The Inhibition of the Degrading Enzyme Fatty Acid Amide Hydrolase Alters the Activity of the Cone System in the Vervet Monkey Retina
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cécyre, B.; Zabouri, N.; Huppé-Gourgues, F.; Bouchard, J.F.; Casanova, C. Roles of cannabinoid receptors type 1 and 2 on the retinal function of adult mice. Invest. Ophthalmol. Vis. Sci. 2013, 54, 8079–8090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zabouri, N.; Bouchard, J.F.; Casanova, C. Cannabinoid Receptor Type 1 Expression During Postnatal Development of the Rat Retina. J. Comp. Neurol. 2011, 519, 1258–1280. [Google Scholar] [CrossRef]
- Bouchard, J.F.; Casanova, C.; Cecyre, B.; Redmond, W.J. Expression and Function of the Endocannabinoid System in the Retina and the Visual Brain. Neural Plast. 2016, 2016, 9247057. [Google Scholar] [CrossRef] [Green Version]
- Bouskila, J.; Palmour, R.; Bouchard, J.-F.; Ptito, M. The Endocannabinoid System in the Vervet Monkey Retina. Primates 2018, Ch 9, 145–162. [Google Scholar] [CrossRef] [Green Version]
- Straiker, A.; Stella, N.; Piomelli, D.; Mackie, K.; Karten, H.J.; Maguire, G. Cannabinoid CB1 receptors and ligands in vertebrate retina: Localization and Function of an Endogenous Signaling System. Proc. Natl. Acad. Sci. USA 1999, 96, 14565–14570. [Google Scholar] [CrossRef] [Green Version]
- Straiker, A.J.; Maguire, G.; Mackie, K.; Lindsey, J. Localization of Cannabinoid CB1 Receptors in the Human Anterior Eye and Retina. Investig. Ophthalmol. Vis. Sci. 1999, 40, 2442–2448. [Google Scholar]
- Yazulla, S. Endocannabinoids in the Retina: From Marijuana to Neuroprotection. Prog. Retin. Eye Res. 2008, 27, 501–526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouskila, J.; Burke, M.W.; Zabouri, N.; Casanova, C.; Ptito, M.; Bouchard, J.F. Expression and Localization of the Cannabinoid Receptor Type 1 and the Enzyme Fatty Acid Amide Hydrolase in the Retina of Vervet Monkeys. Neuroscience 2012, 202, 117–130. [Google Scholar] [CrossRef] [PubMed]
- Bouskila, J.; Javadi, P.; Casanova, C.; Ptito, M.; Bouchard, J.F. Müller Cells Express the Cannabinoid CB2 Receptor in the Vervet Monkey Retina. J. Comp. Neurol. 2013, 521, 2399–2415. [Google Scholar] [CrossRef]
- Bouskila, J.; Javadi, P.; Casanova, C.; Ptito, M.; Bouchard, J.-F. Rod Photoreceptors Express GPR55 in the Adult Vervet Monkey Retina. PLoS ONE 2013, 8, e81080. [Google Scholar] [CrossRef]
- Bouskila, J.; Javadi, P.; Elkrief, L.; Casanova, C.; Bouchard, J.F.; Ptito, M. A Comparative Analysis of the Endocannabinoid System in the Retina of Mice, Tree Shrews, and Monkeys. Neural Plast. 2016, 2016, 3127658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zabouri, N.; Ptito, M.; Casanova, C.; Bouchard, J.F. Fatty Acid Amide Hydrolase Expression during Retinal Postnatal Development in Rats. Neuroscience 2011, 195, 145–165. [Google Scholar] [CrossRef]
- Bouskila, J.; Micaelo-Fernandes, C.; Palmour, R.M.; Bouchard, J.F.; Ptito, M. Transient Receptor Potential Vanilloid Type 1 is Expressed in the Horizontal Pathway of the Vervet Monkey Retina. Sci. Rep. 2020, 10, 12116. [Google Scholar] [CrossRef]
- Bouskila, J.; Harrar, V.; Javadi, P.; Beierschmitt, A.; Palmour, R.; Casanova, C.; Bouchard, J.-F.; Ptito, M. Cannabinoid Receptors CB1 And CB2 Modulate the Electroretinographic Waves in Vervet Monkeys. Neural Plast. 2016, 2016, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Borowska-Fielding, J.; Murataeva, N.; Smith, B.; Szczesniak, A.-M.; Leishman, E.; Daily, L.; Toguri, J.T.; Hillard, C.J.; Romero, J.; Bradshaw, H. Revisiting Cannabinoid Receptor 2 Expression and Function in Murine Retina. Neuropharmacology 2018, 141, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Bouskila, J.; Harrar, V.; Javadi, P.; Casanova, C.; Hirabayashi, Y.; Matsuo, I.; Ohyama, J.; Bouchard, J.-F.; Ptito, M. Scotopic Vision in the Monkey is Modulated by the G Protein-Coupled Receptor 55. Vis. Neurosci. 2016, 33, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cécyre, B.; Bachand, I.; Papineau, F.; Brochu, C.; Casanova, C.; Bouchard, J.-F. Cannabinoids Affect the Mouse Visual Acuity Via the Cannabinoid Receptor Type 2. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- McCulloch, D.L.; Marmor, M.F.; Brigell, M.G.; Hamilton, R.; Holder, G.E.; Tzekov, R.; Bach, M. ISCEV Standard for Full-Field Clinical Electroretinography (2015 Update). Doc. Ophthalmol. 2015, 130, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Alexander, K.R.; Rajagopalan, A.S.; Raghuram, A.; Fishman, G.A. Activation Phase of Cone Phototransduction and the Flicker Electroretinogram in Retinitis Pigmentosa. Vis. Res. 2006, 46, 2773–2785. [Google Scholar] [CrossRef] [Green Version]
- Massof, R.; Johnson, M.; Sunness, J.; Perry, C.; Finkelstein, D. Flicker Electroretinogram in Retinitis Pigmentosa. Doc. Ophthalmol. 1986, 62, 231–245. [Google Scholar] [CrossRef]
- Sandberg, M.A.; Weigel-DiFranco, C.; Rosner, B.; Berson, E.L. The Relationship Between Visual Field Size and Electroretinogram Amplitude in Retinitis Pigmentosa. Investig. Ophthalmol. Vis. Sci. 1996, 37, 1693–1698. [Google Scholar]
- Verma, R.; Pianta, M.J. The Contribution of Human Cone Photoreceptors to the Photopic Flicker Electroretinogram. J. Vis. 2009, 9, 9. [Google Scholar] [CrossRef] [Green Version]
- Zeitz, C.; Van Genderen, M.; Neidhardt, J.; Luhmann, U.F.; Hoeben, F.; Forster, U.; Wycisk, K.; Mátyás, G.; Hoyng, C.B.; Riemslag, F. Mutations in GRM6 Cause Autosomal Recessive Congenital Stationary Night Blindness with a Distinctive Scotopic 15-Hz Flicker Electroretinogram. Investig. Ophthalmol. Vis. Sci. 2005, 46, 4328–4335. [Google Scholar] [CrossRef] [Green Version]
- Kondo, M.; Sieving, P.A. Primate Photopic Sine-Wave Flicker ERG: Vector Modeling Analysis of Component Origins Using Glutamate Analogs. Investig. Ophthalmol. Vis. Sci. 2001, 42, 305–312. [Google Scholar] [PubMed]
- Kondo, M.; Sieving, P.A. Post-Photoreceptoral Activity Dominates Primate Photopic 32-Hz ERG for Sine-, Square-, and Pulsed Stimuli. Investig. Ophthalmol. Vis. Sci. 2002, 43, 2500–2507. [Google Scholar] [PubMed]
- Behl, T.; Kaur, I.; Kotwani, A. Implication of Oxidative Stress in Progression of Diabetic Retinopathy. Surv. Ophthalmol. 2016, 61, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Cairns, E.A.; Toguri, J.T.; Porter, R.F.; Szczesniak, A.M.; Kelly, M.E. Seeing Over the Horizon—Targeting the Endocannabinoid System for the Treatment of Ocular Disease. J. Basic Clin. Physiol. Pharmacol. 2016, 27, 253–265. [Google Scholar] [CrossRef]
- Matias, I.; Wang, J.W.; Moriello, A.S.; Nieves, A.; Woodward, D.F.; Di Marzo, V. Changes in Endocannabinoid and Palmitoylethanolamide Levels in Eye Tissues of Patients with Diabetic Retinopathy and Age-Related Macular Degeneration. Prostaglandins Leukot. Essent. Fat. Acids 2006, 75, 413–418. [Google Scholar] [CrossRef]
- Rapino, C.; Tortolani, D.; Scipioni, L.; Maccarrone, M. Neuroprotection by (endo) cannabinoids in glaucoma and retinal neurodegenerative diseases. Curr. Neuropharmacol. 2018, 16, 959–970. [Google Scholar] [CrossRef] [PubMed]
- Devane, W.A.; Hanus, L.; Breuer, A.; Pertwee, R.G.; Stevenson, L.A.; Griffin, G.; Gibson, D.; Mandelbaum, A.; Etinger, A.; Mechoulam, R. Isolation and Structure of a Brain Constituent that Binds to the Cannabinoid Receptor. Science 1992, 258, 1946–1949. [Google Scholar] [CrossRef]
- Mechoulam, R.; Ben-Shabat, S.; Hanus, L.; Ligumsky, M.; Kaminski, N.E.; Schatz, A.R.; Gopher, A.; Almog, S.; Martin, B.R.; Compton, D.R. Identification of an Endogenous 2-Monoglyceride, Present in Canine Gut, that Binds to Cannabinoid Receptors. Biochem. Pharmacol. 1995, 50, 83–90. [Google Scholar] [CrossRef]
- Sugiura, T.; Kondo, S.; Sukagawa, A.; Nakane, S.; Shinoda, A.; Itoh, K.; Yamashita, A.; Waku, K. 2-Arachidonoylglycerol: A Possible Endogenous Cannabinoid Receptor Ligand in Brain. Biochem. Biophys. Res. Commun. 1995, 215, 89–97. [Google Scholar] [CrossRef]
- Piomelli, D. The Molecular Logic of Endocannabinoid Signalling. Nat. Rev. Neurosci. 2003, 4, 873–884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cravatt, B.F.; Giang, D.K.; Mayfield, S.P.; Boger, D.L.; Lerner, R.A.; Gilula, N.B. Molecular Characterization of an Enzyme that Degrades Neuromodulatory Fatty-Acid Amides. Nature 1996, 384, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Deutsch, D.G.; Chin, S.A. Enzymatic Synthesis and Degradation of Anandamide, a Cannabinoid Receptor Agonist. Biochem. Pharmacol. 1993, 46, 791–796. [Google Scholar] [CrossRef]
- Miller, S.; Daily, L.; Dharla, V.; Gertsch, J.; Malamas, M.S.; Ojima, I.; Kaczocha, M.; Ogasawara, D.; Straiker, A. Endocannabinoid Metabolism and Transport as Targets to Regulate Intraocular Pressure. Exp. Eye Res. 2020, 201, 108266. [Google Scholar] [CrossRef]
- Mor, M.; Rivara, S.; Lodola, A.; Plazzi, P.V.; Tarzia, G.; Duranti, A.; Tontini, A.; Piersanti, G.; Kathuria, S.; Piomelli, D. Cyclohexylcarbamic Acid 3 ‘-Or 4 ‘-Substituted Biphenyl-3-Yl Esters as Fatty Acid Amide Hydrolase Inhibitors: Synthesis, Quantitative Structure—Activity Relationships, and Molecular Modeling Studies. J. Med. Chem. 2004, 47, 4998–5008. [Google Scholar] [CrossRef] [Green Version]
- Nucci, C.; Gasperi, V.; Cerulli, A.; Spanò, A.; Morrone, L.; Bagetta, G.; Maccarrone, M. Evidence that the Endocannabinoid System is Involved in Retinal Ganglion Cell Death Following High Intraocular Pressure-Induced Ischemia in Rat. Investig. Ophthalmol. Vis. Sci. 2007, 48, 2997–3004. [Google Scholar] [CrossRef] [PubMed]
- Nucci, C.; Gasperi, V.; Tartaglione, R.; Cerulli, A.; Terrinoni, A.; Bari, M.; De Simone, C.; Agro, A.F.; Morrone, L.A.; Corasaniti, M.T. Involvement of the Endocannabinoid System in Retinal Damage after High Intraocular Pressure–Induced Ischemia in Rats. Investig. Ophthalmol. Vis. Sci. 2007, 48, 2997–3004. [Google Scholar] [CrossRef]
- Slusar, J. Examination of the Neuroprotective Effects of URB597 in Young and Aged Rat Retina. Master’s Thesis, Dalhousie University, Halifax, NS, Canada, September 2010. [Google Scholar]
- Slusar, J.E.; Cairns, E.A.; Szczesniak, A.-M.; Bradshaw, H.B.; Di Polo, A.; Kelly, M.E. The Fatty Acid Amide Hydrolase Inhibitor, URB597, Promotes Retinal Ganglion Cell Neuroprotection in a Rat Model of Optic Nerve Axotomy. Neuropharmacology 2013, 72, 116–125. [Google Scholar] [CrossRef]
- Su, S.-H.; Wu, Y.-F.; Lin, Q.; Yu, F.; Hai, J. Cannabinoid Receptor Agonist WIN55, 212–2 and Fatty Acid Amide Hydrolase Inhibitor URB597 Suppress Chronic Cerebral Hypoperfusion-Induced Neuronal Apoptosis by Inhibiting C-Jun N-Terminal Kinase Signaling. Neuroscience 2015, 301, 563–575. [Google Scholar] [CrossRef] [PubMed]
- Laprevote, V.; Schwitzer, T.; Giersch, A.; Schwan, R. Flash Electroretinogram and Addictive Disorders. Prog. Neuropsychopharmacol. Biol. Psychiatry 2015, 56, 264. [Google Scholar] [CrossRef] [PubMed]
- Schwitzer, T.; Schwan, R.; Angioi-Duprez, K.; Giersch, A.; Lalanne, L.; Albuisson, E.; Laprevote, V. Delayed Bipolar and Ganglion Cells Neuroretinal Processing in Regular Cannabis Users: The Retina as a Relevant Site to Investigate Brain Synaptic Transmission Dysfunctions. J. Psychiatr. Res. 2018, 103, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Schwitzer, T.; Schwan, R.; Angioi-Duprez, K.; Giersch, A.; Laprevote, V. The Endocannabinoid System in the Retina: From Physiology to Practical and Therapeutic Applications. Neural Plast. 2016, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Schwitzer, T.; Schwan, R.; Angioi-Duprez, K.; Ingster-Moati, I.; Lalanne, L.; Giersch, A.; Laprevote, V. The Cannabinoid System and Visual Processing: A Review on Experimental Findings and Clinical Presumptions. Eur. Neuropsychopharmacol. 2015, 25, 100–112. [Google Scholar] [CrossRef]
- Bouskila, J.; Javadi, P.; Palmour, R.M.; Bouchard, J.F.; Ptito, M. Standardized Full-Field Electroretinography in the Green Monkey (Chlorocebus sabaeus). PLoS ONE 2014, 9, e111569. [Google Scholar] [CrossRef] [Green Version]
- Severns, M.L.; Johnson, M.A.; Merritt, S.A. Automated Estimation of Implicit Time and Amplitude from the Flicker Electroretinogram. Appl. Opt. 1991, 30, 2106–2112. [Google Scholar] [CrossRef]
- Heck, J. The Flicker Electroretinogram of the Human Eye. Acta Physiol. Scand. 1957, 39, 158–166. [Google Scholar] [CrossRef]
- Di Marzo, V. Targeting the Endocannabinoid System: To Enhance or Reduce? Nat. Rev. Drug Discov. 2008, 7, 438–455. [Google Scholar] [CrossRef] [PubMed]
- Lauckner, J.E.; Jensen, J.B.; Chen, H.Y.; Lu, H.C.; Hille, B.; Mackie, K. GPR55 is a Cannabinoid Receptor that Increases Intracellular Calcium and Inhibits M Current. Proc. Natl. Acad. Sci. USA 2008, 105, 2699–2704. [Google Scholar] [CrossRef] [Green Version]
- Silverman, C.A.; Yoshizumi, M.O. Ocular Toxicity of Experimental Intravitreal DMSO. J. Toxicol. Cutaneous Ocul. Toxicol. 1983, 2, 193–200. [Google Scholar] [CrossRef]
- Cristino, L.; Bisogno, T.; Di Marzo, V. Cannabinoids and the Expanded Endocannabinoid System in Neurological Disorders. Nat. Rev. Neurol. 2020, 16, 9–29. [Google Scholar] [CrossRef] [PubMed]
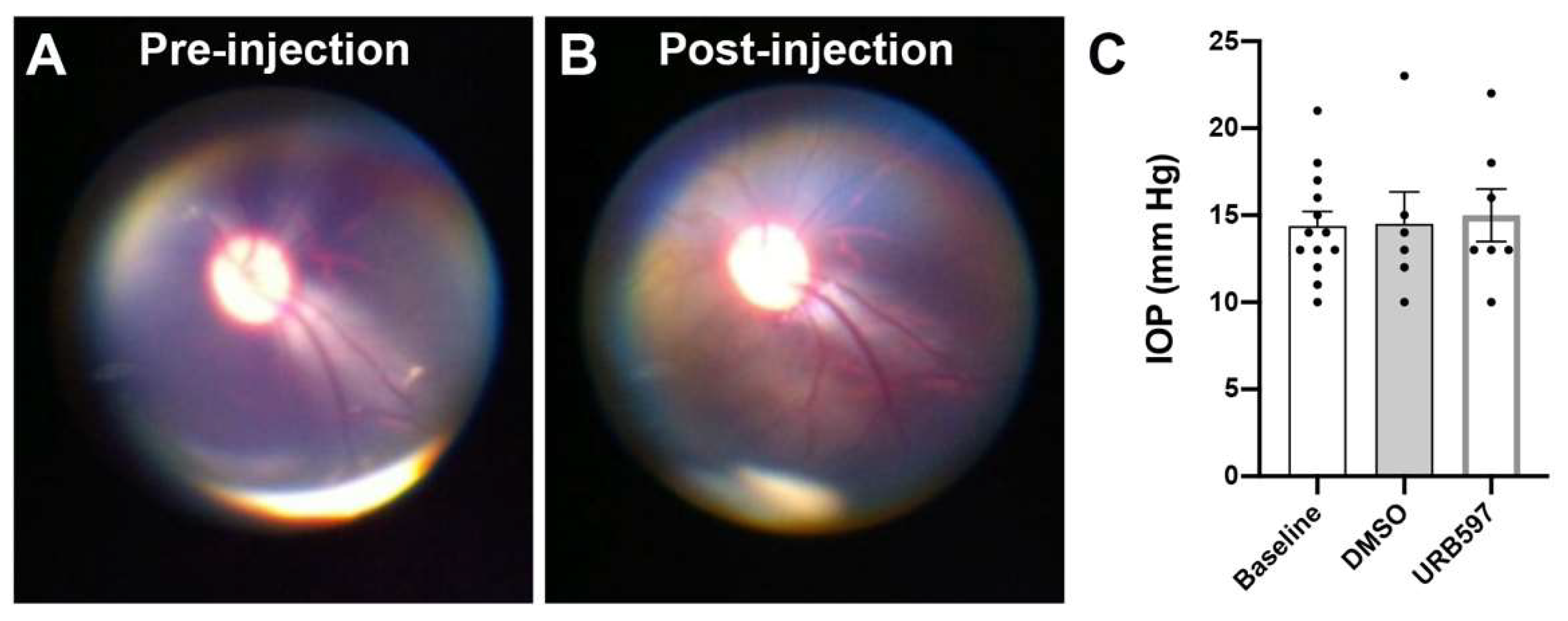

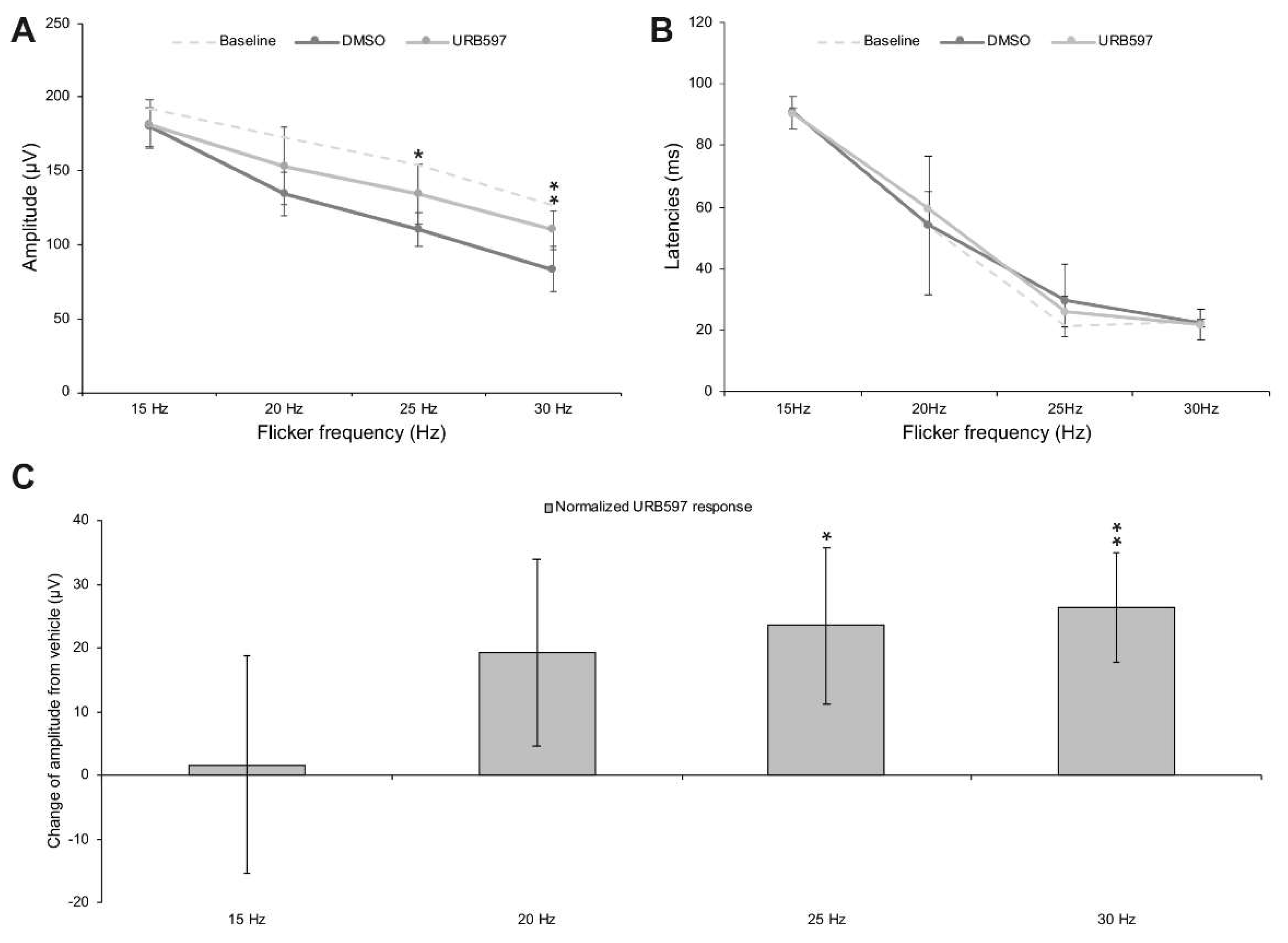

| Animal Subjects | Drug | |||
|---|---|---|---|---|
| ID | Sex | Weight | ||
| 1 | o2011-3-7 | female | 3.33 | Vehicle |
| 2 | o1097-1-3-8 | female | 3.23 | Vehicle |
| 3 | o1787-4-5-4 | female | 3.48 | Vehicle |
| 4 | o1085-7-2-1 | female | 3.36 | Vehicle |
| 5 | o1842-6-5-3 | male | 4.28 | Vehicle |
| 6 | o1986-2-0-5 | male | 3.29 | Vehicle |
| 7 | o1085-7-7 | female | 3.03 | URB597 |
| 8 | o1313-1-2-2-3-2 | female | 2.87 | URB597 |
| 9 | o1669-3-7-1 | female | 2.95 | URB597 |
| 10 | o1645-1-7-2 | female | 2.7 | URB597 |
| 11 | o1083-13-8 | female | 3.06 | URB597 |
| 12 | o1842-4-2-1-2 | male | 3.1 | URB597 |
| 13 | o8711-8-4-1 | male | 2.8 | URB597 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouskila, J.; Bleau, M.; Micaelo-Fernandes, C.; Bouchard, J.-F.; Ptito, M. The Inhibition of the Degrading Enzyme Fatty Acid Amide Hydrolase Alters the Activity of the Cone System in the Vervet Monkey Retina. Brain Sci. 2021, 11, 1418. https://doi.org/10.3390/brainsci11111418
Bouskila J, Bleau M, Micaelo-Fernandes C, Bouchard J-F, Ptito M. The Inhibition of the Degrading Enzyme Fatty Acid Amide Hydrolase Alters the Activity of the Cone System in the Vervet Monkey Retina. Brain Sciences. 2021; 11(11):1418. https://doi.org/10.3390/brainsci11111418
Chicago/Turabian StyleBouskila, Joseph, Maxime Bleau, Catarina Micaelo-Fernandes, Jean-François Bouchard, and Maurice Ptito. 2021. "The Inhibition of the Degrading Enzyme Fatty Acid Amide Hydrolase Alters the Activity of the Cone System in the Vervet Monkey Retina" Brain Sciences 11, no. 11: 1418. https://doi.org/10.3390/brainsci11111418
APA StyleBouskila, J., Bleau, M., Micaelo-Fernandes, C., Bouchard, J.-F., & Ptito, M. (2021). The Inhibition of the Degrading Enzyme Fatty Acid Amide Hydrolase Alters the Activity of the Cone System in the Vervet Monkey Retina. Brain Sciences, 11(11), 1418. https://doi.org/10.3390/brainsci11111418








