The Influence of Intraocular Lens Implantation and Alterations in Blue Light Transmittance Level on the Brain Functional Network Architecture Reorganization in Cataract Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. IOL and Blue Light Transmittance
2.3. Behavioral Measurements
2.4. MRI Data Acquisition
2.5. Imaging Data Preprocessing
2.6. Parcellation
2.7. Graph Metrics
2.8. Statistical Analysis
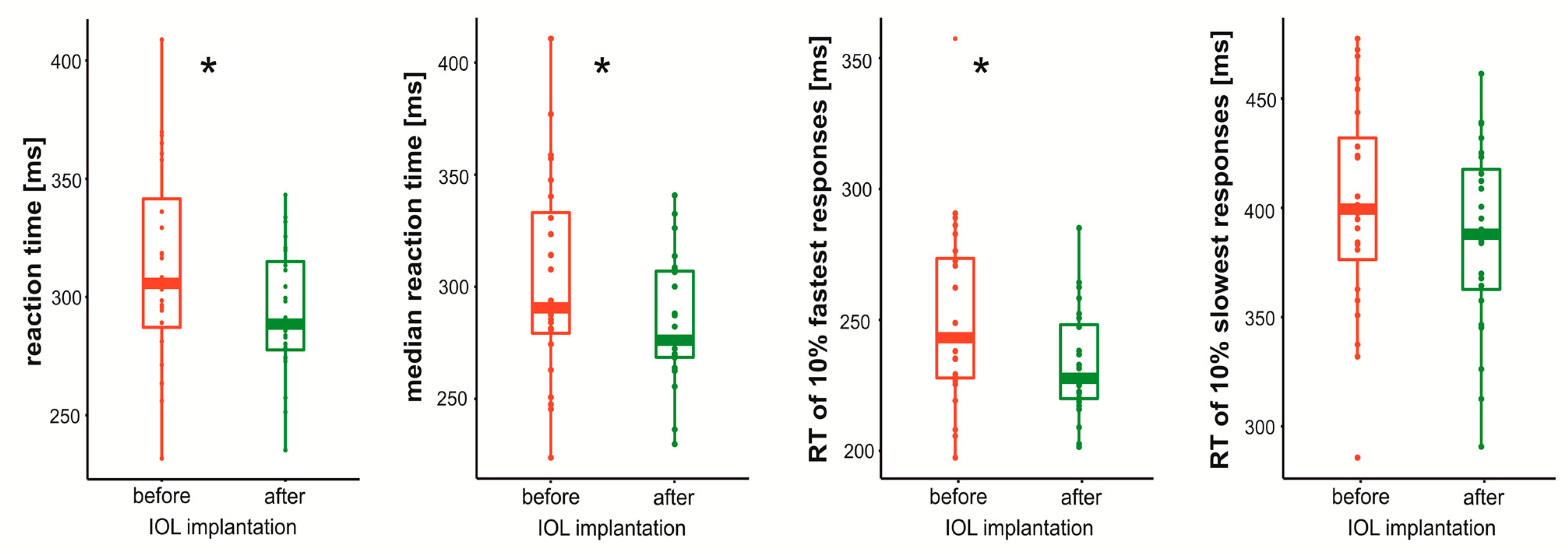
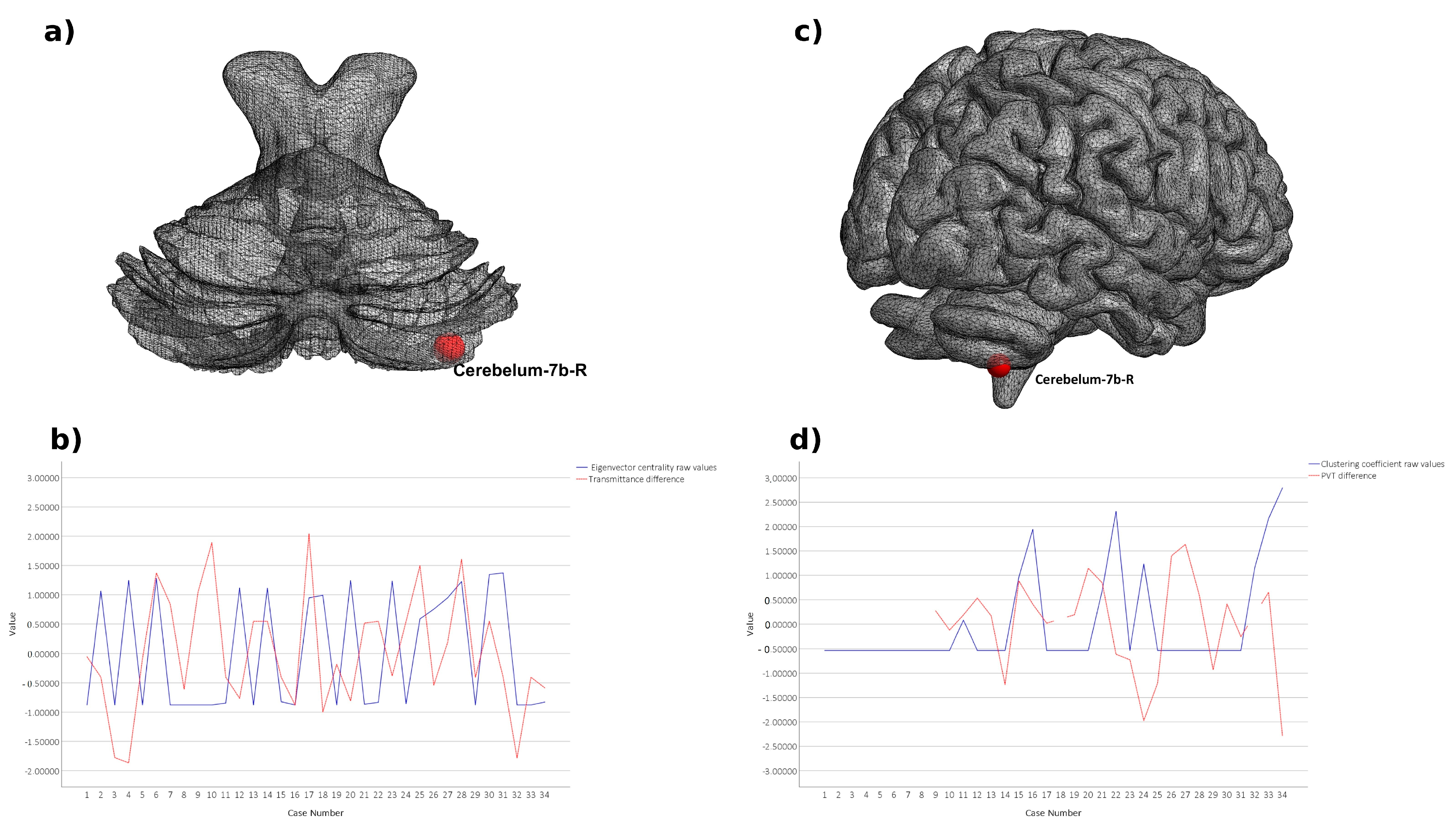
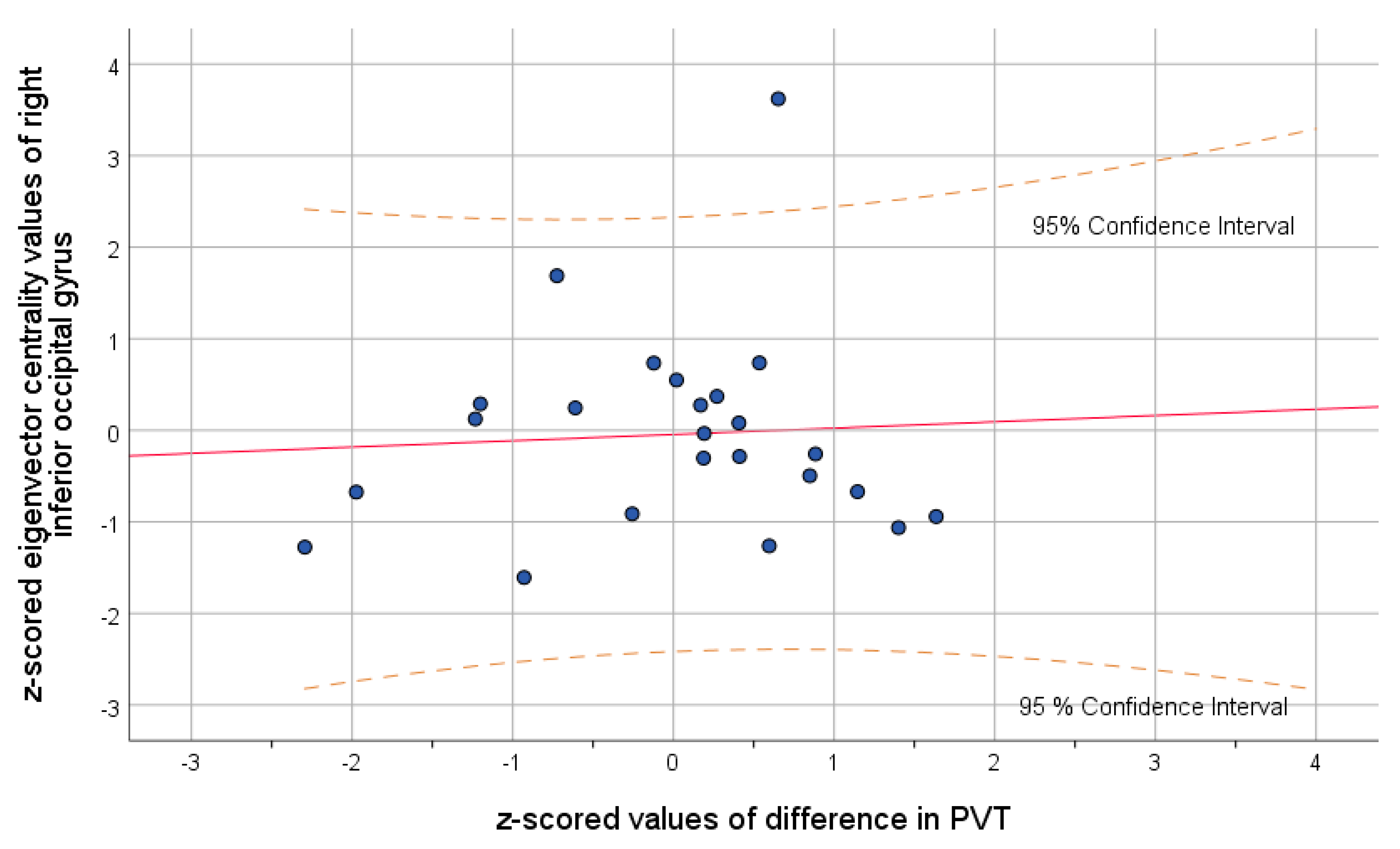
3. Results
4. Discussion
5. Limitation
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schnitzer, M.J.; Meister, M. Multineuronal Firing Patterns in the Signal from Eye to Brain. Neuron 2003, 37, 499–511. [Google Scholar] [CrossRef]
- Chen, C.; Bickford, M.E.; Hirsch, J.A. Untangling the Web between Eye and Brain. Cell 2016, 165, 20–21. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pascolini, D.; Mariotti, S.P. Global estimates of visual impairment: 2010. Br. J. Ophthalmol. 2011, 96, 614–618. [Google Scholar] [CrossRef]
- Linn, L.; Kahn, R.L.; Coles, R.; Cohen, J.; Marshall, D.; Weinstein, E.A. Patterns of behavior disturbance following cataract extraction. Am. J. Psychiatry 1953, 110, 281–289. [Google Scholar] [CrossRef]
- Lai, S.-W.; Lin, C.-L.; Liao, K.-F. Cataract may be a non-memory feature of Alzheimer’s disease in older people. Eur. J. Epidemiol. 2014, 29, 405–409. [Google Scholar] [CrossRef]
- Kessel, L.; Lundeman, J.H.; Herbst, K.; Andersen, T.V.; Larsen, M. Age-related changes in the transmission properties of the human lens and their relevance to circadian entrainment. J. Cataract. Refract. Surg. 2010, 36, 308–312. [Google Scholar] [CrossRef]
- Cajochen, C. Alerting effects of light. Sleep Med. Rev. 2007, 11, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Davison, J.A.; Patel, A.S.; Cunha, J.P.; Schwiegerling, J.; Muftuoglu, O. Recent studies provide an updated clinical perspective on blue light-filtering IOLs. Graefe’s Arch. Clin. Exp. Ophthalmol. 2011, 249, 957–968. [Google Scholar] [CrossRef][Green Version]
- Daneault, V.; Hébert, M.; Albouy, G.; Doyon, J.; Dumont, M.; Carrier, J.; Vandewalle, G. Aging Reduces the Stimulating Effect of Blue Light on Cognitive Brain Functions. Sleep 2014, 37, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Ishii, K.; Kabata, T.; Oshika, T. The Impact of Cataract Surgery on Cognitive Impairment and Depressive Mental Status in Elderly Patients. Am. J. Ophthalmol. 2008, 146, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Zhang, L.; Lin, D.; Chen, W.; Zhu, Y.; Chen, C.; Chan, K.C.W.; Liu, Y.; Chen, W. Visual Restoration after Cataract Surgery Promotes Functional and Structural Brain Recovery. EBioMedicine 2018, 30, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Fagerström, R. Correlations of Memory and Learning with Vision in Aged Patients before and after a Cataract Operation. Psychol. Rep. 1992, 71, 675–686. [Google Scholar] [CrossRef]
- Hall, T.A.; McGwin, G., Jr.; Owsley, C. Effect of cataract surgery on cognitive function in older adults. J. Am. Geriatr. Soc. 2005, 53, 2140–2144. [Google Scholar] [CrossRef] [PubMed]
- Fukuoka, H.; Sutu, C.; Afshari, N.A. The impact of cataract surgery on cognitive function in an aging population. Curr. Opin. Ophthalmol. 2016, 27, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Gray, C.S.; Karimova, G.; Hildreth, A.J.; Crabtree, L.; Allen, D.; O’Connell, J.E. Recovery of visual and functional disability following cataract surgery in older people: Sunderland Cataract Study. J. Cataract. Refract. Surg. 2006, 32, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Medaglia, J.D. Functional Neuroimaging in Traumatic Brain Injury: From Nodes to Networks. Front. Neurol. 2017, 8, 407. [Google Scholar] [CrossRef]
- Xu, T.; Cullen, K.R.; Mueller, B.; Schreiner, M.W.; Lim, K.O.; Schulz, S.C.; Parhi, K.K. Network analysis of functional brain connectivity in borderline personality disorder using resting-state fMRI. NeuroImage Clin. 2016, 11, 302–315. [Google Scholar] [CrossRef]
- Sadeghi, M.; Khosrowabadi, R.; Bakouie, F.; Mahdavi, H.; Eslahchi, C.; Pouretemad, H. Screening of autism based on task-free fMRI using graph theoretical approach. Psychiatry Res. Neuroimaging 2017, 263, 48–56. [Google Scholar] [CrossRef]
- Xing, M.; Tadayonnejad, R.; MacNamara, A.; Ajilore, O.; DiGangi, J.; Phan, K.L.; Leow, A.; Klumpp, H. Resting-state theta band connectivity and graph analysis in generalized social anxiety disorder. NeuroImage Clin. 2016, 13, 24–32. [Google Scholar] [CrossRef]
- Bromis, K.; Gkiatis, K.; Karanasiou, I.; Matsopoulos, G.; Karavasilis, E.; Papathanasiou, M.; Efstathopoulos, E.; Kelekis, N.; Kouloulias, V. Altered Brain Functional Connectivity in Small-Cell Lung Cancer Patients after Chemotherapy Treatment: A Resting-State fMRI Study. Comput. Math. Methods Med. 2017, 2017, 1–12. [Google Scholar] [CrossRef]
- Macpherson, H.; Formica, M.; Harris, E.; Daly, R.M. Brain functional alterations in Type 2 Diabetes—A systematic review of fMRI studies. Front. Neuroendocr. 2017, 47, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Sobczak, A.M.; Bohaterewicz, B.; Fafrowicz, M.; Zyrkowska, A.; Golonka, N.; Domagalik, A.; Beldzik, E.; Oginska, H.; Rekas, M.; Bronicki, D.; et al. Brain Functional Network Architecture Reorganization and Alterations of Positive and Negative Affect, Experiencing Pleasure and Daytime Sleepiness in Cataract Patients after Intraocular Lenses Implantation. Brain Sci. 2021, 11, 1275. [Google Scholar] [CrossRef] [PubMed]
- Siik, S.; Chylack, L.T., Jr.; Friend, J.; Wolfe, J.; Teikari, J.; Nieminen, H.; Airaksinen, P.J. Lens autofluorescence and light scatter in relation to the lens opacities classification system, LOCS III. Acta Ophthalmol. Scand. 1999, 77, 509–514. [Google Scholar] [CrossRef]
- Dinges, D.F.; Powell, J.W. Microcomputer analyses of performance on a portable, simple visual RT task during sustained operations. Behav. Res. Methods Instrum. Comput. 1985, 17, 652–655. [Google Scholar] [CrossRef]
- Basner, M.; Dinges, D.F. Maximizing Sensitivity of the Psychomotor Vigilance Test (PVT) to Sleep Loss. Sleep 2011, 34, 581–591. [Google Scholar] [CrossRef]
- Yan, C.-G.; Wang, X.-D.; Zuo, X.-N.; Zang, Y.-F. DPABI: Data Processing & Analysis for (Resting-State) Brain Imaging. Neuroinformatics 2016, 14, 339–351. [Google Scholar] [CrossRef]
- Behzadi, Y.; Restom, K.; Liau, J.; Liu, T.T. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. NeuroImage 2007, 37, 90–101. [Google Scholar] [CrossRef]
- Liu, T.T.; Nalci, A.; Falahpour, M. The global signal in fMRI: Nuisance or Information? NeuroImage 2017, 150, 213–229. [Google Scholar] [CrossRef] [PubMed]
- Mazoyera, N.; Landeau, B.; Papathanassiou, D.; Crivello, F.; Etard, O.; Delcroix, N.; Tzourio-Mazoyer, N.; Joliot, M. Automated Anatomical Labeling of Activations in SPM Using a Macroscopic Anatomical Parcellation of the MNI MRI Single-Subject Brain. NeuroImage 2002, 15, 273–289. [Google Scholar] [CrossRef]
- Power, J.D.; Cohen, A.; Nelson, S.M.; Wig, G.S.; Barnes, K.A.; Church, J.; Vogel, A.C.; Laumann, T.O.; Miezin, F.M.; Schlaggar, B.L.; et al. Functional Network Organization of the Human Brain. Neuron 2011, 72, 665–678. [Google Scholar] [CrossRef]
- Heuvel, M.P.V.D.; de Lange, S.C.; Zalesky, A.; Seguin, C.; Yeo, B.T.; Schmidt, R. Proportional thresholding in resting-state fMRI functional connectivity networks and consequences for patient-control connectome studies: Issues and recommendations. NeuroImage 2017, 152, 437–449. [Google Scholar] [CrossRef] [PubMed]
- Gamboa, O.; Tagliazucchi, E.; von Wegner, F.; Jurcoane, A.; Wahl, M.; Laufs, H.; Ziemann, U. Working memory performance of early MS patients correlates inversely with modularity increases in resting state functional connectivity networks. NeuroImage 2014, 94, 385–395. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B (Methodol.) 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Watts, D.J.; Strogatz, S.H. Collective dynamics of ‘small-world’ networks. In The Structure and Dynamics of Networks; Princeton University Press: Princeton, NJ, USA, 2011; pp. 301–303. [Google Scholar] [CrossRef]
- Brier, M.R.; Thomas, J.B.; Fagan, A.M.; Hassenstab, J.; Holtzman, D.M.; Benzinger, T.L.; Morris, J.C.; Ances, B.M. Functional connectivity and graph theory in preclinical Alzheimer’s disease. Neurobiol. Aging 2014, 35, 757–768. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liang, M.; Zhou, Y.; He, Y.; Hao, Y.; Song, M.; Yu, C.; Liu, H.; Liu, Z.; Jiang, T. Disrupted small-world networks in schizophrenia. Brain 2008, 131, 945–961. [Google Scholar] [CrossRef]
- Filippi, M.; Basaia, S.; Canu, E.; Imperiale, F.; Meani, A.; Caso, F.; Magnani, G.; Falautano, M.; Comi, G.; Falini, A.; et al. Brain network connectivity differs in early-onset neurodegenerative dementia. Neurology 2017, 89, 1764–1772. [Google Scholar] [CrossRef]
- Masuda, N.; Sakaki, M.; Ezaki, T.; Watanabe, T. Clustering Coefficients for Correlation Networks. Front. Aging Neurosci. 2018, 12, 7. [Google Scholar] [CrossRef] [PubMed]
- Davey, J.; Cornelissen, P.L.; Thompson, H.E.; Sonkusare, S.; Hallam, G.P.; Smallwood, J.; Jefferies, E. Automatic and Controlled Semantic Retrieval: TMS Reveals Distinct Contributions of Posterior Middle Temporal Gyrus and Angular Gyrus. J. Neurosci. 2015, 35, 15230–15239. [Google Scholar] [CrossRef]
- Van Overwalle, F.; Ma, Q.; Heleven, E. The posterior crus II cerebellum is specialized for social mentalizing and emotional self-experiences: A meta-analysis. Soc. Cogn. Affect. Neurosci. 2020, 15, 905–928. [Google Scholar] [CrossRef]
- Patla, A.E. Understanding the roles of vision in the control of human locomotion. Gait Posture 1997, 5, 54–69. [Google Scholar] [CrossRef]
- Inoue, S.; Kawashima, M.; Hiratsuka, Y.; Nakano, T.; Tamura, H.; Ono, K.; Murakami, A.; Tsubota, K.; Yamada, M. Assessment of physical inactivity and locomotor dysfunction in adults with visual impairment. Sci. Rep. 2018, 8, 12032. [Google Scholar] [CrossRef] [PubMed]
- Brissenden, J.A.; Levin, E.J.; Osher, D.E.; Halko, M.A.; Somers, D.C. Functional Evidence for a Cerebellar Node of the Dorsal Attention Network. J. Neurosci. 2016, 36, 6083–6096. [Google Scholar] [CrossRef]
- Fletcher, J.M.; Wennekers, T. From Structure to Activity: Using Centrality Measures to Predict Neuronal Activity. Int. J. Neural Syst. 2018, 28, 1750013. [Google Scholar] [CrossRef]
- Skouras, S.; Falcon, C.; Tucholka, A.; Rami, L.; Sanchez-Valle, R.; Lladó, A.; Gispert, J.D.; Molinuevo, J.L. Mechanisms of functional compensation, delineated by eigenvector centrality mapping, across the pathophysiological continuum of Alzheimer’s disease. NeuroImage: Clin. 2019, 22, 101777. [Google Scholar] [CrossRef]
- Bachmann, C.; Tetzlaff, T.; Duarte, R.; Morrison, A. Firing rate homeostasis counteracts changes in stability of recurrent neural networks caused by synapse loss in Alzheimer’s disease. PLoS Comput. Biol. 2020, 16, e1007790. [Google Scholar] [CrossRef]
- Sjoerds, Z.; Stufflebeam, S.M.; Veltman, D.J.; Brink, W.V.D.; Penninx, B.W.J.H.; Douw, L. Loss of brain graph network efficiency in alcohol dependence. Addict. Biol. 2015, 22, 523–534. [Google Scholar] [CrossRef] [PubMed]
- Guell, X.; Schmahmann, J.D.; De Gabrieli, J.; Ghosh, S.S. Functional gradients of the cerebellum. eLife 2018, 7, e36652. [Google Scholar] [CrossRef]
- Sang, L.; Qin, W.; Liu, Y.; Han, W.; Zhang, Y.; Jiang, T.; Yu, C. Resting-state functional connectivity of the vermal and hemispheric subregions of the cerebellum with both the cerebral cortical networks and subcortical structures. NeuroImage 2012, 61, 1213–1225. [Google Scholar] [CrossRef]
- Seeley, W.W. The Salience Network: A Neural System for Perceiving and Responding to Homeostatic Demands. J. Neurosci. 2019, 39, 9878–9882. [Google Scholar] [CrossRef] [PubMed]
- Lehrl, S.; Gerstmeyer, K.; Jacob, J.H.; Frieling, H.; Henkel, A.W.; Meyrer, R.; Wiltfang, J.; Kornhuber, J.; Bleich, S. Blue light improves cognitive performance. J. Neural Transm. 2007, 114, 457–460. [Google Scholar] [CrossRef] [PubMed]
- Chellappa, S.; Steiner, R.; Blattner, P.; Oelhafen, P.; Götz, T.; Cajochen, C. Non-Visual Effects of Light on Melatonin, Alertness and Cognitive Performance: Can Blue-Enriched Light Keep Us Alert? PLoS ONE 2011, 6, e16429. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Yang, J.; Ren, X.; Yu, Z.; Zhang, R.; Li, X. Evaluation of eye movements and visual performance in patients with cataract. Sci. Rep. 2020, 10, 9875. [Google Scholar] [CrossRef]
| p-Value | ||||
|---|---|---|---|---|
| ROI (Names) | AAL Label | Threshold | Clustering Coefficient | Eigenvector Centrality |
| Preoperative-Postoperative | ||||
| Left Supplementary Motor Area | SMA.L | 0.2 | 0.035 | |
| Right Supplementary Motor Area | SMA.R | 0.4 | 0.027 | |
| Left Inferior Occipital Gyrus | IOG.L | 0.4 | 0.034 | |
| Right Inferior Occipital Gyrus | IOG.R | 0.3 | 0.009 | |
| 0.4 | 0.02 | |||
| 0.5 | 0.015 | |||
| Left Superior Parietal Gyrus | SPG.L | 0.15 | 0.042 | |
| 0.2 | 0.017 | |||
| 0.3 | 0.038 | |||
| Right Superior Parietal Gyrus | SPG.R | 0.15 | 0.016 | |
| Right Supramarginal Gyrus | SMG.R | 0.15 | 0.004 | |
| 0.35 | 0.029 | |||
| Left Cerebellum Crus 2 | CRBLCrus2.L | 0.2 | 0.046 | |
| 0.3 | 0.019 | |||
| 0.4 | 0.014 | |||
| 0.5 | 0.016 | |||
| Left Cerebellum 7b | CER7b.L | 0.35 | 0.024 | |
| Right Cerebellum 7b | CER7b.R | 0.1 | 0.018 | |
| 0.15 | 0.02 | |||
| 0.2 | 0.017 | |||
| 0.25 | 0.027 | |||
| 0.3 | 0.04 | |||
| Left Cerebellum 8 | CER8.L | 0.1 | 0.03 | |
| Right Cerebellum 8 | CER8.R | 0.15 | 0.048 | |
| 0.45 | 0.03 | |||
| Left Cerebellum 9 | CER9.L | 0.45 | 0.006 | |
| Right Cerebellum 9 | CER9.R | 0.25 | 0.011 | |
| Vermis 8 | VER8 | 0.1 | 0.0002 | |
| Postoperative-Preoperative | ||||
| Right Superior Parietal Gyrus | SPG.R | 0.45 | 0.027 | |
| 0.5 | 0.046 | |||
| Left Inferior Parietal Gyrus | IPL.L | 0.2 | 0.022 | |
| 0.25 | 0.021 | |||
| 0.35 | 0.014 | |||
| 0.4 | 0.014 | |||
| Temporal Pole: Left Middle Temporal Gyrus | TPOmid.L | 0.4 | 0.006 | |
| 0.45 | 0.008 | |||
| 0.5 | 0.014 | |||
| Temporal Pole: Right Middle Temporal Gyrus | TPOmid.R | 0.35 | 0.023 | |
| Left Cerebellum Crus 2 | CRBLCrus2.L | 0.15 | 0.016 | |
| 0.2 | 0.015 | |||
| 0.25 | 0.048 | |||
| 0.3 | 0.016 | |||
| Right Cerebellum Crus 2 | CRBLCrus2.R | 0.1 | 0.022 | |
| 0.25 | 0.015 | |||
| Right Cerebellum 7b | CER7b.R | 0.35 | 0.049 | |
| Left Cerebellum 8 | CER8.L | 0.15 | 0.048 | |
| Left Cerebellum 10 | CER10.L | 0.5 | 0.01 | |
| Vermis 8 | VER8 | 0.2 | 0.033 | |
| 0.25 | 0.034 | |||
| 0.3 | 0.023 | |||
| 0.35 | 0.025 | |||
| 0.4 | 0.022 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sobczak, A.M.; Bohaterewicz, B.; Fafrowicz, M.; Domagalik, A.; Beldzik, E.; Oginska, H.; Golonka, N.; Rekas, M.; Bronicki, D.; Romanowska-Dixon, B.; et al. The Influence of Intraocular Lens Implantation and Alterations in Blue Light Transmittance Level on the Brain Functional Network Architecture Reorganization in Cataract Patients. Brain Sci. 2021, 11, 1400. https://doi.org/10.3390/brainsci11111400
Sobczak AM, Bohaterewicz B, Fafrowicz M, Domagalik A, Beldzik E, Oginska H, Golonka N, Rekas M, Bronicki D, Romanowska-Dixon B, et al. The Influence of Intraocular Lens Implantation and Alterations in Blue Light Transmittance Level on the Brain Functional Network Architecture Reorganization in Cataract Patients. Brain Sciences. 2021; 11(11):1400. https://doi.org/10.3390/brainsci11111400
Chicago/Turabian StyleSobczak, Anna Maria, Bartosz Bohaterewicz, Magdalena Fafrowicz, Aleksandra Domagalik, Ewa Beldzik, Halszka Oginska, Natalia Golonka, Marek Rekas, Dominik Bronicki, Bożena Romanowska-Dixon, and et al. 2021. "The Influence of Intraocular Lens Implantation and Alterations in Blue Light Transmittance Level on the Brain Functional Network Architecture Reorganization in Cataract Patients" Brain Sciences 11, no. 11: 1400. https://doi.org/10.3390/brainsci11111400
APA StyleSobczak, A. M., Bohaterewicz, B., Fafrowicz, M., Domagalik, A., Beldzik, E., Oginska, H., Golonka, N., Rekas, M., Bronicki, D., Romanowska-Dixon, B., Bolsega-Pacud, J., Karwowski, W., Farahani, F. V., & Marek, T. (2021). The Influence of Intraocular Lens Implantation and Alterations in Blue Light Transmittance Level on the Brain Functional Network Architecture Reorganization in Cataract Patients. Brain Sciences, 11(11), 1400. https://doi.org/10.3390/brainsci11111400









