Time-Dependent Analysis of Plasmalogens in the Hippocampus of an Alzheimer’s Disease Mouse Model: A Role of Ethanolamine Plasmalogen
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Animals
2.3. Phospholipid Extraction
2.4. Liquid Chromatography-Targeted Selected Reaction Monitoring Mass Spectrometry (LC-SRM/MS) Analysis
2.5. Tissue Preparation for Immunohistochemistry
2.6. Immunofluorescence Staining
2.7. Western Blotting Analysis
2.8. Reactive Oxygen Species (ROS) Level Measurement
2.9. Statistical Analyses
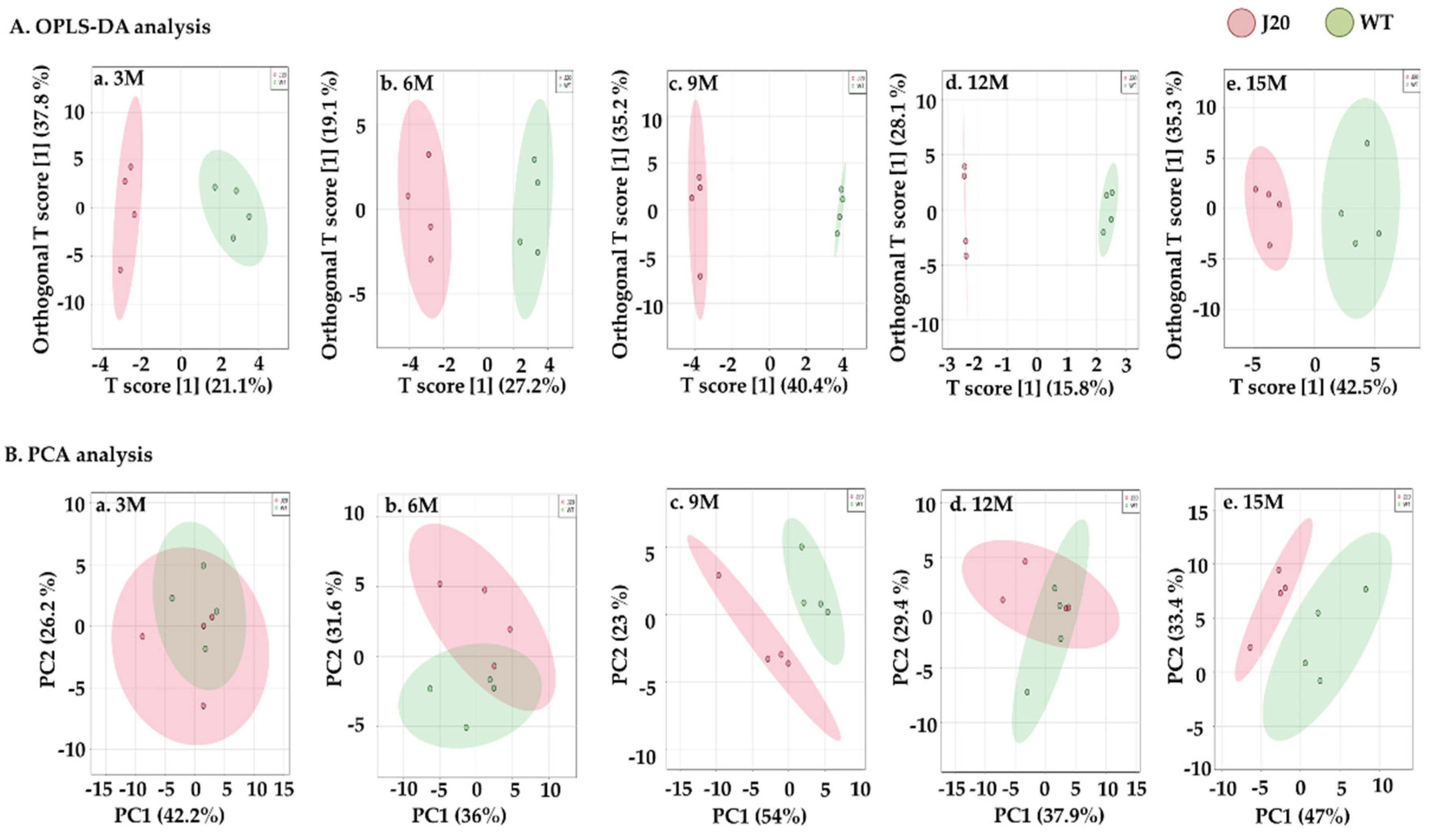
3. Results
3.1. Total Pls-PE, Alkyl-Acyl-PE, Pls-PC, and Alkyl-Acyl-PC in the Hippocampus of J20 and WT Mice
3.2. Molecular Species of Pls-PE, Alkyl-Acyl-PE, Pls-PC, and Alkyl-Acyl-PC in the Hippocampus of J20 and WT Mice
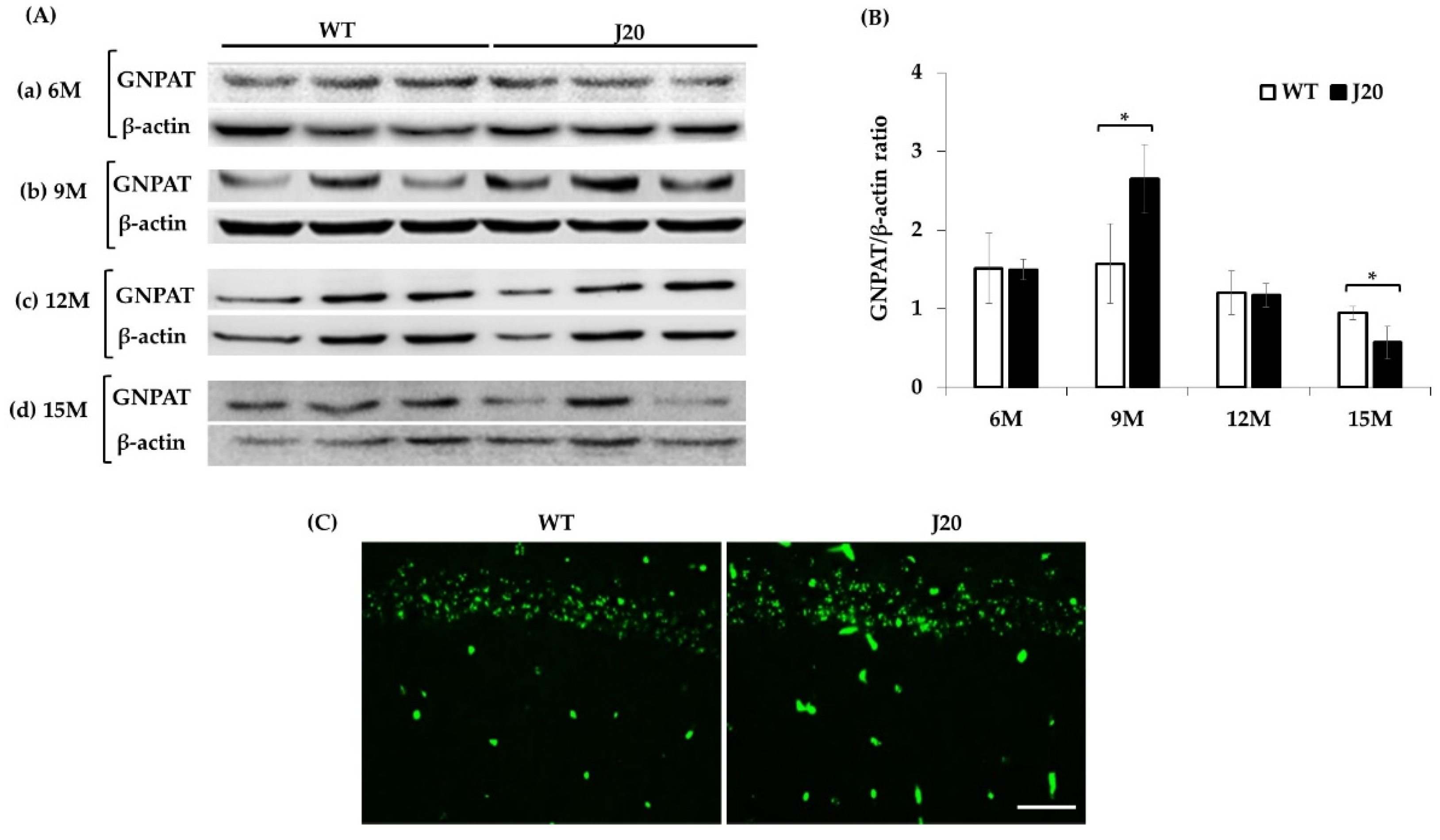
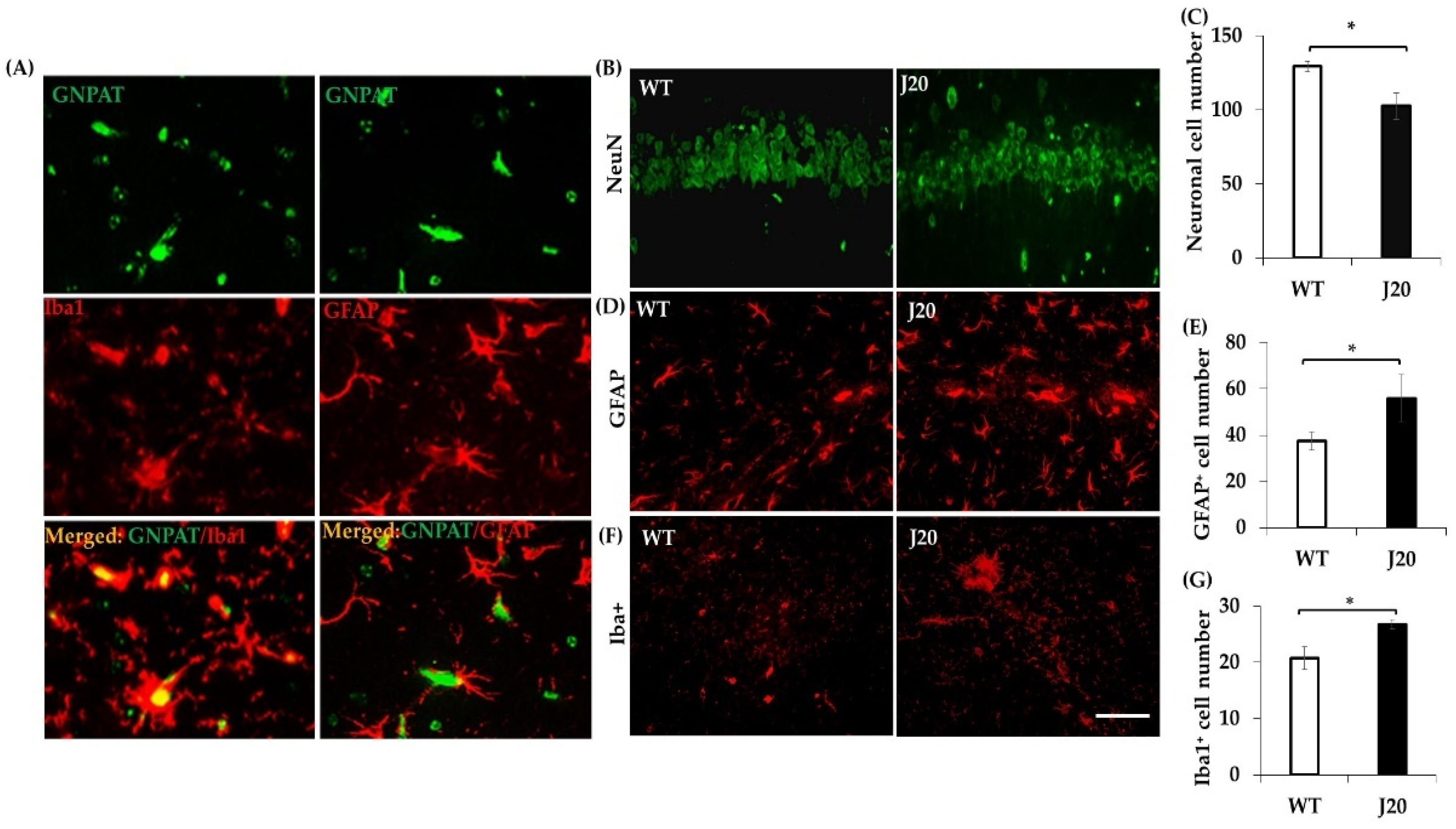
3.3. GNPAT Protein Expression and its Localization in the Hippocampus of J20 and WT Mice
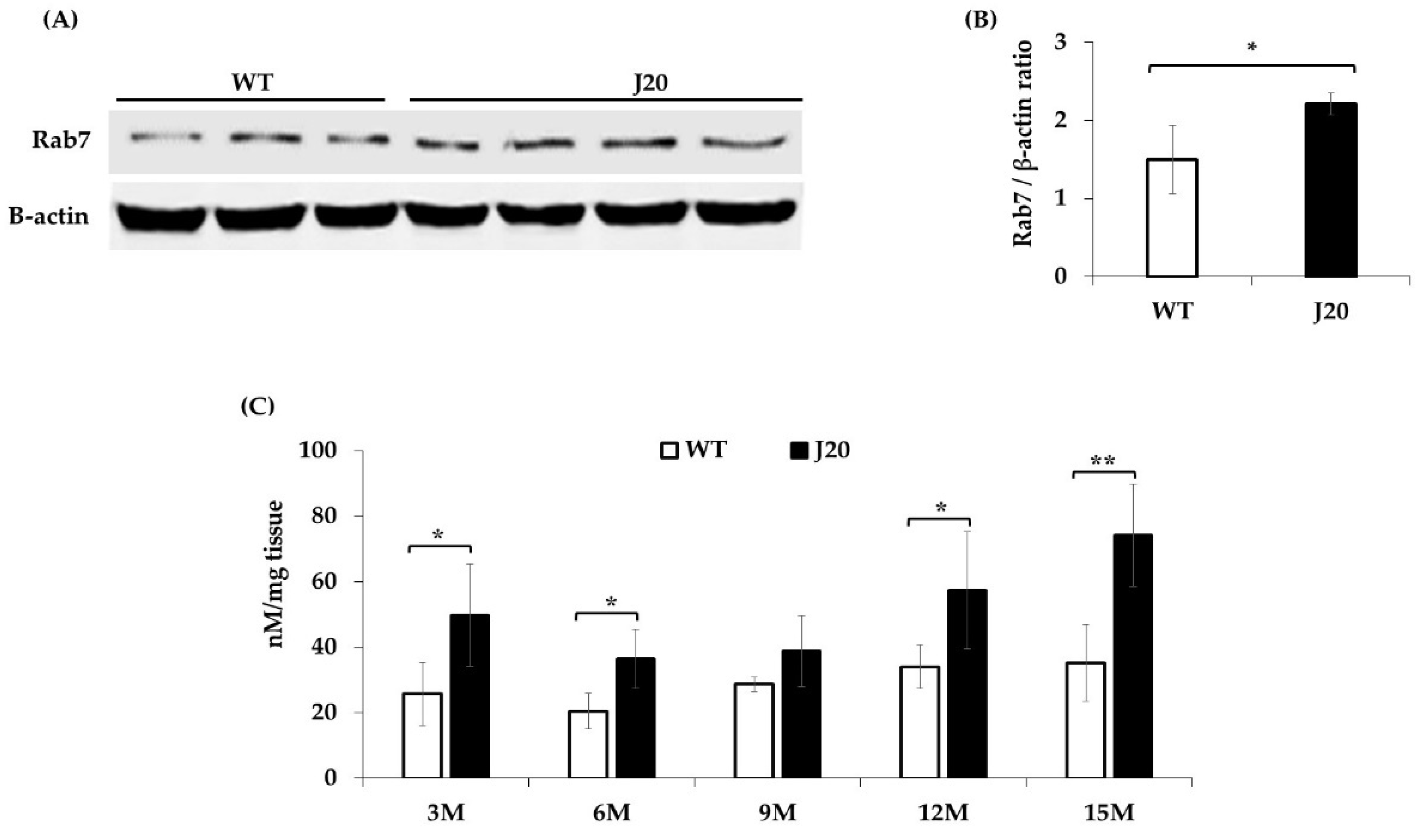
3.4. Change of Rab7 Protein at the 9 Months in the Hippocampus of J20 and WT Mice
3.5. Oxidative Stress Level Changes in the Hippocampus of J20 and WT Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paul, S.; Lancaster, G.I.; Meikle, P.J. Plasmalogens: A potential therapeutic target for neurodegenerative and cardiometabolic disease. Prog. Lipid Res. 2019, 74, 186–195. [Google Scholar] [CrossRef]
- Maeba, R.; Ueta, N. A novel antioxidant action of ethanolamine plasmalogens in lowering the oxidizability of membranes. Biochem. Soc. Trans. 2004, 32, 141–143. [Google Scholar] [CrossRef] [Green Version]
- Glaser, P.E.; Gross, R.W. Plasmenylethanolamine facilitates rapid membrane fusion: A stopped-flow kinetic investigation correlating the propensity of a major plasma membrane constituent to adopt an HII phase with its ability to promote membrane fusion. Biochemistry 1994, 33, 5805–5812. [Google Scholar] [CrossRef]
- Rog, T.; Koivuniemi, A. The biophysical properties of ethanolamine plasmalogens revealed by atomistic molecular dynamics simulations. Biochim. Biophys. Acta—Biomembr. 2016, 1858, 97–103. [Google Scholar] [CrossRef] [Green Version]
- Wood, P.L.; Khan, M.A.; Mankidy, R.; Smith, T.; Goodenowe, D.B. Plasmalogen deficit: A new and testable hypothesis for the etiology of Alzheimer’s disease. In Alzheimer’s Disease Pathogenesis-Core Concepts, Shifting Paradigms and Therapeutic Targets; De La Monte, S., Ed.; InTechOpen: London, UK, 2011. [Google Scholar] [CrossRef] [Green Version]
- Senanayake, V.; Goodenowe, D.B. Plasmalogen deficiency and neuropathology in Alzheimer’s disease: Causation or coincidence? Alzheimers Dement. Transl. Res. Clin. Interv. 2019, 5, 524–532. [Google Scholar] [CrossRef]
- Messias, M.C.F.; Mecatti, G.C.; Priolli, D.G.; De Oliveira Carvalho, P. Plasmalogen lipids: Functional mechanism and their involvement in gastrointestinal cancer. Lipids Health Dis. 2018, 17, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Rubio, J.M.; Astudillo, A.M.; Casas, J.; Balboa, M.A.; Balsinde, J. Regulation of phagocytosis in macrophages by membrane ethanolamine plasmalogens. Front. Immunol. 2018, 9, 1723. [Google Scholar] [CrossRef]
- Ding, Y.; Caberoy, N.B.; Guo, F.; LeBlanc, M.E.; Zhang, C.; Wang, W.; Wang, F.; Chen, R.; Li, W. Reticulocalbin-1 facilitates microglial phagocytosis. PLoS ONE 2015, 10, e0126993. [Google Scholar] [CrossRef] [Green Version]
- Harrison, R.E.; Bucci, C.; Vieira, O.V.; Schroer, T.A.; Grinstein, S. Phagosomes fuse with late endosomes and/or lysosomes by extension of membrane protrusions along microtubules: Role of Rab7 and RILP. Mol. Cell. Biol. 2003, 23, 6494–6506. [Google Scholar] [CrossRef] [Green Version]
- Lizard, G. (Ed.) Peroxisome Biology: And Neurological Peroxisomal Disorders Experimental Models, Disease. Adv. Exp. Med. Biol. 2020, 1299, 171–193. [Google Scholar] [CrossRef]
- Su, X.Q.; Wang, J.; Sinclair, A.J. Plasmalogens and Alzheimer’s disease: A review. Lipids Health Dis. 2019, 18, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Duyckaerts, C.; Delatour, B.; Potier, M.C. Classification and basic pathology of Alzheimer disease. Acta Neuropathol. 2009, 118, 5–36. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P.; Levine, H. Alzheimer’s disease and the amyloid-β peptide. J. Alzheimers Dis. 2010, 19, 311–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.C.M.; Askarova, S.; Yang, X. Impacts of membrane biophysics in Alzheimer’s disease: From amyloid precursor protein processing to Aβ peptide-induced membrane changes. Int. J. Alzheimers Dis. 2011, 2011, 12. [Google Scholar] [CrossRef] [Green Version]
- Kaya, I.; Jennische, E.; Lange, S.; Tarik Baykal, A.; Malmberg, P.; Fletcher, J.S. Brain region-specific amyloid plaque-associated myelin lipid loss, APOE deposition and disruption of the myelin sheath in familial Alzheimer’s disease mice. J. Neurochem. 2020, 154, 84–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kou, J.; Kovacs, G.G.; Höftberger, R.; Kulik, W.; Brodde, A.; Forss-Petter, S.; Hönigschnabl, S.; Gleiss, A.; Brügger, B.; Wanders, R.; et al. Peroxisomal alterations in Alzheimer’s disease. Acta Neuropathol. 2011, 122, 271–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grimm, M.O.W.; Kuchenbecker, J.; Rothhaar, T.L.; Grösgen, S.; Hundsdörfer, B.; Burg, V.K.; Friess, P.; Müller, U.; Grimm, H.S.; Riemenschneider, M.; et al. Plasmalogen synthesis is regulated via alkyl-dihydroxyacetonephosphate- synthase by amyloid precursor protein processing and is affected in Alzheimer’s disease. J. Neurochem. 2011, 116, 916–925. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.S.; Abe, Y.; Ali, F.; Youssef, M.; Honsho, M.; Fujiki, Y.; Katafuchi, T. Reduction of ether-type glycerophospholipids, plasmalogens, by NF-κB signal leading to microglial activation. J. Neurosci. 2017, 37, 4074–4092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hartmann, T.; Kuchenbecker, J.; Grimm, M.O.W. Alzheimer’s disease: The lipid connection. J. Neurochem. 2007, 103, 159–170. [Google Scholar] [CrossRef]
- Han, X.; Holtzman, D.M.; McKeel, D.W., Jr. Plasmalogen deficiency in early Alzheimer’s disease subjects and in animal models: Molecular characterization using electrospray ionization mass spectrometry. J. Neurochem. 2001, 77, 1168–1180. [Google Scholar] [CrossRef] [PubMed]
- Guan, Z.; Wang, Y.; Cairns, N.J.; Lantos, P.L.; Dallner, G.; Sindelar, P.J. Decrease and structural modifications of phosphatidylethanolamine plasmalogen in the brain with Alzheimer disease. J. Neuropathol. Exp. Neurol. 1999, 58, 740–747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Igarashi, M.; Ma, K.; Gao, F.; Kim, H.; Rapoport, S.I.; Rao, J.S. Disturbed choline plasmalogen and phospholipid fatty acid concentrations in Alzheimer disease prefrontal cortex. Brain 2011, 24, 507–517. [Google Scholar] [CrossRef]
- Rothhaar, T.L.; Grösgen, S.; Haupenthal, V.J.; Burg, V.K.; Hundsdörfer, B.; Mett, J.; Riemenschneider, M.; Grimm, H.S.; Hartmann, T.; Grimm, M.O.W. Plasmalogens inhibit APP processing by directly affecting γ -secretase activity in Alzheimer’s disease. Sci. World J. 2012, 2012, 15. [Google Scholar] [CrossRef] [Green Version]
- Dacremont, G.; Vincent, G. Assay of plasmalogens and Polyunsaturated Fatty Acids (PUFA) in erythrocytes and fibroblasts. J. Inherit. Metab. Dis. 1995, 18, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Zemski Berry, K.A.; Murphy, R.C. Electrospray ionization tandem mass spectrometry of glycerophosphoethanolamine plasmalogen phospholipids. J. Am. Soc. Mass Spectrom. 2004, 15, 1499–1508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mawatari, S.; Okuma, Y.; Fujino, T. Separation of intact plasmalogens and all other phospholipids by a single run of high-performance liquid chromatography. J. Anal. Biochem. 2007, 370, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Otoki, Y.; Nakagawa, K.; Kato, S.; Miyazawa, T. MS/MS and LC-MS/MS analysis of choline/ethanolamine plasmalogens via promotion of alkali metal adduct formation. J. Chromatogr. B 2015, 1004, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Azad, A.K.; Kobayashi, H.; Sheikh, A.M.; Osago, H.; Sakai, H.; Haque, M.A.; Yano, S.; Nagai, A. Rapid identification of plasmalogen molecular species using targeted multiplexed selected reaction monitoring mass spectrometry. J. Mass Spectrom. Adv. Clin. Lab. 2021, 22, 26–33. [Google Scholar] [CrossRef]
- Galvan, V.; Gorostiza, O.F.; Banwait, S.; Ataie, M.; Logvinova, A.V.; Sitaraman, S.; Carlson, E.; Sagi, S.A.; Chevallier, N.; Jin, K.; et al. Reversal of Alzheimer’s-like pathology and behavior in human APP transgenic mice by mutation of Asp664. Proc. Natl. Acad. Sci. USA 2006, 103, 7130–7135. [Google Scholar] [CrossRef] [Green Version]
- Wright, A.L.; Zinn, R.; Hohensinn, B.; Konen, L.M.; Beynon, S.B.; Tan, R.P.; Clark, I.A.; Abdipranoto, A.; Vissel, B. Neuroinflammation and neuronal loss precede Aβ plaque deposition in the hAPP-J20 mouse model of Alzheimer’s disease. PLoS ONE 2013, 8, e59586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ameen-Ali, K.E.; Simpson, J.E.; Wharton, S.B.; Heath, P.R.; Sharp, P.; Brezzo, G.; Berwick, J. The time course of recognition memory impairment and glial pathology in the hAPP-J20 mouse model of Alzheimer’s disease. J. Alzheimers Dis. 2019, 68, 609–624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbott, S.K.; Jenner, A.M.; Mitchell, T.W.; Brown, S.H.J.; Halliday, G.M.; Garner, B. An improved high-throughput lipid extraction method for the analysis of human brain lipids. Lipids 2013, 48, 307–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hashimoto, M.; Hossain, S.; Shimada, T.; Sugioka, K.; Yamasaki, H.; Fujii, Y.; Ishibashi, Y.; Oka, J.I.; Shido, O. Docosahexaenoic acid provides protection from impairment of learning ability in Alzheimer’s disease model rats. J. Neurochem. 2002, 81, 1084–1091. [Google Scholar] [CrossRef] [PubMed]
- Gamaleldin, N.M.; Bakeer, W.; Sayed, A.M.; Shamikh, Y.I.; El-Gendy, A.O.; Hassan, H.M.; Horn, H.; Abdelmohsen, U.R.; Hozzein, W.N. Exploration of chemical diversity and antitrypanosomal activity of some red sea-derived actinomycetes using the OSMAC approach supported by LC-MS-based metabolomics and molecular modelling. Antibiotics 2020, 9, 629. [Google Scholar] [CrossRef] [PubMed]
- Hatazawa, Y.; Senoo, N.; Tadaishi, M.; Ogawa, Y.; Ezaki, O.; Kamei, Y.; Miura, S. Metabolomic analysis of the skeletal muscle mice overexpressing PGC-1α. PLoS ONE 2015, 10, e0129084. [Google Scholar] [CrossRef]
- Wood, P.L.; Mankidy, R.; Ritchie, S.; Heath, D.; Wood, J.A.; Flax, J.; Goodenowe, D.B. Circulating plasmalogen levels and Alzheimer disease assessment scale-cognitive scores in Alzheimer patients. J. Psychiatry Neurosci. 2010, 35, 59–62. [Google Scholar] [CrossRef] [Green Version]
- Goodenowe, D.B.; Cook, L.L.; Liu, J.; Lu, Y.; Jayasinghe, D.A.; Ahiahonu, P.W.K.; Heath, D.; Yamazaki, Y.; Flax, J.; Krenitsky, K.F.; et al. Peripheral ethanolamine plasmalogen deficiency: A logical causative factor in Alzheimer’s disease and dementia. J. Lipid Res. 2007, 48, 2485–2498. [Google Scholar] [CrossRef] [Green Version]
- Goodenowe, D.B.; Senanayake, V. Relation of serum plasmalogens and APOE genotype to cognition and dementia in older persons in a cross-sectional study. Brain Sci. 2019, 9, 92. [Google Scholar] [CrossRef] [Green Version]
- Wood, P.L.; Locke, V.A.; Herling, P.; Passaro, A.; Vigna, G.B.; Volpato, S.; Valacchi, G.; Cervellati, C.; Zuliani, G. Targeted lipidomics distinguishes patient subgroups in Mild Cognitive Impairment (MCI) and Late Onset Alzheimer’s Disease (LOAD). BBA Clin. 2016, 5, 25–28. [Google Scholar] [CrossRef] [Green Version]
- Ginsberg, L.; Rafique, S.; Xuereb, J.H.; Rapoport, S.I.; Gershfeld, N.L. Disease and anatomic specificity of ethanolamine plasmalogen deficiency in Alzheimer’s disease brain. Brain Res. 1995, 698, 223–226. [Google Scholar] [CrossRef]
- Wood, P.L.; Barnette, B.L.; Kaye, J.A.; Quinn, J.F.; Woltjer, R.L. Non-targeted lipidomics of CSF and frontal cortex grey and white matter in control, mild cognitive impairment, and Alzheimer’s disease subjects. Acta Neuropsychiatr. 2015, 27, 270–278. [Google Scholar] [CrossRef]
- Otoki, Y.; Kato, S.; Nakagawa, K.; Harvey, D.J.; Jin, L.W.; Dugger, B.N.; Taha, A.Y. Lipidomic analysis of postmortem prefrontal cortex phospholipids reveals changes in choline plasmalogen containing docosahexaenoic acid and stearic acid between cases with and without Alzheimer’s disease. NeuroMol. Med. 2021, 23, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Pomilio, C.; Pavia, P.; Gorojod, R.M.; Vinuesa, A.; Alaimo, A.; Galvan, V.; Kotler, M.L.; Beauquis, J.; Saravia, F. Glial alterations from early to late stages in a model of Alzheimer’s disease: Evidence of autophagy involvement in Aβ internalization. Hippocampus 2016, 26, 194–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Youssef, M.; Ibrahim, A.; Hossain, S. PUFA-plasmalogens attenuate the LPS-induced nitric oxide production by inhibiting the NF-kB, p38 MAPK and JNK pathways in microglial cells. Neuroscience 2019, 397, 18–30. [Google Scholar] [CrossRef]
- Ali, F.; Hossain, M.S.; Abdeen, A.; Uddin, M.S.; Gaber, A.; Alsanie, W.F.; Waheed, R.; Shanab, O.; Ahmed, H. Plasmalogens ensure the stability of non-neuronal (microglial) cells during long-term cytotoxicity. Environ. Sci. Pollut. Res. 2021, 28, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Thai, T.-P.; Rodemer, C.; Jauch, A.; Hunziker, A.; Moser, A.; Gorgas, K.; Just, W.W. Impaired membrane traffic in defective ether lipid biosynthesis. Hum. Mol. Genet. 2001, 10, 127–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gil-de-Gómez, L.; Astudillo, A.M.; Lebrero, P.; Balboa, M.A.; Balsinde, J. Essential role for ethanolamine plasmalogen hydrolysis in bacterial lipopolysaccharide priming of macrophages for enhanced arachidonic acid release. Front. Immunol. 2017, 8, 1251. [Google Scholar] [CrossRef] [PubMed]
- Walsh, D.M.; Klyubin, I.; Fadeeva, J.V.; Cullen, W.K.; Anwyl, R.; Wolfe, M.S.; Rowan, M.J.; Selkoe, D.J. Naturally secreted oligomers of amyloid β protein potently inhibit hippocampal long-term potentiation in vivo. Nature 2002, 416, 535–539. [Google Scholar] [CrossRef]
- Selkoe, D.J. Soluble oligomers of the amyloid β-protein impair synaptic.pdf. Behav. Brain Res. 2008, 192, 106–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drake, J.; Link, C.D.; Butterfield, D.A. Oxidative stress precedes fibrillar deposition of Alzheimer’s disease amyloid β-peptide (1-42) in a transgenic Caenorhabditis elegans model. Neurobiol. Aging 2003, 24, 415–420. [Google Scholar] [CrossRef]
- Meletis, C.D. Alkyl-acylglycerols and the important clinical ramifications of raising plasmalogens in dementia and Alzheimer’s disease. Integr. Med. 2020, 19, 12–16. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azad, A.K.; Sheikh, A.M.; Haque, M.A.; Osago, H.; Sakai, H.; Shibly, A.Z.; Yano, S.; Michikawa, M.; Hossain, S.; Tabassum, S.; et al. Time-Dependent Analysis of Plasmalogens in the Hippocampus of an Alzheimer’s Disease Mouse Model: A Role of Ethanolamine Plasmalogen. Brain Sci. 2021, 11, 1603. https://doi.org/10.3390/brainsci11121603
Azad AK, Sheikh AM, Haque MA, Osago H, Sakai H, Shibly AZ, Yano S, Michikawa M, Hossain S, Tabassum S, et al. Time-Dependent Analysis of Plasmalogens in the Hippocampus of an Alzheimer’s Disease Mouse Model: A Role of Ethanolamine Plasmalogen. Brain Sciences. 2021; 11(12):1603. https://doi.org/10.3390/brainsci11121603
Chicago/Turabian StyleAzad, Abul Kalam, Abdullah Md. Sheikh, Md. Ahsanul Haque, Harumi Osago, Hiromichi Sakai, Abu Zaffar Shibly, Shozo Yano, Makoto Michikawa, Shahdat Hossain, Shatera Tabassum, and et al. 2021. "Time-Dependent Analysis of Plasmalogens in the Hippocampus of an Alzheimer’s Disease Mouse Model: A Role of Ethanolamine Plasmalogen" Brain Sciences 11, no. 12: 1603. https://doi.org/10.3390/brainsci11121603





