkinson, L.Z.; Tessari, L.; Banaschewski, T.; Coghill, D.; et al. Comparative efficacy and tolerability of medications for attention-deficit hyperactivity disorder in children, adolescents, and adults: A systematic review and network meta-analysis. Lancet Psychiatry 2018, 5, 727–738. [Google Scholar] [CrossRef] [PubMed]
- Whalen, C.K.; Henker, B.; Granger, D.A. Social judgment processes in hyperactive boys: Effects of methylphenidate and comparisons with normal peers. J. Abnorm. Child Psychol. 1990, 18, 297–316. [Google Scholar] [CrossRef] [PubMed]
- Whalen, C.K.; Henker, B. Social impact of stimulant treatment for hyperactive children. J. Learn. Disabil. 1991, 24, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Demirci, E.; Erdogan, A. Is emotion recognition the only problem in ADHD? Effects of pharmacotherapy on face and emotion recognition in children with ADHD. ADHD-Atten. Deficit Hyperact. Disord. 2016, 8, 197–204. [Google Scholar] [CrossRef]
- Golubchik, P.; Weizman, A. The Possible Effect of Methylphenidate Treatment on Empathy in Children Diagnosed with Attention-Deficit/Hyperactivity Disorder, Both with and Without Comorbid Oppositional Defiant Disorder. J. Child Adolesc. Psychopharmacol. 2017, 27, 429–432. [Google Scholar] [CrossRef]
- Gumustas, F.; Yilmaz, I.; Yulaf, Y.; Gokce, S.; Sabuncuoglu, O. Empathy and facial expression recognition in children with and without attention-deficit/hyperactivity disorder: Effects of stimulant medication on empathic skills in children with attention-deficit/hyperactivity disorder. J. Child Adolesc. Psychopharmacol. 2017, 27, 433–439. [Google Scholar] [CrossRef]
- Levi-Shachar, O.; Gvirts, H.Z.; Goldwin, Y.; Bloch, Y.; Shamay-Tsoory, S.; Zagoory-Sharon, O.; Feldman, R.; Maoz, H. The effect of methylphenidate on social cognition and oxytocin in children with attention deficit hyperactivity disorder. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2020, 45, 367–373. [Google Scholar] [CrossRef]
- Fantozzi, P.; Muratori, P.; Caponi, M.C.; Levantini, V.; Nardoni, C.; Pfanner, C.; Ricci, F.; Sesso, G.; Tacchi, A.; Milone, A.; et al. Treatment with Methylphenidate Improves Affective but Not Cognitive Empathy in Youths with Attention-Deficit/Hyperactivity Disorder. Children 2021, 8, 596. [Google Scholar] [CrossRef]
- Zoratto, F.; Franchi, F.; Macri, S.; Laviola, G. Methylphenidate administration promotes sociability and reduces aggression in a mouse model of callousness. Psychopharmacology 2019, 236, 2593–2611. [Google Scholar] [CrossRef] [PubMed]
- Uekermann, J.; Kraemer, M.; Krankenhaus, A.K.; Abdel-Hamid, M.; Hebebrand, J.; Uekermann, J.; Kraemer, M.; Abdel-Hamid, M.; Schimmelmann, B.G.; Hebebrand, J.; et al. Social Cognition in Attention-Deficit Hyperactivity Disorder (ADHD) Essener Interview zur Schulzeitbezogenen Biographie bei ADHS im Erwachsenenalter View Project COMPAS Study View Project Social Cognition in Attention-Deficit Hyperactivity Disorder (ADHD); Elsevier: Amsterdam, The Netherlands, 2009. [Google Scholar] [CrossRef]
- Coelho, L.F.; Barbosa, D.L.F.; Rizzutti, S.; Bueno, O.F.A.; Miranda, M.C. Group cognitive behavioral therapy for children and adolescents with ADHD. Psicol. Reflex. Crit. Rev. Semest. Dep. Psicol. UFRGS 2017, 30, 11. [Google Scholar] [CrossRef]
- Golubchik, P.; Weizman, A. Poor performance of the “child Reading the Mind in the Eyes Test” correlates with poorer social-emotional functioning in children with attention-deficit/hyperactivity disorder. Int. Clin. Psychopharmacol. 2020, 35, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Levi-Shachar, O.; Gvirts, H.Z.; Goldwin, Y.; Bloch, Y.; Shamay-Tsoory, S.; Boyle, D.; Maoz, H. The association between symptom severity and theory of mind impairment in children with attention deficit/hyperactivity disorder. Psychiatry Res. 2021, 303, 114092. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.W.; Peterson, A.D.; Webster, R.E.; Bolen, L.M.; Brown, M.B. Perception of nonverbal social cues by regular education, adhd, and adhd/ld students cathy w. hall, andrea d. peterson, raymond e. webster, larry m. bolen, and michael b. brown. Psychology 1999, 36, 505–514. [Google Scholar]
- Schulz, K.P.; Krone, B.; Adler, L.A.; Bédard, A.C.V.; Duhoux, S.; Pedraza, J.; Mahagabin, S.; Newcorn, J.H. Lisdexamfetamine Targets Amygdala Mechanisms That Bias Cognitive Control in Attention-Deficit/Hyperactivity Disorder. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2018, 3, 686–693. [Google Scholar] [CrossRef]
- Schwenck, C.; Schneider, T.; Schreckenbach, J.; Zenglein, Y.; Gensthaler, A.; Taurines, R.; Freitag, C.M.; Schneider, W.; Romanos, M. Emotion recognition in children and adolescents with attention-deficit/hyperactivity disorder (ADHD). ADHD Atten. Deficit Hyperact. Disord. 2013, 5, 295–302. [Google Scholar] [CrossRef]
- Meier, S.M.; Pavlova, B.; Dalsgaard, S.; Nordentoft, M.; Mors, O.; Mortensen, P.B.; Uher, R. Attention-deficit hyperactivity disorder and anxiety disorders as precursors of bipolar disorder onset in adulthood. Br. J. Psychiatry 2018, 213, 555–560. [Google Scholar] [CrossRef]
- Shaw, M.; Hodgkins, P.; Caci, H.; Young, S.; Kahle, J.; Woods, A.G.; Arnold, L.E. A systematic review and analysis of long-term outcomes in attention deficit hyperactivity disorder: Effects of treatment and non-treatment. BMC Med. 2012, 10, 99. [Google Scholar] [CrossRef]
- Cortese, S.; Castellanos, F.X. Neuroimaging of attention-deficit/hyperactivity disorder: Current neuroscience-informed perspectives for clinicians. Curr. Psychiatry Rep. 2012, 14, 568–578. [Google Scholar] [CrossRef]
- Frodl, T.; Skokauskas, N. Meta-analysis of structural MRI studies in children and adults with attention deficit hyperactivity disorder indicates treatment effects. Acta Psychiatr. Scand. 2012, 125, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Greven, C.U.; Bralten, J.; Mennes, M.; O’Dwyer, L.; Van Hulzen, K.J.E.; Rommelse, N.; Schweren, L.J.S.; Hoekstra, P.J.; Hartman, C.A.; Heslenfeld, D.; et al. Developmentally stable whole-brain volume reductions and developmentally sensitive caudate and putamen volume alterations in those with attention-deficit/hyperactivity disorder and their unaffected siblings. JAMA Psychiatry 2015, 72, 490–499. [Google Scholar] [CrossRef]
- Hoogman, M.; Bralten, J.; Hibar, D.P.; Mennes, M.; Zwiers, M.P.; Schweren, L.S.J.; van Hulzen, K.J.E.; Medland, S.E.; Shumskaya, E.; Jahanshad, N.; et al. Subcortical brain volume differences in participants with attention deficit hyperactivity disorder in children and adults: A cross-sectional mega-analysis. Lancet Psychiatry 2017, 4, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Franke, B.; Michelini, G.; Asherson, P.; Banaschewski, T.; Bilbow, A.; Buitelaar, J.K.; Cormand, B.; Faraone, S.V.; Ginsberg, Y.; Haavik, J.; et al. Live fast, die young? A review on the developmental trajectories of ADHD across the lifespan. Eur. Neuropsychopharmacol. 2018, 28, 1059–1088. [Google Scholar] [CrossRef]
- Faraone, S.V. The pharmacology of amphetamine and methylphenidate: Relevance to the neurobiology of attention-deficit/hyperactivity disorder and other psychiatric comorbidities. Neurosci. Biobehav. Rev. 2018, 87, 255–270. [Google Scholar] [PubMed]
- Spencer, T.J.; Brown, A.; Seidman, L.J.; Valera, E.M.; Makris, N.; Lomedico, A.; Faraone, S.V.; Biederman, J. Effect of psychostimulants on brain structure and function in ADHD: A qualitative literature review of magnetic resonance imaging-based neuroimaging studies. J. Clin. Psychiatry 2013, 74, 902–917. [Google Scholar] [CrossRef] [PubMed]
- Faraone, S.V.; Asherson, P.; Banaschewski, T.; Biederman, J.; Buitelaar, J.K.; Ramos-Quiroga, J.A.; Rohde, L.A.; Sonuga-Barke, E.J.S.; Tannock, R.; Franke, B. Attention-deficit/hyperactivity disorder. Nat. Rev. Dis. Prim. 2015, 1, 15020. [Google Scholar] [CrossRef]
- Rubia, K. Functional brain imaging across development. Eur. Child Adolesc. Psychiatry 2013, 22, 719–731. [Google Scholar] [CrossRef]
- Sonuga-Barke, E.J.S.; Sergeant, J.A.; Nigg, J.; Willcutt, E. Executive Dysfunction and Delay Aversion in Attention Deficit Hyperactivity Disorder: Nosologic and Diagnostic Implications. Child Adolesc. Psychiatr. Clin. N. Am. 2008, 17, 367–384. [Google Scholar]
- Rubia, K. “Cool” inferior frontostriatal dysfunction in attention-deficit/hyperactivity disorder versus “hot” ventromedial orbitofrontal-limbic dysfunction in conduct disorder: A review. Biol. Psychiatry 2011, 69, e69–e87. [Google Scholar] [CrossRef]
- Pievsky, M.A.; McGrath, R.E. The Neurocognitive Profile of Attention-Deficit/Hyperactivity Disorder: A Review of Meta-Analyses. Arch. Clin. Neuropsychol. 2018, 33, 143–157. [Google Scholar]
- Rubia, K.; Halari, R.; Christakou, A.; Taylor, E. Impulsiveness as a tinning disturbance: Neurocognitive abnormalities in attention-deficit hyperactivity disorder during temporal processes and normalization with methylphenidate. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 1919–1931. [Google Scholar] [CrossRef] [PubMed]
- Noreika, V.; Falter, C.M.; Rubia, K. Timing deficits in attention-deficit/hyperactivity disorder (ADHD): Evidence from neurocognitive and neuroimaging studies. Neuropsychologia 2013, 51, 235–266. [Google Scholar] [CrossRef]
- Plichta, M.M.; Scheres, A. Ventral-striatal responsiveness during reward anticipation in ADHD and its relation to trait impulsivity in the healthy population: A meta-analytic review of the fMRI literature. Neurosci. Biobehav. Rev. 2014, 38, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Rubia, K.; Criaud, M.; Wulff, M.; Alegria, A.; Brinson, H.; Barker, G.; Stahl, D.; Giampietro, V. Functional connectivity changes associated with fMRI neurofeedback of right inferior frontal cortex in adolescents with ADHD. Neuroimage 2019, 188, 43–58. [Google Scholar] [CrossRef] [PubMed]
- Posner, J.; Kass, E.; Hulvershorn, L. Using Stimulants to Treat ADHD-Related Emotional Lability. Curr. Psychiatry Rep. 2014, 16, 478. [Google Scholar] [CrossRef] [PubMed]
- Lenzi, F.; Cortese, S.; Harris, J.; Masi, G. Neuroscience and Biobehavioral Reviews Pharmacotherapy of emotional dysregulation in adults with ADHD: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2018, 84, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Retz, W.; Stieglitz, R.D.; Corbisiero, S.; Retz-Junginger, P.; Rösler, M. Emotional dysregulation in adult ADHD: What is the empirical evidence? Expert Rev. Neurother. 2012, 12, 1241–1251. [Google Scholar] [PubMed]
- Barkley, R.A.; Murphy, K.R. Impairment in occupational functioning and adult ADHD: The predictive utility of executive function (EF) ratings versus EF tests. Arch. Clin. Neuropsychol. 2010, 25, 157–173. [Google Scholar] [CrossRef]
- Tamminga, H.G.H.; Reneman, L.; Huizenga, H.M.; Geurts, H.M. Effects of methylphenidate on executive functioning in attention-deficit/hyperactivity disorder across the lifespan: A meta-regression analysis. Psychol. Med. 2016, 46, 1791–1807. [Google Scholar]
- Arioli, M.; Cattaneo, Z.; Ricciardi, E.; Canessa, N. Overlapping and specific neural correlates for empathizing, affective mentalizing, and cognitive mentalizing: A coordinate-based meta-analytic study. Hum. Brain Mapp. 2021, 42, 4777–4804. [Google Scholar] [CrossRef]
- Jones, A.P.; Happé, F.G.E.; Gilbert, F.; Burnett, S.; Viding, E. Feeling, caring, knowing: Different types of empathy deficit in boys with psychopathic tendencies and autism spectrum disorder. J. Child Psychol. Psychiatry 2010, 51, 1188–1197. [Google Scholar] [CrossRef] [PubMed]
- Visser, J.C.; Rommelse, N.N.J.; Greven, C.U.; Buitelaar, J.K. Autism spectrum disorder and attention-deficit/hyperactivity disorder in early childhood: A review of unique and shared characteristics and developmental antecedents. Neurosci. Biobehav. Rev. 2016, 65, 229–263. [Google Scholar] [CrossRef]
- Polderman, T.J.C.; Hoekstra, R.A.; Vinkhuyzen, A.A.E.; Sullivan, P.F.; Van Der Sluis, S.; Posthuma, D. Attentional switching forms a genetic link between attention problems and autistic traits in adults. Psychol. Med. 2013, 43, 1985–1996. [Google Scholar] [CrossRef] [PubMed]
- Sokolova, E.; Oerlemans, A.M.; Rommelse, N.N.; Groot, P.; Hartman, C.A.; Glennon, J.C.; Claassen, T.; Heskes, T.; Buitelaar, J.K. A causal and mediation analysis of the comorbidity between attention deficit hyperactivity disorder (ADHD) and autism spectrum disorder (ASD). J. Autism Dev. Disord. 2017, 47, 1595–1604. [Google Scholar] [CrossRef] [PubMed]
| Study | N | Gender | Age | ADHD | Comorbidity | Treatment | Assessment | Outcome |
|---|---|---|---|---|---|---|---|---|
| Coelho et al., 2017 [65] | 60 ADHD (30 C, 30 I) | 48/12 | 7–14 (unimodal group = 10.13) (multimodal group = 10.2) | no other medications when recruited | ID excluded | unimodal-medication only vs. multimodal medication + CBT for 20 weeks (prolonged release-MPH 20 mg) | Children’s Social Skills Multimedia System | Multimodal treatment showed more improvement in frequency indicators on empathy. |
| Demirci and Erdogan, 2016 [58] | 60 ADHD (21 C, 17 H/I, 22 I) 60 HCs | 35/25 ADHD 35/25 HCs | 8–15 (ADHD = 10.8) (HCs = 10.8) | drug-naive | ID, ASD, CD excluded | pharmacological treatment for 12 weeks: −38 OROS-MPH (final dose 1.2 mg/kg/day) −32 ATX (final dose 1.2 mg/kg/day) | RMET | The ADHD sample had significantly lower scores in RMET than HCs. ADHD-H/I had a lower number of correct answers in the RMET than ADHD-I. After OROS-MPH/ATX treatment, the ADHD sample showed a significant improvement in RMET. |
| Fantozzi et al., 2021 [62] | 61 ADHD (50 C, 11 I) | 51/10 | 6–17 (10.3) | drug-naive | ID, ASD excluded 14 SLD; 9 ODD; 4 MD; 2 LD; 1 AD; 1 tics; 1 dyspraxia | MPH treatment for 6 months (final dosage 31.6 ± 15.1 mg/day) | BES | Significant improvement in AE and CE. Changes in attention symptoms predicted changes in AE but not in CE. |
| Golubchik and Weizman, 2017 [59] | 52 ADHD | 8–18 | psychostimulant-medication naive | ID, ASD, schizophrenia, bipolar disorder, suicidal ideation excluded 26 ODD | MPH treatment for 12 weeks (0.5–1 mg/kg/day) | EQ-C | Significant improvement in EQ scores in both groups (ADHD and ADHD/ODD). Only in the ADHD group, a significant correlation between changes in ADHD-RS and in EQ-C was found. | |
| Golubchik and Weizman, 2019 [66] | 25 ADHD | 21/4 | 7–17 (10.8) | ID, ASD, psychosis, bipolar disorder excluded | single dose of MPH (1 mg/kg) | RMET | No improvement of RMET. | |
| Gumustas et al., 2017 [60] | 65 ADHD 61 HCs | 53/12 ADHD 46/15 HCs | 8–14 (ADHD = 10.86) (HCs = 11.21) | drug-naive | ID, ASD, psychosis, mood disorders, anxiety disorders, ODD excluded | OROS-MPH treatment for 12 weeks (0.83 ± 0.21 mg/kg/day) | BEI (trait empathy) GEM-PR (trait empathy) ERT (state empathy) | No significant statistical differences in trait and in state empathy skills in the two groups. Following the MPH treatment, the ADHD group showed a significant increase in the ERT (state empathy) interpretation sub-score. |
| Levi-Shachar et al., 2019 [61] | 50 ADHD 40 HCs | 28/22 ADHD 22/18 HCs | 6–12 (ADHD = 9.42) (HCs = 8.95) | psychotropic medication free | psychosis, affective disorders, CD, substance abuse disorder excluded | single dose of short-acting MPH (0.3–0.5 mg/kg) | ToM test | The ADHD sample displayed significantly poorer ToM performance compared with HCs. Following MPH administration, the ToM performance of the ADHD sample normalized. |
| Levi-Shachar et al., 2021 [67] | 50 ADHD | 28/22 ADHD | 6–12 (ADHD = 9.42) | psychotropic medication free | psychosis, affective disorders, CD, substance abuse disorder excluded | single dose of short-acting MPH (0.3–0.5 mg/kg) | ToM test FPR | Negative association between severity of behavioral ADHD domains and impairment in ToM. Administration of MPH improved ToM performance, with the greatest improvement in children with more severe behavioral symptoms. |
| Maoz et al., 2013 [47] | 24 ADHD (11 C, 13 I) | 16/8 | 6–12 (10.2) | ID, psychosis, bipolar disorder, major depression, DBD, substance abuse disorder excluded | single-dose of long-acting MPH | IRI FRP TCT | Significant improvement in ToM performance. | |
| Maoz et al., 2019 [46] | 24 ADHD 36 HCs | 6/8 ADHD 19/17 HCs | 6–12 (ADHD = 10.29) (HCs = 9.37) | psychotropic medication free | ID, psychosis, bipolar disorder, major depression, CD, substance abuse disorder excluded | single dose of long-acting MPH | IRIFRP | The ADHD sample showed lower levels of self-reported empathy and FRP scores compared with HCs. In ADHD sample, MPH administration improved FRP scores to a level equal to that in HCs. |
| Study | N | Gender | Age | ADHD | Comorbidity | Treatment | Assessment | Outcome |
|---|---|---|---|---|---|---|---|---|
| Demrici and Erdogan, 2016 [58] | 60 ADHD (21 C, 17 H/I, 22 I) 60 HCs | 35/25 ADHD 35/25 HCs | 8–15 years (ADHD = 10.8) (HCs = 10.8) | drug-naive | ID, ASD, CD excluded | pharmacological treatment for 12 weeks: −38 OROS-MPH (final dose 1.2 mg/kg/day) −32 ATX (final dose 1.2 mg/kg/day) | BFRT | ADHD sample had significantly lower scores in BFRT than HCs. ADHD-H/I had a lower number of correct answers in BRFT than ADHD-C and I. After OROS-MPH/ATX treatment, the ADHD sample showed a significant improvement in BFRT. |
| Gumustas et al., 2017a [60] | 65 ADHD 61 HCs | 53/12 ADHD 46/15 HCs | 8–14 years (ADHD = 10.86)(HCs = 11.21) | drug-naive | ID, ASD, psychosis, mood disorders, anxiety disorders, ODD excluded | OROS-MPH treatment for 12 weeks (0.83 ± 0.21 mg/kg/day) | DANVA-2 | No significant statistical differences in facial expression recognition skills in the two groups. Following the MPH treatment, the ADHD group showed a significant decrease in the recognition error of anger and sadness expressions. |
| Hall et al., 1999 [68] | 15 ADHD (13 C, 2 H/I) 15 ADHD/LD (14 C, 1 H/I) 15 no ADHD or LD | 36/9 | 7–10 years | the ADHD sample was taken MPH (Ritalin) for at least a month at the time of the study | ID excluded | the DANVA was administered twice to each child in the ADHD and ADHD/LD groups: once while the ADHD and ADHD/LD participants were on medication and once off medication | DANVA SPBRS | The ADHD/LD group demonstrated significant difficulty in comparison to their peers in perceiving paralanguage cues effectively. The ADHD/LD group showed significant improvement on the Postures and Paralanguage subtests during on-medication conditions. |
| Schulz et al., 2018 [69] | 25 ADHD (17C, 8I) | 14/9 | 19–52 years (34.8 ± 9.8) | 2 participants were on medication at intake, 9 had a history of previous stimulant treatment (2 of whom had also previously been treated with nonstimulant medication) | psychosis, BD, PTSD, substance use disorderexcluded | 3 to 4 weeks of LDX (mean maintenance dose = 64 mg/day–SD = 13 mg) treatment and 3 weeks of medication in a randomized, counterbalanced, hybrid crossover design | participants were scanned twice with event-related fMRI while performing an emotional go/no-go task | No significant differences between the two treatment arms. LDX was associated with an increase in fMRI activation in the right amygdala and reduced interactions with the orbital aspect of the left inferior frontal gyrus specifically for responses to sad faces. |
| Schwenck et al., 2013 [70] | 56 ADHD (10C,2H/I,44I) 28 ADHD-MD− 28 ADHD-MD+ 28 CG | 19/9 | 8.2–17.3 years (MD− = 12.36) (MD+ = 12.31) (CG = 12.49) | 47 children in the ADHD group were taken MPH at the time of the study (one child was additionally taken ATX), 6 drug-naive | ID, ASD, ODD, CD excluded | cross-sectional design study | MT | No differences found between ADHD-MD−, ADHD-MD+ and CG on emotion recognition. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fantozzi, P.; Sesso, G.; Muratori, P.; Milone, A.; Masi, G. Biological Bases of Empathy and Social Cognition in Patients with Attention-Deficit/Hyperactivity Disorder: A Focus on Treatment with Psychostimulants. Brain Sci. 2021, 11, 1399. https://doi.org/10.3390/brainsci11111399
Fantozzi P, Sesso G, Muratori P, Milone A, Masi G. Biological Bases of Empathy and Social Cognition in Patients with Attention-Deficit/Hyperactivity Disorder: A Focus on Treatment with Psychostimulants. Brain Sciences. 2021; 11(11):1399. https://doi.org/10.3390/brainsci11111399
Chicago/Turabian StyleFantozzi, Pamela, Gianluca Sesso, Pietro Muratori, Annarita Milone, and Gabriele Masi. 2021. "Biological Bases of Empathy and Social Cognition in Patients with Attention-Deficit/Hyperactivity Disorder: A Focus on Treatment with Psychostimulants" Brain Sciences 11, no. 11: 1399. https://doi.org/10.3390/brainsci11111399
APA StyleFantozzi, P., Sesso, G., Muratori, P., Milone, A., & Masi, G. (2021). Biological Bases of Empathy and Social Cognition in Patients with Attention-Deficit/Hyperactivity Disorder: A Focus on Treatment with Psychostimulants. Brain Sciences, 11(11), 1399. https://doi.org/10.3390/brainsci11111399







