Can Evoked Potential Changes during the Superficial Temporal Artery-Middle Cerebral Artery Bypass Surgery Predict Postoperative Improvement of Cerebral Perfusion and Functional Status?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Inclusion and Clinical Assessment
2.2. Surgical Procedures and Anesthesia
2.3. Intraoperative Neurophysiological Monitoring Protocol
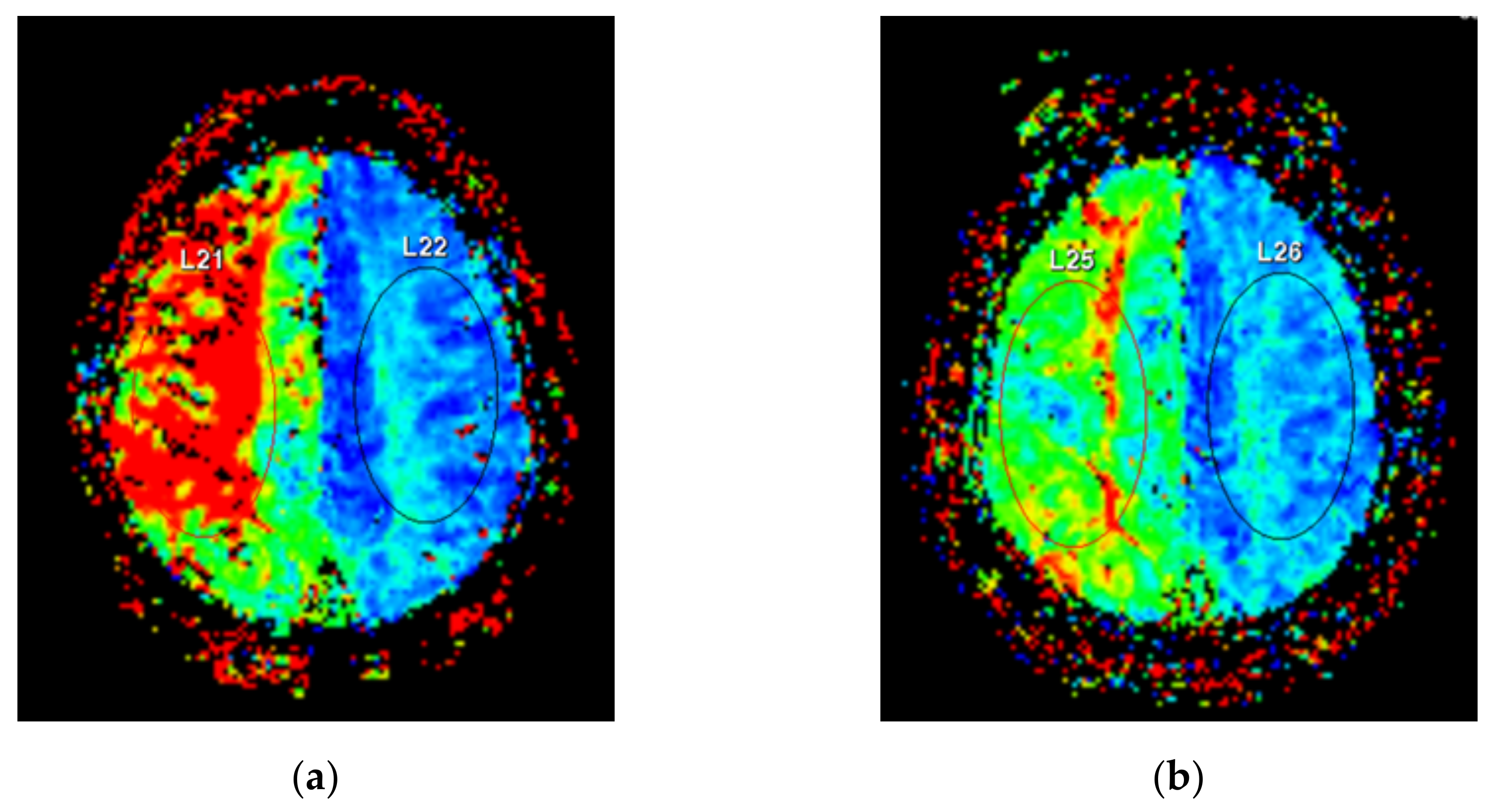
2.4. Perfusion-Weighted Imaging Protocol
ΔTTP = Preoperative TTP − Postoperative TTP
MTT AI = affected side MTT/unaffected side MTT
TTP AI = affected side TTP/unaffected side TTP
ΔMTT AI = ((Preoperative MTT AI − Postoperative MTT AI)/Preoperative MTT AI) × 100
ΔTTP AI = ((Preoperative TTP AI − Postoperative TTP AI)/Preoperative TTP AI) × 100
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics and Propensity Score Matching
3.2. Comparison of EP Changes between the MB and MC Group
3.3. Changes in Examined Parameters and Their Correlations in the MB Group
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stapleton, C.J.; Charbel, F.T. Protective STA-MCA bypass to prevent brain ischemia during high-flow bypass surgery: Case series of 10 patients. Acta Neurochir. 2019, 161, 1205–1206. [Google Scholar] [CrossRef] [Green Version]
- Meybodi, A.T.; Huang, W.; Benet, A.; Kola, O.; Lawton, M.T. Bypass surgery for complex middle cerebral artery aneurysms: An algorithmic approach to revascularization. J. Neurosurg. 2017, 127, 463–479. [Google Scholar] [CrossRef]
- Pandey, P.; Steinberg, G.K. Neurosurgical Advances in the Treatment of Moyamoya Disease. Stroke 2011, 42, 3304–3310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sekhar, L.N.; Natarajan, S.K.; Ellenbogen, R.G.; Ghodke, B. Cerebral revascularization for ischemia, aneurysms, and cranial base tumors. Neurosurgery 2008, 62, SHC1373–SHC1410. [Google Scholar] [CrossRef] [PubMed]
- Soufiany, I.; Hijrat, K.A.; Soufiany, S.; Chen, L. Bypass Surgery for Ischemic Stroke Caused by Intracranial Artery Stenosis or Occlusion. Brain Sci. Adv. 2018, 4, 49–60. [Google Scholar] [CrossRef]
- Pandey, P.; Ambekar, S.; Babu, A.; Devi, I.B. Intraoperative assessment of STA-MCA bypass patency using near-infrared indocyanine green video-angiography: A preliminary study. Neurol. India 2012, 60, 604–607. [Google Scholar] [CrossRef]
- Dengler, J.; Cabraja, M.; Faust, K.; Picht, T.; Kombos, T.; Vajkoczy, P. Intraoperative neurophysiological monitoring of extracranial-intracranial bypass procedures. J. Neurosurg. 2013, 119, 207–214. [Google Scholar] [CrossRef]
- Della Puppa, A.; Rossetto, M.; Volpin, F.; Rustemi, O.; Grego, A.; Gerardi, A.; Ortolan, R.; Causin, F.; Munari, M.; Scienza, R. Microsurgical Clipping of Intracranial Aneurysms Assisted by Neurophysiological Monitoring, Microvascular Flow Probe, and ICG-VA: Outcomes and Intraoperative Data on a Multimodal Strategy. World Neurosurg. 2018, 113, e336–e344. [Google Scholar] [CrossRef] [PubMed]
- Caramia, F.; Santoro, A.; Pantano, P.; Passacantilli, E.; Guidetti, G.; Pierallini, A.; Fantozzi, L.M.; Cantore, G.P.; Bozzao, L. Cerebral Hemodynamics on MR Perfusion Images before and after Bypass Surgery in Patients with Giant Intracranial Aneurysms. Am. J. Neuroradiol. 2001, 22, 1704–1710. [Google Scholar]
- Bozzao, A.; Fasoli, F.; Finocchi, V.; Santoro, G.; Romano, A.; Fantozzi, L.M. Long term evaluation of brain perfusion with magnetic resonance in high flow extracranial-intracranial saphenous graft bypass. Eur. Radiol. 2006, 17, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhou, P.; Xiong, Z.; Ma, Z.; Wang, S.; Bian, H.; Chen, J. Perfusion-Weighted Magnetic Resonance Imaging Used in Assessing Hemodynamics following Superficial Temporal Artery-Middle Cerebral Artery Bypass in Patients with Moyamoya Disease. Cerebrovasc. Dis. 2013, 35, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kim, D.Y.; Bin Kim, S.; Kim, W.; Kang, M.-R.; Kim, H.-J.; Lee, K.H.; Yoo, M.; Choi, B.-S.; Kim, J.S.; et al. Predictive value of neurophysiologic monitoring during neurovascular intervention for postoperative new neurologic deficits. Neuroradiology 2018, 61, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Park, D.; Kim, B.H.; Lee, S.-E.; Jeong, E.; Cho, K.; Park, J.K.; Choi, Y.-J.; Jin, S.; Hong, D.; Kim, M.-C. Usefulness of Intraoperative Neurophysiological Monitoring During the Clipping of Unruptured Intracranial Aneurysm: Diagnostic Efficacy and Detailed Protocol. Front. Surg. 2021, 8, 631053. [Google Scholar] [CrossRef]
- Jo, K.-I.; Kim, H.R.; Yeon, J.Y.; Hong, S.-C.; Kim, J.-S. Treatment outcomes of surgical clipping for unruptured anterior circulation aneurysm—single institute experiences in the era of neurophysiologic monitoring and endovascular treatment. Neurosurg. Rev. 2015, 38, 677–682. [Google Scholar] [CrossRef]
- Zhu, F.; Chui, J.; Herrick, I.; Martin, J. Intraoperative evoked potential monitoring for detecting cerebral injury during adult aneurysm clipping surgery: A systematic review and meta-analysis of diagnostic test accuracy. BMJ Open 2019, 9, e022810. [Google Scholar] [CrossRef] [Green Version]
- Chung, J.; Park, W.; Hong, S.H.; Park, J.C.; Ahn, J.S.; Kwun, B.D.; Lee, S.-A.; Kim, S.-H.; Jeon, J.-Y. Intraoperative use of transcranial motor/sensory evoked potential monitoring in the clipping of intracranial aneurysms: Evaluation of false-positive and false-negative cases. J. Neurosurg. 2019, 130, 936–948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akbari, K.K.; Badikillaya, V.; Venkatesan, M.; Hegde, S.K. Do Intraoperative Neurophysiological Changes During Decompressive Surgery for Cervical Myeloradiculopathy Affect Functional Outcome? A Prospective Study. Glob. Spine J. 2020, 2192568220951779. [Google Scholar] [CrossRef]
- Clark, A.J.; Ziewacz, J.E.; Safaee, M.; Lau, D.; Lyon, R.; Chou, D.; Weinstein, P.R.; Ames, C.P.; Clark, J.P.; Mummaneni, P.V. Intraoperative neuromonitoring with MEPs and prediction of postoperative neurological deficits in patients undergoing surgery for cervical and cervicothoracic myelopathy. Neurosurg. Focus 2013, 35, E7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greve, T.; Wagner, A.; Ille, S.; Wunderlich, S.; Ikenberg, B.; Meyer, B.; Zimmer, C.; Shiban, E.; Kreiser, K. Motor evoked potentials during revascularization in ischemic stroke predict motor pathway ischemia and clinical outcome. Clin. Neurophysiol. 2020, 131, 2307–2314. [Google Scholar] [CrossRef]
- Lee, Y.; Park, D.; Kim, B.H.; Lee, S.E.; Lee, J.W. Changes in Evoked Potentials during Superficial Temporal Artery-Middle Cerebral Artery Bypass Surgery: A Case Series Report. J. Electrodiagn. Neuromuscul. Dis. 2020, 22, 109–114. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, G.; Huang, G.; Wang, Z.; Tan, H.; Liu, J.; Li, A. Intraoperative Combined Use of Somatosensory Evoked Potential, Microvascular Doppler Sonography, and Indocyanine Green Angiography in Clipping of Intracranial Aneurysm. Med. Sci. Monit. 2016, 22, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Ho, D.E.; Imai, K.; King, G.; Stuart, E. MatchIt: Nonparametric Preprocessing for Parametric Causal Inference. J. Stat. Softw. 2011, 42, 1–28. [Google Scholar] [CrossRef] [Green Version]
- Shiban, E.; Wunderlich, S.; Kreiser, K.; Lehmberg, J.; Hemmer, B.; Prothmann, S.; Zimmer, C.; Meyer, B.; Ringel, F. Predictive value of transcranial evoked potentials during mechanical endovascular therapy for acute ischaemic stroke: A feasibility study. J. Neurol. Neurosurg. Psychiatry 2016, 87, 598–603. [Google Scholar] [CrossRef]
- Park, M.K.; Lee, S.J.; Kim, S.B.; Lee, K.W.; Lee, H.-J.; Han, E.Y.; Kim, B.R. The effect of positive changes during intraoperative monitoring of the functional improvement in patients with cervical compressive myelopathy. Clin. Interv. Aging 2018, 13, 1211–1218. [Google Scholar] [CrossRef] [Green Version]
- Esposito, G.; Amin-Hanjani, S.; Regli, L. Role of and Indications for Bypass Surgery After Carotid Occlusion Surgery Study (COSS)? Stroke 2016, 47, 282–290. [Google Scholar] [CrossRef]
- Rodríguez-Hernández, A.; Josephson, S.A.; Lawton, M.T. Bypass surgery for the prevention of ischemic stroke: Current indications and techniques. Neurocirugía 2012, 23, 5–14. [Google Scholar] [CrossRef]
- Horiuchi, K.; Suzuki, K.; Sasaki, T.; Matsumoto, M.; Sakuma, J.; Konno, Y.; Oinuma, M.; Itakura, T.; Kodama, N. Intraoperative monitoring of blood flow insufficiency during surgery of middle cerebral artery aneurysms. J. Neurosurg. 2005, 103, 275–283. [Google Scholar] [CrossRef]
- Neuloh, G.; Schramm, J. Monitoring of motor evoked potentials compared with somatosensory evoked potentials and microvascular Doppler ultrasonography in cerebral aneurysm surgery. J. Neurosurg. 2004, 100, 389–399. [Google Scholar] [CrossRef]
- Holland, N.R. Subcortical Strokes From Intracranial Aneurysm Surgery: Implications for Intraoperative Neuromonitoring. J. Clin. Neurophysiol. 1998, 15, 439–446. [Google Scholar] [CrossRef]
- Gross, B.A.; Du, R. STA-MCA bypass. Acta Neurochir. 2012, 154, 1463–1467. [Google Scholar] [CrossRef] [PubMed]
- Aboukais, R.; Verbraeken, B.; Leclerc, X.; Gautier, C.; Vermandel, M.; Bricout, N.; Lejeune, J.-P.; Menovsky, T. Protective STA-MCA bypass to prevent brain ischemia during high-flow bypass surgery: Case series of 10 patients. Acta Neurochir. 2019, 161, 1207–1214. [Google Scholar] [CrossRef] [PubMed]
- Bogousslavsky, J.; Regli, F. Centrurn ovale infarcts: Subcortical infarction in the superficial territory of the middle cerebral artery. Neurology 1992, 42, 1992. [Google Scholar] [CrossRef] [PubMed]
- Newell, D.W. Superficial Temporal Artery to Middle Cerebral Artery Bypass. Semin. Neurol. 2005, 15, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.H.; Lee, J.J.; Lee, S.M.; Park, M.N.; Park, S.K.; Seo, D.W.; Chung, I.S. Comparison of motor-evoked potentials monitoring in response to transcranial electrical stimulation in subjects undergoing neurosurgery with partial vs no neuromuscular block. Br. J. Anaesth. 2013, 110, 567–576. [Google Scholar] [CrossRef] [Green Version]
- Choi, H.H.; Ha, E.J.; Cho, W.-S.; Kang, H.-S.; Kim, J.E. Effectiveness and Limitations of Intraoperative Monitoring with Combined Motor and Somatosensory Evoked Potentials During Surgical Clipping of Unruptured Intracranial Aneurysms. World Neurosurg. 2017, 108, 738–747. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Zhao, J.; Zhao, Y.; Zhang, D.; Wang, R.; Qiao, H.; Wang, S. Multiple Intraoperative Monitoring-Assisted Microneurosurgical Treatment for Anterior Circulation Cerebral Aneurysm. J. Int. Med. Res. 2011, 39, 891–903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.; Robinson, K.; Lodge, M.; Theroux, M.; Miller, F.; Akins, R. Resistance to Neuromuscular Blockade by Rocuronium in Surgical Patients with Spastic Cerebral Palsy. J. Pers. Med. 2021, 11, 765. [Google Scholar] [CrossRef] [PubMed]
- Lyon, R.; Feiner, J.; Lieberman, J.A. Progressive suppression of motor evoked potentials during general anesthesia: The phenomenon of “anesthetic fade”. J. Neurosurg. Anesthesiol. 2005, 17, 13–19. [Google Scholar] [PubMed]
- Ugawa, R.; Takigawa, T.; Shimomiya, H.; Ohnishi, T.; Kurokawa, Y.; Oda, Y.; Shiozaki, Y.; Misawa, H.; Tanaka, M.; Ozaki, T. An evaluation of anesthetic fade in motor evoked potential monitoring in spinal deformity surgeries. J. Orthop. Surg. Res. 2018, 13, 227. [Google Scholar] [CrossRef]
- Zaro-Weber, O.; Moeller-Hartmann, W.; Siegmund, D.; Kandziora, A.; Schuster, A.; Heiss, W.-D.; Sobesky, J. MRI-based mismatch detection in acute ischemic stroke: Optimal PWI maps and thresholds validated with PET. Br. J. Pharmacol. 2016, 37, 3176–3183. [Google Scholar] [CrossRef] [Green Version]
- Meagher, R.; Shankar, J.J.S. CT Perfusion in Acute Stroke: “Black Holes” on Time-to-Peak Image Maps Indicate Unsalvageable Brain. J. Neuroimaging 2016, 26, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xu, W.; Zhanguo, S.; Shi, Z.; Sun, Z.; Gao, L.; Jin, F.; Wang, J.; Chen, W.; Yang, Y. CT perfusion assessment of Moyamoya syndrome before and after direct revascularization (superficial temporal artery to middle cerebral artery bypass). Eur. Radiol. 2016, 26, 254–261. [Google Scholar] [CrossRef]
- Moody, D.M.; A Bell, M.; Challa, V.R. Features of the cerebral vascular pattern that predict vulnerability to perfusion or oxygenation deficiency: An anatomic study. Am. J. Neuroradiol. 1990, 11, 431–439. [Google Scholar] [PubMed]
- Kluytmans, M.; Van Der Grond, J.; Folkers, P.J.M.; Mali, W.P.T.M.; Viergever, M.A. Differentiation of gray matter and white matter perfusion in patients with unilateral internal carotid artery occlusion. J. Magn. Reson. Imaging 1998, 8, 767–774. [Google Scholar] [CrossRef]
- Yamauchi, H.; Fukuyama, H.; Nagahama, Y.; Katsumi, Y.; Hayashi, T.; Okazawa, H.; Yonekura, Y. Selective Cerebral Hematocrit Decrease in the Centrum Semiovale after Carotid Artery Occlusion: A PET Study. Br. J. Pharmacol. 1999, 19, 109–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| MC Group | MB Group | ||||
|---|---|---|---|---|---|
| Total (n = 154) | p | PSM (n = 22) | p | (n = 22) | |
| Age, years | 61.5 ± 8.9 | 0.074 | 61.5 ± 10.1 | 0.233 | 65.2 ± 10.4 |
| Sex, male (%) | 41 (26.6) | 0.255 | 9 (40.9) | >0.999 | 9 (40.9) |
| Side, right (%) | 99 (64.3) | 0.023 | 10 (45.5) | 0.759 | 8 (36.4) |
| Vascular risk factors | |||||
| Hypertension, n (%) | 80 (51.9) | 0.005 | 18 (81.8) | >0.999 | 19 (86.4) |
| Diabetes, n (%) | 17 (11.0) | <0.001 | 9 (40.9) | 0.546 | 12 (54.5) |
| Hyperlipidemia, n (%) | 33 (21.4) | 0.255 | 4 (18.2) | 0.664 | 2 (9.1) |
| Cardiac problemsa, n (%) | 10 (6.5) | 0.211 | 3 (13.6) | >0.999 | 3 (13.6) |
| Smoking, n (%) | 21 (13.6) | 0.114 | 6 (27.3) | >0.999 | 6 (27.3) |
| Time-related factors | |||||
| TOD, min | 210.1 ± 54.5 | <0.001 | 242.7 ± 56.3 | 0.060 | 273.4 ± 48.8 |
| TBE, min | 57.0 (47.0, 64.0) | <0.001 | 69.0 (60.0, 85.0) | 0.018 | 85.0 (73.0, 99.0) |
| EPI, min | 153.3 ± 49.5 | <0.001 | 170.8 ± 51.1 | 0.331 | 183.7 ± 34.4 |
| MB Group (n = 22) | MC Group (n = 22) | p-Value | |
|---|---|---|---|
| ΔMedian SSEP (%) | 13.8 (0.6, 41.3) | −4.3 (−19.8, 11.7) | 0.027 |
| ΔTibial SSEP (%) | 9.3 ± 26.8 | 1.5 ± 23.7 | 0.318 |
| ΔAPB-MEP (%) | 20.7 (5.6, 71.6) | 2.5 (−14.5, 12.9) | 0.006 |
| ΔAH-MEP (%) | 29.2 (8.6, 55.2) | 9.7 (−5.2, 28.2) | 0.015 |
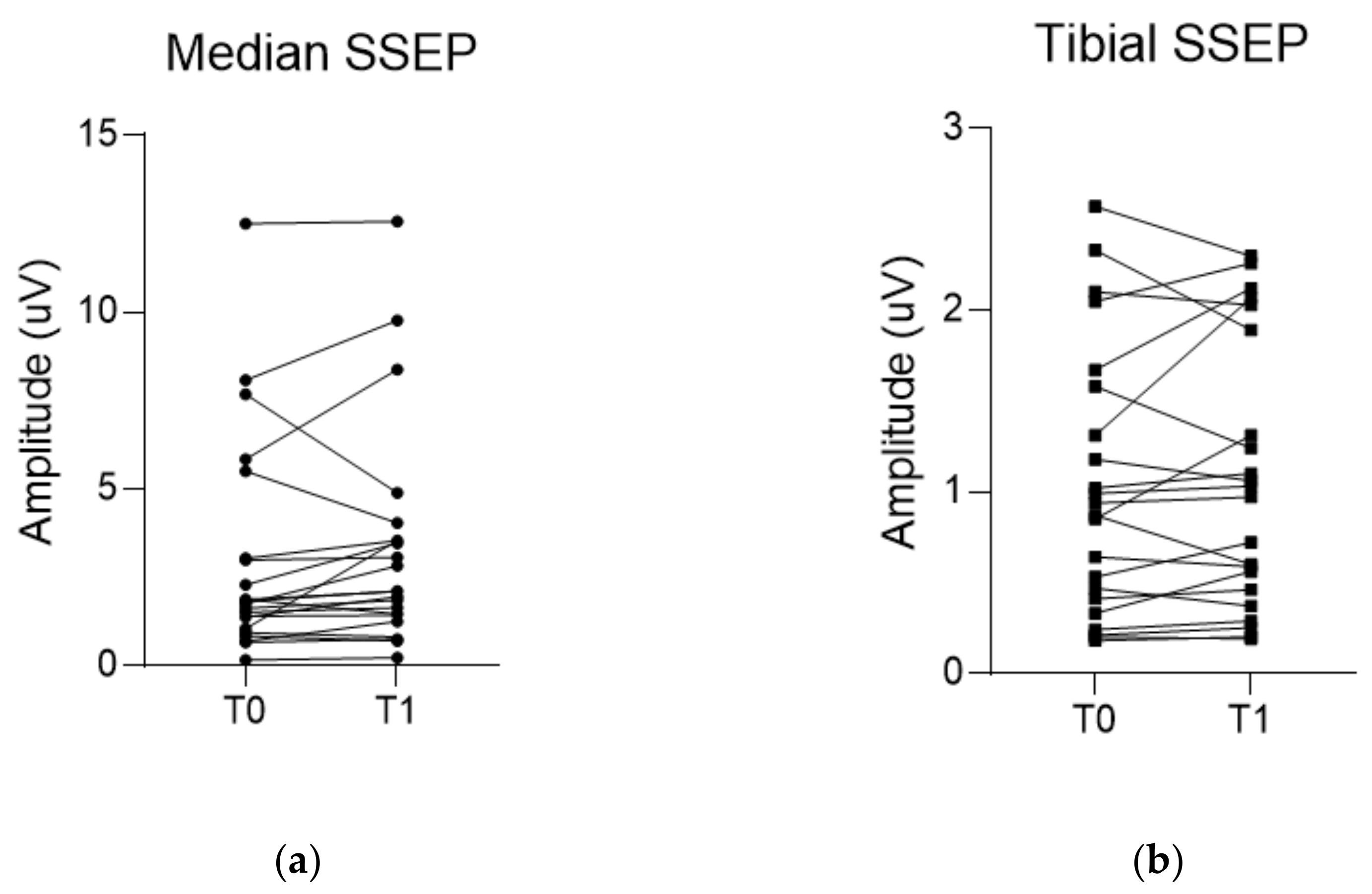
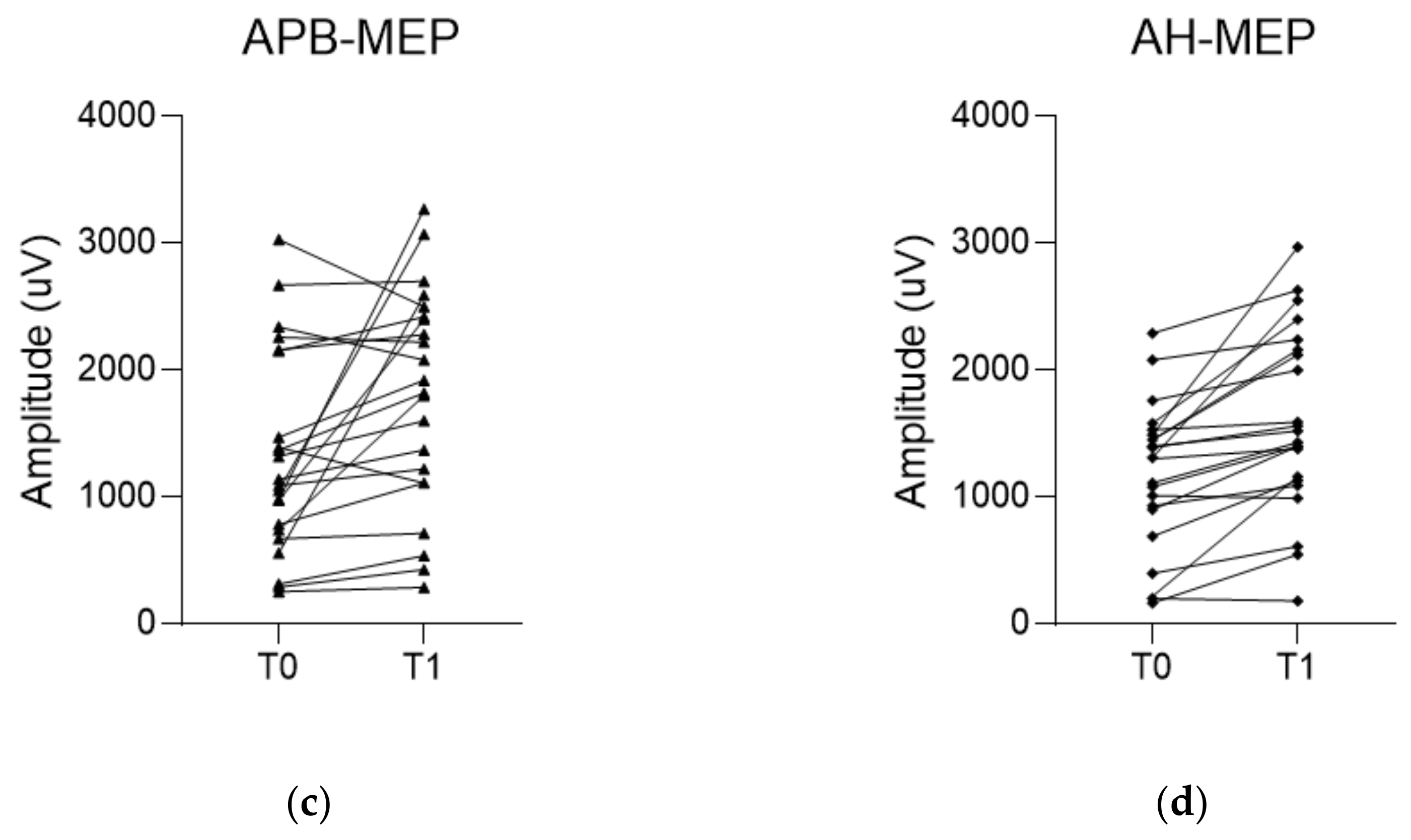
| T0 a | T1 b | p-Value | |
|---|---|---|---|
| Median SSEP (µV) | 1.8 (1.0, 3.0) | 2.1 (1.4, 3.5) | 0.051 |
| Tibial SSEP (µV) | 0.9 (0.4, 1.6) | 1.0 (0.5, 1.9) | 0.604 |
| APB-MEP (µV) | 1318.9 ± 796.1 | 1793.2 ± 856.0 | 0.010 |
| AH-MEP (µV) | 1169.9 ± 576.2 | 1593.8 ± 721.6 | <0.001 |
| MTT (s) | 12.4 (10.1, 14.1) | 10.7 (9.7, 12.6) | 0.026 |
| TTP (s) | 32.4 ± 6.3 | 29.8 ± 5.8 | 0.012 |
| MTT AI c | 1.2 (1.1, 1.5) | 1.1 (1.1, 1.2) | 0.010 |
| TTP AI c | 1.1 (1.1, 1.2) | 1.1 (1.0, 1.1) | <0.001 |
| mRS, n (%) | <0.001 | ||
| 0 | 0 (0.0) | 1 (4.5) | |
| 1 | 0 (0.0) | 9 (40.9) | |
| 2 | 5 (22.7) | 6 (27.3) | |
| 3 | 7 (31.8) | 3 (13.6) | |
| 4 | 10 (45.5) | 3 (13.6) |
| ΔMTT a (s) | ΔTTP a (s) | ΔMTT AI b (%) | ΔTTP AI b (%) | ΔmRS c at 1 M | ΔmRS c at 6 M | |
|---|---|---|---|---|---|---|
| ΔMedian SSEP d (%) | 0.102 (0.651) | 0.194 (0.388) | −0.069 (0.759) | 0.112 (0.619) | 0.374 (0.087) | 0.422 (0.050) |
| ΔTibial SSEP d (%) | −0.139 (0.536) | −0.108 (0.633) | −0.124 (0.584) | 0.130 (0.563) | −0.060 (0.794) | −0.116 (0.608) |
| ΔAPB-MEP d (%) | 0.429 (0.047) | 0.043 (0.848) | 0.348 (0.112) | 0.573 (0.005) | 0.514 (0.015) | 0.271 (0.222) |
| ΔAH-MEP d (%) | 0.415 (0.055) | 0.325 (0.140) | 0.344 (0.117) | 0.617 (0.002) | 0.332 (0.131) | 0.183 (0.416) |
| ΔMTT a (s) | ΔTTP a (s) | ΔMTT AI b (%) | ΔTTP AI b (%) | |||||
|---|---|---|---|---|---|---|---|---|
| β ± SE | p | β ± SE | p | β ± SE | p | β ± SE | p | |
| ΔMedian SSEPc (%) | −0.080 ± 0.083 | 0.346 | 0.007 ± 0.051 | 0.890 | −0.032 ± 0.063 | 0.618 | −0.156 ± 0.026 | 0.562 |
| ΔTibial SSEP c (%) | −0.164 ± 0.173 | 0.355 | −0.072 ± 0.106 | 0.508 | −0.082 ± 0.131 | 0.533 | −0.014 ± 0.056 | 0.808 |
| ΔAPB-MEP c (%) | 0.021 ± 0.049 | 0.667 | 0.001 ± 0.030 | 0.975 | 0.025 ± 0.036 | 0.505 | 0.010 ± 0.015 | 0.532 |
| ΔAH-MEP c (%) | 0.034 ± 0.048 | 0.487 | 0.019 ± 0.029 | 0.532 | 0.013 ± 0.036 | 0.727 | 0.012 ± 0.015 | 0.449 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, D.; Jin, S.; Kim, Y.; Choi, Y.-J.; Hong, D.; Kim, B.H.; Lee, S.-E.; Cho, K.; Park, J.K.; Kim, M.-C. Can Evoked Potential Changes during the Superficial Temporal Artery-Middle Cerebral Artery Bypass Surgery Predict Postoperative Improvement of Cerebral Perfusion and Functional Status? Brain Sci. 2021, 11, 1478. https://doi.org/10.3390/brainsci11111478
Park D, Jin S, Kim Y, Choi Y-J, Hong D, Kim BH, Lee S-E, Cho K, Park JK, Kim M-C. Can Evoked Potential Changes during the Superficial Temporal Artery-Middle Cerebral Artery Bypass Surgery Predict Postoperative Improvement of Cerebral Perfusion and Functional Status? Brain Sciences. 2021; 11(11):1478. https://doi.org/10.3390/brainsci11111478
Chicago/Turabian StylePark, Dougho, Suntak Jin, Youngsoo Kim, Yeon-Ju Choi, Daeyoung Hong, Byung Hee Kim, Sang-Eok Lee, Kwansang Cho, Ji Kang Park, and Mun-Chul Kim. 2021. "Can Evoked Potential Changes during the Superficial Temporal Artery-Middle Cerebral Artery Bypass Surgery Predict Postoperative Improvement of Cerebral Perfusion and Functional Status?" Brain Sciences 11, no. 11: 1478. https://doi.org/10.3390/brainsci11111478
APA StylePark, D., Jin, S., Kim, Y., Choi, Y.-J., Hong, D., Kim, B. H., Lee, S.-E., Cho, K., Park, J. K., & Kim, M.-C. (2021). Can Evoked Potential Changes during the Superficial Temporal Artery-Middle Cerebral Artery Bypass Surgery Predict Postoperative Improvement of Cerebral Perfusion and Functional Status? Brain Sciences, 11(11), 1478. https://doi.org/10.3390/brainsci11111478






